Published online Oct 18, 2015. doi: 10.5312/wjo.v6.i9.680
Peer-review started: June 1, 2015
First decision: June 24, 2015
Revised: July 5, 2015
Accepted: August 10, 2015
Article in press: August 11, 2015
Published online: October 18, 2015
Processing time: 144 Days and 10.8 Hours
Imaging of the spine is of paramount importance for the recognition of osteoporotic vertebral fractures (VFs), and standard radiography (SR) of the spine is the suggested diagnostic method but is not routinely used because of the cost and radiation exposure considerations. VF assessment (VFA) is an efficient, low radiation method for identifying VFs at the time of bone mineral density (BMD) measurement. Prediction models used to indicate the need for VFA may have little predictive power in subspecialty referral populations such as rheumatologic patients or patients who underwent kyphoplasty. Rheumatologic patients are frequently at increased risk for VFs, and VFA should be performed on an individual basis, also taking in account the guidelines for the general population. Kyphoplasty is a new minimal invasive procedure for the treatment of VFs and is being performed with increasing frequency. Following kyphoplasty, there may be a risk of new VFs in adjacent vertebrae. The assessment and follow-up of patients who underwent kyphoplasty requires repetitive X-ray imaging with the known limitations of SR. Thus, VFA may facilitate the evaluation of VFs in these patients because most of the kyphoplasty patients would fulfill the criteria. In a pilot study, we measured the BMD and performed VFA in 28 patients treated with kyphoplasty. Ratios of anterior to posterior (A/P) and middle to posterior (M/P) height were measured, and Genant’s method was used to classify vertebrae accordingly. Intraobserver and interobserver reliability for A/P, M/P and the Genant’s method were determined. Only 1 patient did not meet the criteria for VFA. Of the 364 available vertebrae, 295 could be analyzed. Most missing data (concerning 69 vertebrae) occurred in the upper thoracic region. Three of the 69 non-eligible vertebrae were lumbar vertebrae with cement leakage from the kyphoplasty procedure. In our hands, VFA was highly reproducible, demonstrating very good agreement in terms of intraobserver and interobserver reliability. Agreement was very good on the vertebral level, “vertebrae with kyphoplasty” level and “2 above and 1 below the kyphoplasty vertebrae” level. The application of Genant’s method to these patients also resulted in perfect agreement. We believe that the potential value of VFA in patients treated with kyphoplasty requires further evaluation, particularly comparing VFA with SR and performing a longitudinal follow-up. More research will help to adopt care processes that determine which patients require VFA and how often VFA should be performed, while also considering the impact of this technique on the cost of healthcare organizations.
Core tip: Vertebral fracture assessment (VFA) is an efficient, low radiation method of identifying vertebral fractures at the time of bone mineral density measurement. Models used to indicate the need for VFA may have little predictive power in subgroups of the general population such as patients with kyphoplasty. In our hands, VFA applied in patients with kyphoplasty was highly reproducible, demonstrating very good agreement in terms of intraobserver and interobserver reliability. More research will help the adoption of care processes to determine when and how often VFA should be performed, considering also the impact of such cost on healthcare organizations.
- Citation: Drampalos E, Nikolopoulos K, Baltas C, Balanika A, Galanos A, Papaioannou N, Pneumaticos S. Vertebral fracture assessment: Current research status and application in patients with kyphoplasty. World J Orthop 2015; 6(9): 680-687
- URL: https://www.wjgnet.com/2218-5836/full/v6/i9/680.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i9.680
Vertebral fracture (VF) is the emblem of osteoporosis and is associated with increased mortality and morbidity[1]. The clinical symptoms include pain (acute and chronic), impaired pulmonary function, thoracic kyphosis, height loss, depression and deterioration of quality of life. Thoracic and lumbar fractures are unique because most of the fractures do not present clinical symptoms at the time of their occurrence[2,3].
VFs commonly occur in postmenopausal women and older men, with an estimated prevalence of 10%-26% in both men and women age 50 and older, depending on the population and definition of VF utilized[3-6]. Prevalent VFs anticipate new fractures independently of bone mineral density (BMD), as patients with one or more fractures have a 4-fold increased risk of subsequent hip fractures and a 5-fold increased risk of further VFs[7]. Furthermore, VFs that do not come to medical attention seem to be associated with increased back pain and functional limitation[8].
Despite all of these facts, in a multicenter, multinational prospective study (the IMPACT trial), underdiagnosis of VFs was observed in all geographic regions (false-negative rates: North America, 45.2%; Latin America, 46.5%; and Europe/South Africa/Australia, 29.5%) with a global false-positive rate of 5%. According to the same study, underdiagnosis of VFs is a worldwide problem attributable, in part, to a lack of radiographic detection, use of ambiguous terminology in the radiology report, or both[9].
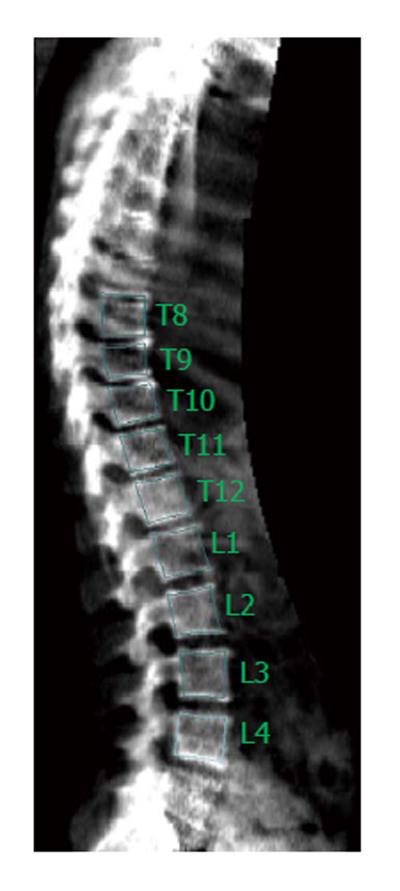
Imaging of the spine is of paramount importance for the recognition of VFs. Standard radiography (SR) of the spine is the reference method for identifying VFs but is not used routinely because of the cost and radiation exposure considerations. Dual-energy X-ray absorptiometry (DXA) systems equipped with special software can be used for the detection of VFs obtaining lateral views of the thoracic and lumbar spine (Figure 1). Densitometric imaging of the spine allows VF analysis to be efficiently performed simultaneously, improving the overall assessment of an individual’s future fracture risk with a very low radiation dose (10 microsieverts vs 800 microsieverts for standard anteroposterior and lateral radiographs of the thoracic and lumbar spine)[10]. Although lateral imaging of the spine with the use of X-ray absorptiometry has been previously described with different terms from the manufacturers (lateral vertebral assessment, dual vertebral assessment and instant vertebral assessment), the ISCD 2005 Official Positions replaced these terms with the label “Vertebral Fracture Assessment” or VF assessment (VFA)[11]. Furthermore, vertebrae with an appearance consistent with a prevalent fracture on VFA images or radiographs are often characterized as deformities, implying that some of the “deformities” identified may not really be VFs[11].
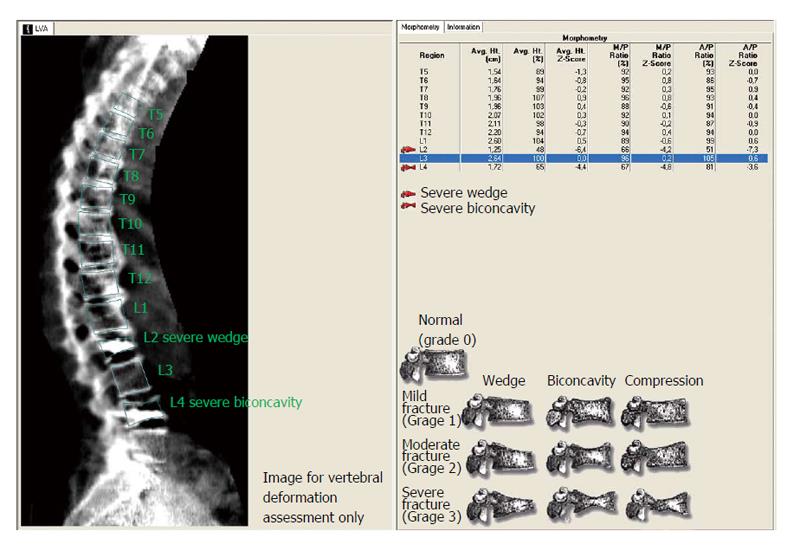
There are several methods available to determine vertebral deformities (VDs) and distinguish them from vertebrae with a normal shape. The semi-quantitative technique developed by Genant et al[12] is one of the most common approaches. The qualitative feature of the vertebrae shape is considered together with the approximate loss of vertebrae height. This method uses fixed values of loss of height (0.60, 0.75 and 0.80).
A plethora of quantitative morphometric methods of detecting VFs has also been described. That study determined a VF based on reductions of anterior (A) and middle height (M) relative to the posterior height (P) within the vertebra and/or on reductions of these heights relative to corresponding heights of adjacent vertebrae. Most studies also used appropriate population-based samples of men and women. McCloskey et al[13] developed a quantitative technique that takes into account the size of adjacent vertebrae into assessing VFs. Eastell et al[14] defined a fracture based on the deviation of the vertebral height of > 3 SD compared with a population-based sample. Melton et al[15] used the ratio of 0.85 for the definition of VFs, with the vertebral dimensions being adjusted for the specific level. Ross et al[16] developed a method that uses Z-scores to identify VFs. A modified approach to visual diagnosis of VFs, known as the algorithm-based qualitative method (ABQ), was proposed by Jiang et al[17]. This technique focuses on the appearance of the central vertebral endplate to identify VFs without a minimum threshold of vertebral height reduction.
When used for VFA images, the Genant’s method, quantitative morphometry and the most recent ABQ method result in good intraobserver and interobserver reliability, approximating that of SR[18-20]. In a multicenter study, including 203 postmenopausal women with imaging of their spine by both DXA and SR that were independently evaluated by three experienced radiologists on two different occasions, there was good agreement with kappa values ranging from 0.64 to 0.77[21]. Furthermore, VFA has been shown to be both sensitive and specific in several studies. In 205 women age 65 and older undergoing bone densitometry for BMD measurement, VFA compared to SR was 87%-93% sensitive and 93%-95% specific[18]. In another study including 80 postmenopausal women, clinicians utilizing VFA with a Genant semi-quantitative method identified the vast majority of grade 2 or 3 VFs and normal vertebral bodies (with a false negative rate of 6%) but only identified 50% of grade 1 fractures[22].
VFA has also a good negative predictive value (NPV) that reaches even 95% in some studies[23]. However, visualization of the upper thoracic spine is of poorer quality for VFA compared to SR, and most of the studies have noted a larger percentage of vertebrae that are not visualized clearly with VFA[24]. Non-eligible vertebrae are particularly common superior to the 7th thoracic vertebra (T7) but there are relatively few osteoporotic VFs above T7.
According to the 2007 Official Positions of the International Society for Clinical Densitometry (ISCD), the candidate indications for VFA were established on the basis that documentation of a prevalent VF may alter treatment of that individual and that there is a reasonable pre-test probability that a prevalent VF is found on VFA[11]. SR following VFA should be performed when grade 2 or milder VFs are present without more severe VFs, VDs are present in patients with a known history of malignancy and when VDs cannot be assigned to benign causes[11].
The 2013 updated positions of the ISCD simplified the indications, and spine imaging with VFA or SR is recommended when the T-score is less than -1.0 and if one or more of the following factors are present[25]: (1) Women age > 70 years or men age > 80 years; (2) Historical height loss > 4 cm (> 1.5 in.); (3) Self-reported but undocumented prior vertebral fracture; and (4) Glucocorticoid therapy equivalent to > 5 mg of prednisolone or equivalent per day for > 3 mo.
One of the aims of this study was to develop a regression-based prediction model to be integrated into the densitometric software so that bone technicians can proceed to a VFA in addition to the standard bone density measurement if the likelihood of prevalent VF is > 10%[25]. In fact, Schousboe et al[11] developed an algorithm to be used by technologists to identify patients for whom VFA should be performed, simplifying the criteria from the ISCD 2007 position statement (T-score -1.5 or worse and one of the following factors: age 65 years or older, historical height loss > 1.5 in, or systemic glucocorticoid therapy at the time of their DXA test). They concluded that such use can be feasible in clinical practice and that documentation of VFs increased the prescription of fracture prevention medication[26]. However, there are no recommendations about the role of VFA in detecting incident fractures in patients receiving osteoporosis treatment or the interval between consecutive VFAs.
Models used to form indications for VFA have been developed in general populations and may have little predictive power in special populations such as patients with ankylosing spondylitis (AS), rheumatoid arthritis (RA) or inflammatory bowel disease.
In rheumatology practice VFA has been shown to be useful for revealing VFs[27]. Patients with AS or RA are at increased risk for VFs compared to the general population[28,29]. Known risk factors include age, low BMD, use of glucocorticoids, disease duration and high levels of disease activity[28-33].
Mohammad et al[31] performed VFA scans in a cohort of 603 patients with RA of age > 40 years. For the entire cohort, 13% (77/603) of patients had one or more vertebral deformities identified on VFA imaging: 58% of these patients were female with a mean age of 56 years. The prevalence of osteoporosis and osteopenia was 59% and 40%, respectively, with the prevalence and severity of VFs showing significant correlation with spine T-scores and femoral T-scores. In multivariable analyses VFs were significantly and independently associated with a longer duration of RA, markers of disease activity and severity[31].
In one of the recent studies on the role of VFA in RA, 100 women underwent lateral imaging of the thoraco-lumbar spine by SR and VFA. All patients with a history of previous VF (n = 13) were visualized with VFA. The sensitivity, positive predictive value, specificity and NPV of VFA compared to SR were 57.3%, 30.9%, 89.1% and 96.1%, respectively, for the total vertebrae[34].
There is less research conducted with regards to VFA in patients with AS. As in rheumatoid arthritis, VFA needs to be validated. Vosse et al[35] compared VFA with SR in 30 patients with AS. Although the agreement between methods in measuring vertebral wedging [expressed as (mean) A/P ratio] was good, agreement between methods in assessing whether there is a fracture was insufficient. However, as the NPV was high (97%), VFA could be of clinical value to select patients for further evaluation by SR.
According to the 2013 official positions of ISCD, VFA for these subsets populations should be performed on an individual basis taking also in account the guidelines for the general population. On 2012, the French Society for Rheumatology and the Osteoporosis Research and Information Group updated their recommendations for the pharmacological treatment of post-menopausal osteoporosis and suggested the same indications for VFA as recommended by the ISCD[36]. Finally, the 2010 American College of Rheumatology recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis suggest considering lateral imaging of the spine with SR or VFA for patients starting or currently receiving prednisolone > 5 mg/d[37].
Kyphoplasty is new minimal invasive procedure for the treatment of osteoporotic VFs and is being performed with increasing frequency. The technique was developed to provide relief to patients with painful VFs and entails inflation of a percutaneously delivered balloon in the vertebral body, followed by the percutaneous injection of bone cement into the cavity created by the balloon. Chemotoxicity, thermal necrosis during exothermic polymerization, and mechanical stability provided by the cured bone cement are the most likely mechanisms for pain relief in kyphoplasty[38-41].
Through the use of a balloon, kyphoplasty is intended to provide restoration of vertebral body height. Based on current evidence, kyphoplasty does not restore substantial vertebral body height in most patients, and the intrinsic value of vertebral body height restoration remains speculative. Patients treated with kyphoplasty are generally quite satisfied with their pain relief and rarely express disappointment in a lack of height restoration[40,41].
There may be an additional risk of new VFs developing in adjacent vertebrae subsequent to kyphoplasty[42]. Because new VFs can occur in osteoporotic patients simply due to disease progression rather than as a result of the kyphoplasty, it is difficult to determine the added risk of fracture resulting from this procedure[38-41].
The assessment and follow-up of patients who underwent kyphoplasty requires repetitive X-ray imaging with the limitations of radiography in terms of radiation, cost, patient positioning and geometric distortion within vertebrae located above or below the central point of the beam (parallax effect because the endplates are projected obliquely, giving them an elliptical appearance)[10,43,44]. Thus, VFA is a technique that may facilitate the evaluation of VFs in these patients. In fact, patients treated with kyphoplasty frequently are: (1) Osteopenic or osteoporotic patients with a T-score less than -1.0 and need regular BMD measurements; (2) Women or men age > 70 or even 80; (3) Patients that present with height loss; (4) Patients that have a history of prior VF; or (5) Patients that having an additional risk for new VFs because of the natural course of the osteoporosis or of the kyphoplasty.
Furthermore, chronic rheumatologic patients under treatment with glucocorticoids have an increased risk for VFs[28-33] and can be treated with kyphoplasty. Considering the above criteria, most of the kyphoplasty patients would fulfill the criteria for VFA according to the 2013 ISCD Official Position[25].
Before any data collection, local ethics committee approval was obtained for the present cross sectional study.
Twenty-eight patients treated with kyphoplasty for acute symptomatic osteoporotic VFs were included. The patients were evaluated according to a standard protocol, which included measurement of BMD (of both nondominant hip and lumbar spine) and densitometric images of the spine with the patient in the lateral position using a Lunar Prodigy Advance densitometer (GE Healthcare Buckinghamshire, United Kingdom). For the calculation of the mean T-score, the least value between the lumbar spine and hip (femoral neck, or total proximal femur) was included for every patient. At the lumbar spine, at least 3 vertebrae (between L1 and L4) had to be evaluable, that is without kyphoplasty, for the T-score to be taken into consideration. For VFA images, the Lunar software of the densitometer was used to set markers on the vertebral margins by two observers (XM and ED), and anterior (A), middle (M) and posterior (P) heights together with their ratios were measured (Figure 2). VFs were assessed by calculating the A/P, M/P ratios.
To measure intraobserver reliability for A/P and M/P ratios, each observer read every densitometric image twice in a random order, evaluating vertebrae from T4 to L4. To assess interobserver reliability, VFA images were read independently from the two observers (XM and ED), who were blinded to each other’s assessment.
We estimated the intraobserver and interobserver reliability for A/P and M/P ratios using the intra-class correlation coefficient (ICC) on the “vertebral level” (n = 364), as well as on the “vertebrae with kyphoplasty” level (n = 49) and adjacent to the kyphoplasty vertebrae level (“2 above and 1 below the kyphoplasty vertebrae”, n = 82). Furthermore, following Genant’s method, we used the cut-off ratios of 0.60, 0.75 and 0.80 for the A/P ratio to categorize the vertebrae as grade 0 (non fracture), grade 1 (mild), grade 2 (moderate) and grade 3 (severe) fractures and calculated Cohen’s kappa value (κ) with quadratic weighting. The κ value was determined on a vertebral level with 95% confidence intervals. To interpret the level of agreement based upon Cohen’s κ results the Landis and Koch guidelines were followed[45]. Thus, a Cohen’s κ value greater than 0.81 was considered almost perfect agreement; between 0.8 and 0.61, substantial agreement; between 0.6 and 0.4, moderate agreement; between 0.4 and 0.2, fair agreement; between 0.2 and 0, slight agreement; and less than zero, poor agreement. All tests were two-sided and statistical significance was set at P < 0.05. All analyses were carried out using the computer software SPSS for Windows (IBM SPSS Statistics 21 NY, United States).
Ten patients were male, 18 patients were female and the mean age was 72.4 years (range, 54-84). Only 1 patient did not meet the 2013 ISCD criteria for VFA[25] because of a T score > -1. The mean T-score was -2.28 (range, -4.1 to -0.8), and the mean height was 1.52 m (range, 1.38-1.71 m). Furthermore, the mean weight was 70 kg (range, 51-98 kg), the mean body mass index was 30 (range, 22-41) and the mean follow-up from the operation date was 34 mo (range, 14-60 mo).
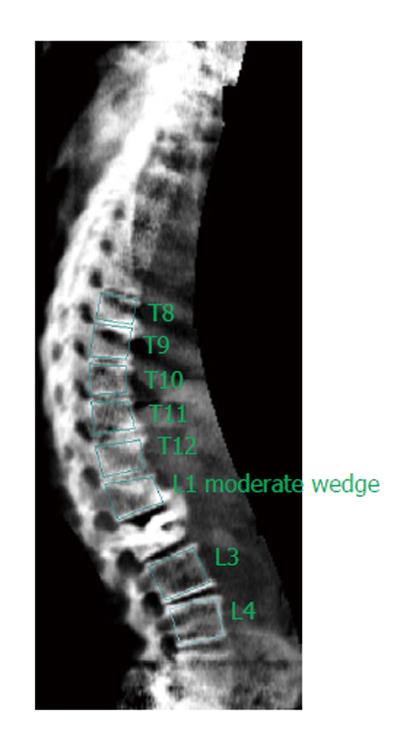
Of the 364 available vertebrae, 295 could be analyzed. Most instances of missing data (concerning 69 vertebrae) occurred in the thoracic T4-T6 region and were equally distributed across the two readers. Three of the 69 non-eligible vertebrae were vertebrae that had been treated with kyphoplasty. Both of the readers characterized these vertebrae as non-eligible. These vertebrae were three lumbar vertebrae (one L1 and two L2) of three different patients and had the presence of cement leakage from the kyphoplasty in common.
Intraobserver agreement for A/P ratios was very good for both readers (XM and ED), with ICC = 0.98 (95%CI: 0.977-0.988) for XM and ICC = 0.96 (95%CI: 0.957-0.973) for ED. Intraobserver agreement for M/P ratios was also very good for both readers, with ICC = 0.977 (95%CI: 0.971-0.982) for XM and ICC = 0.945 (95%CI: 0.93-0.956) for ED. Interobserver reliability (first assessments of the two readers were compared) for A/P and M/P ratios, the results were again very good, with ICC = 0.951 (95%CI: 0.938-0.961) for A/P and ICC = 0.947 (95%CI: 0.933-0.958) for M/P.
Agreements on the “vertebrae with kyphoplasty” level and on the “2 above and 1 below the kyphoplasty vertebrae”, were also very good. There was very good interobserver reliability for the A/P ratio on the “vertebrae with kyphoplasty” level with ICC = 0.94 (95%CI: 0.894-0.966) and for the A/P ratio on the “2 above and 1 below the kyphoplasty vertebrae” level with ICC = 0.969 (95%CI: 0.951-0.98).
With regards to Genant’s method, after using the cut-off ratios of 0.60, 0.75 and 0.80 for A/P ratio and classifying the vertebrae, interobserver agreement was almost perfect calculating a κ value with Quadratic Weighting = 0.833 (95%CI: 0.82-0.95). Furthermore, using Genant’s method and taking in account the first observer’s measurements (XM), 15 VFs were found on the “2 above and 1 below the kyphoplasty vertebrae” level. More specifically, there were nine VFs on the adjacent-superior to the kyphoplasty vertebrae, one VF on the adjacent-inferior to the kyphoplasty vertebrae and five VFs were identified in two vertebrae above the kyphoplasty. Eleven of these 15 VFs were at the thoracolumbar junction (T12-L1), three were on T11 and one was on T9. Seven more VFs were found on the rest of those not adjacent to the kyphoplasty vertebrae.
In our hands, VFA was highly reproducible when applied to patients treated with kyphoplasty, demonstrating very good agreement in terms of intraobserver and interobserver reliability. Agreement was very good on the vertebral level, “vertebrae with kyphoplasty” level and “2 above and 1 below the kyphoplasty vertebrae” level. Application of Genant’s method on these patients also resulted in perfect agreement.
With regards to the patient who did not meet the criteria for VFA, the lowest T-score = -0.9 of the hip was considered. She was a woman aged 68 years with two kyphoplasty vertebrae (T11 and T12). Being under treatment with intravenous zoledronic acid for the last two years, post-kyphoplasty may have improved her BMD. The 69 individual non-eligible vertebrae (most of them in the thoracic T4-T6 region) were in concordance also with studies where VFA was applied in general population groups or in subgroups such as patients with ankylosing spondylitis[24,35]. Three of the 69 non-eligible vertebrae were kyphoplasty vertebrae in 3 patients presenting cement leakage (Figure 3). In fact, cement extravasation alters vertebral shape, and as a result, it is extremely difficult for the reader to assess the vertebra.
It is still a matter of debate as to whether vertebrae neighboring the kyphoplasty are more likely to fracture[42]. According to our database, most of the fractures on the vertebrae without kyphoplasty were on the “2 above and 1 below the kyphoplasty vertebrae” level (15 VFs), but we have no data on whether these were new fractures or present before the kyphoplasty.
We believe that the potential value of VFA in patients treated with kyphoplasty needs further evaluation, in particular, evaluations comparing VFA with SR and including longitudinal follow-up. Although we did not perform longitudinal VFA assessments, VFA has the potential to be a very useful test for longitudinal follow-up. Our pilot study demonstrated that VFA could be a valuable technique in terms of intraobserver and interobserver reliability for the determination of the height ratios of the vertebrae or using Genant’s method. A larger study, comparing the agreement between VFA and SR in patients who underwent kyphoplasty is currently underway. Moreover, considering the guidelines for monitoring BMD in populations at high risk for VFs, patients who underwent kyphoplasty should have their DXA measurement regularly at least every 1 or 2 years[46]. In an era in which all dimensions of pathology should be accounted for, serial BMD testing and VFA should probably be part of routine practice in this at-risk population, and having a single exam that entails minimal irradiation and allows for the assessment of major structural changes certainly warrants further systematic evaluation.
Overall, assessing structural damage is essential in patients treated with kyphoplasty and has key implications for treatment outcomes. SR remains the gold standard to evaluate the spines of these patients, despite all its disadvantages and limitations. Our study demonstrates a potential for VFA in these patients. The technique of VFA is much less irradiating than standard radiographs, easily available during the BMD determination and inexpensive in the modern era of economic crisis.
Future research is needed to determine if other predictors can be established that may improve the efficiency of lateral spine imaging to identify those with clinically unrecognized VFs. Because models used to form general indications for VFA may have little predictive power in subsets of populations, such as patients with RA or kyphoplasty, further studies are needed on new care processes within healthcare systems to identify those who should have VFA. Furthermore, although VFA has the potential to be a very useful test for longitudinal follow-up; there are currently no recommendations on how often the assessment should be repeated. It is important to also mention that, according to evidence, VFA studies revealing a VF do affect patient’s and physician’s fracture prevention behavior[47,48]. In conclusion, more research will help the adoption of care processes to determine which patients require VFA and how often VFA should be performed, while also considering the impact of such cost on healthcare organizations.
P- Reviewer: Roellinghoff M, Vokes T S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2507] [Cited by in RCA: 2579] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 2. | Fink HA, Milavetz DL, Palermo L, Nevitt MC, Cauley JA, Genant HK, Black DM, Ensrud KE. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res. 2005;20:1216-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 265] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 3. | Melton LJ, Lane AW, Cooper C, Eastell R, O’Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993;3:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 334] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Bouxsein ML, Melton LJ, Riggs BL, Muller J, Atkinson EJ, Oberg AL, Robb RA, Camp JJ, Rouleau PA, McCollough CH. Age- and sex-specific differences in the factor of risk for vertebral fracture: a population-based study using QCT. J Bone Miner Res. 2006;21:1475-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Jinbayashi H, Aoyagi K, Ross PD, Ito M, Shindo H, Takemoto T. Prevalence of vertebral deformity and its associations with physical impairment among Japanese women: The Hizen-Oshima Study. Osteoporos Int. 2002;13:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Ling X, Cummings SR, Mingwei Q, Xihe Z, Xioashu C, Nevitt M, Stone K. Vertebral fractures in Beijing, China: the Beijing Osteoporosis Project. J Bone Miner Res. 2000;15:2019-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1351] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 8. | Nevitt MC, Ettinger B, Black DM, Stone K, Jamal SA, Ensrud K, Segal M, Genant HK, Cummings SR. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 554] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Delmas PD, van de Langerijt L, Watts NB, Eastell R, Genant H, Grauer A, Cahall DL. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res. 2005;20:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 366] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 10. | Genant HK, Li J, Wu CY, Shepherd JA. Vertebral fractures in osteoporosis: a new method for clinical assessment. J Clin Densitom. 2000;3:281-290. [PubMed] |
| 11. | Schousboe JT, Vokes T, Broy SB, Ferrar L, McKiernan F, Roux C, Binkley N. Vertebral Fracture Assessment: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:92-108. [PubMed] |
| 12. | Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137-1148. [PubMed] |
| 13. | McCloskey EV, Spector TD, Eyres KS, Fern ED, O’Rourke N, Vasikaran S, Kanis JA. The assessment of vertebral deformity: a method for use in population studies and clinical trials. Osteoporos Int. 1993;3:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 334] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Eastell R, Cedel SL, Wahner HW, Riggs BL, Melton LJ. Classification of vertebral fractures. J Bone Miner Res. 1991;6:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 382] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Melton LJ, Kan SH, Frye MA, Wahner HW, O’Fallon WM, Riggs BL. Epidemiology of vertebral fractures in women. Am J Epidemiol. 1989;129:1000-1011. [PubMed] |
| 16. | Ross PD, Yhee YK, He YF, Davis JW, Kamimoto C, Epstein RS, Wasnich RD. A new method for vertebral fracture diagnosis. J Bone Miner Res. 1993;8:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Jiang G, Eastell R, Barrington NA, Ferrar L. Comparison of methods for the visual identification of prevalent vertebral fracture in osteoporosis. Osteoporos Int. 2004;15:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Schousboe JT, Debold CR. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int. 2006;17:281-289. [PubMed] |
| 19. | Rea JA, Chen MB, Li J, Marsh E, Fan B, Blake GM, Steiger P, Smith IG, Genant HK, Fogelman I. Vertebral morphometry: a comparison of long-term precision of morphometric X-ray absorptiometry and morphometric radiography in normal and osteoporotic subjects. Osteoporos Int. 2001;12:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Pavlov L, Gamble GD, Reid IR. Comparison of dual-energy X-ray absorptiometry and conventional radiography for the detection of vertebral fractures. J Clin Densitom. 2005;8:379-385. [PubMed] |
| 21. | Fuerst T, Wu C, Genant HK, von Ingersleben G, Chen Y, Johnston C, Econs MJ, Binkley N, Vokes TJ, Crans G. Evaluation of vertebral fracture assessment by dual X-ray absorptiometry in a multicenter setting. Osteoporos Int. 2009;20:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Binkley N, Krueger D, Gangnon R, Genant HK, Drezner MK. Lateral vertebral assessment: a valuable technique to detect clinically significant vertebral fractures. Osteoporos Int. 2005;16:1513-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Damiano J, Kolta S, Porcher R, Tournoux C, Dougados M, Roux C. Diagnosis of vertebral fractures by vertebral fracture assessment. J Clin Densitom. 2006;9:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Rea JA, Steiger P, Blake GM, Fogelman I. Optimizing data acquisition and analysis of morphometric X-ray absorptiometry. Osteoporos Int. 1998;8:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Rosen HN, Vokes TJ, Malabanan AO, Deal CL, Alele JD, Olenginski TP, Schousboe JT. The Official Positions of the International Society for Clinical Densitometry: vertebral fracture assessment. J Clin Densitom. 2013;16:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Schousboe J, McKiernan F, Fuehrer J, Binkley N. Use of a performance algorithm improves utilization of vertebral fracture assessment in clinical practice. Osteoporos Int. 2014;25:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Ghazi M, Kolta S, Briot K, Fechtenbaum J, Paternotte S, Roux C. Prevalence of vertebral fractures in patients with rheumatoid arthritis: revisiting the role of glucocorticoids. Osteoporos Int. 2012;23:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3104-3112. [PubMed] |
| 29. | Prieto-Alhambra D, Muñoz-Ortego J, De Vries F, Vosse D, Arden NK, Bowness P, Cooper C, Diez-Perez A, Vestergaard P. Ankylosing spondylitis confers substantially increased risk of clinical spine fractures: a nationwide case-control study. Osteoporos Int. 2015;26:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Ghozlani I, Ghazi M, Nouijai A, Mounach A, Rezqi A, Achemlal L, Bezza A, El Maghraoui A. Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone. 2009;44:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Mohammad A, Lohan D, Bergin D, Mooney S, Newell J, O’Donnell M, Coughlan RJ, Carey JJ. The prevalence of vertebral fracture on vertebral fracture assessment imaging in a large cohort of patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Orstavik RE, Haugeberg G, Uhlig T, Mowinckel P, Falch JA, Halse JI, Kvien TK. Incidence of vertebral deformities in 255 female rheumatoid arthritis patients measured by morphometric X-ray absorptiometry. Osteoporos Int. 2005;16:35-42. [PubMed] |
| 33. | Lodder MC, Haugeberg G, Lems WF, Uhlig T, Orstavik RE, Kostense PJ, Dijkmans BA, Kvien TK, Woolf AD. Radiographic damage associated with low bone mineral density and vertebral deformities in rheumatoid arthritis: the Oslo-Truro-Amsterdam (OSTRA) collaborative study. Arthritis Rheum. 2003;49:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Lee JH, Cho SK, Han M, Lee S, Kim JY, Ryu JA, Choi YY, Bae SC, Sung YK. Validity and role of vertebral fracture assessment in detecting prevalent vertebral fracture in patients with rheumatoid arthritis. Joint Bone Spine. 2014;81:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Vosse D, Heijckmann C, Landewé R, van der Heijde D, van der Linden S, Geusens P. Comparing morphometric X-ray absorptiometry and radiography in defining vertebral wedge fractures in patients with ankylosing spondylitis. Rheumatology (Oxford). 2007;46:1667-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Briot K, Cortet B, Thomas T, Audran M, Blain H, Breuil V, Chapuis L, Chapurlat R, Fardellone P, Feron JM. 2012 update of French guidelines for the pharmacological treatment of postmenopausal osteoporosis. Joint Bone Spine. 2012;79:304-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, Curtis JR, Furst DE, McMahon M, Patkar NM. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2010;62:1515-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 487] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 38. | Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila Pa 1976). 2001;26:1631-1638. [PubMed] |
| 39. | McGraw JK, Lippert JA, Minkus KD, Rami PM, Davis TM, Budzik RF. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and follow-up. J Vasc Interv Radiol. 2002;13:883-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | McKiernan F, Faciszewski T, Jensen R. Reporting height restoration in vertebral compression fractures. Spine (Phila Pa 1976). 2003;28:2517-2521; disucssion 3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Cloft HJ, Jensen ME. Kyphoplasty: an assessment of a new technology. AJNR Am J Neuroradiol. 2007;28:200-203. [PubMed] |
| 42. | Berlemann U, Ferguson SJ, Nolte LP, Heini PF. Adjacent vertebral failure after vertebroplasty. A biomechanical investigation. J Bone Joint Surg Br. 2002;84:748-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 238] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 43. | Banks L, van Kuik C, Genant H. Radiographic technique for assessing osteoporotic vertebral deformity. ) Vertebral fracture in osteoporosis. USA: Radiology Research and Education Foundation, San Francisco, CA 1995; 131-147. |
| 44. | van Bodegom JW, Kuiper JW, van Rijn RR, van Kuijk C, Zwamborn AW, Grashuis JL. Vertebral dimensions: influence of X-ray technique and patient size on measurements. Calcif Tissue Int. 1998;62:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. |
| 46. | Schousboe JT, Shepherd JA, Bilezikian JP, Baim S. Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom. 2013;16:455-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 383] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 47. | Schousboe JT, Davison ML, Dowd B, Thiede Call K, Johnson P, Kane RL. Predictors of patients’ perceived need for medication to prevent fracture. Med Care. 2011;49:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Schousboe JT, McKiernan FE, Binkley N. A performance algorithm improves appropriate vertebral fracture assessment use among those referred for DXA and improves utilization of fracture prevention medication for those with prevalent vertebral fracture. J Bone Miner Res. 2011;27 (1Suppl). |