INTRODUCTION
The incidence of shoulder instability in the population is estimated to be as high as 2%[1]. While many first-time dislocators can be managed conservatively, there are specific patient groups that have a higher risk for dislocation after a single event and may benefit from surgical stabilization. For example, Taylor et al[2] found increased risk of recurrence in overhead athletes and participants in contact sports. In addition, hyper-laxity has been an identified risk factor[3]. Of the risk factors for recurrence, the most predictive is age at the time of first dislocation. Increasing instability risk has been found to be inversely proportional to the age of the patient[4]. For example, in older patients the risk of instability ranges from 10% to 20%[5]; yet in skeletally immature patients, Marans et al[6] found a re-dislocation rate of up to 100%. While demographics play a major role in anterior instability, intra-articular pathology also has a strong association.
The most difficult dislocators to treat are those with bony deficits. After first time anterior dislocation, glenohumeral deficiency (humeral head defect, glenoid defect or combination of both) has been found in up to 70% of patients[7]. While small defects tend to have limited implications on overall stability, there is a significantly increased risk of instability as the size of the humeral head lesion or glenoid deficiency increases[8,9]. Historically, these large defects had been treated with isolated soft tissue procedures, but further biomechanical and clinical studies have led to treatment algorithms that focus more on addressing the bone loss. Given these concerns, our purpose is to review recent data on surgical management of anterior instability with associated bone loss.
TYPES OF BONE LOSS
In bony anterior instability, both articulations of the glenohumeral joint have been associated with increased risk of further dislocations. Defects can occur on the glenoid side (i.e., Bony Bankart lesions), on the humeral side (i.e., Hill Sachs lesions), or on both sides.
Glenoid deficits
Glenoid deficiency has been found in up to 22% of patients after initial dislocation[10]. In recurrent instability cases their incidence ranges from 46% to 86%[11,12]. To understand the biomechanics of the glenoid deficiency, initial discussion should be begin with the discussion of small defects. First described by Dr. Arthur Bankart, these anterior labral lesions (known as Bankart lesions) increase the risk of instability. If a small piece of the anterior glenoid rim is concomitant with these labral tears some refer to this as “bony Bankart lesions. As the pieces become large the propensity for dislocation increases. As these defects approach greater than 20% to 25% of the glenoid the glenoid appearance changes. Burkhart et al[13] first described this glenoid appearance as an “inverted pear”. His colleagues found in larger defects the standard pear shaped appearance of the glenoid was reversed. As a result the glenoid is wider superiorly than inferiorly, giving it an inverted pear appearance. When this occurs, they described a disruption in the arc of motion with abduction and external rotation of the arm, creating an increased risk of recurrent dislocation.
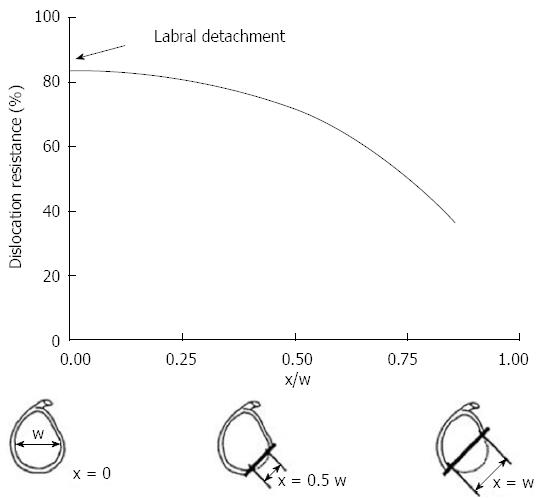
Gerber et al[14] confirmed this theory in their biomechanical study. They found with subsequent loss of anterior inferior glenoid arc the resistances to dislocation decreased exponentially (Figure 1). Newer biomechanical studies have further described this “glenoid track”. This concept has shifted the previous paradigm from engaging defects to track-off track mismatch. Yamamoto et al[15] evaluated 9 cadaveric shoulders and found dislocation was most likely with disruption of the medial margin of this track.
Figure 1 The graph demonstrates the relationship between the size of the glenoid rim and the dislocation risk.
When defect (×) measure more than 50% of the glenoid width there is a significant drop in dislocation resistance. Adapted with permission from Clin Orthop Related Res 2002; 400: 65-76.
While understanding the biomechanics of glenoid defects is necessary, Bigliani’s classification of the glenoid deficit best defines clinical prognostic features[11]. He defined four types of glenoid defects: type 1 involves a non-displaced anterior glenoid fragment, type 2 is a small detached anterior fragment, and type 3a involves anterior glenoid deficits of < 25%, while type 3b involves defects greater than 25%. These distinctions determine the need for bony reconstruction. They recommended soft tissue procedures for types 1, 2, 3a while type 3b defects should have glenoid augmentation. Mologne et al[16] also recommend glenoid restoration for defects greater than 20% to 25% of the glenoid surface. They reached this conclusion after performing soft tissue repair on 23 patients with glenoid defects greater than 20% and had a 14% failure rate at 34 mo follow up when bony incorporation did not occur. An additional study by Burkhart et al[17], who performed 194 consecutive arthroscopic Bankart repairs and found in 18 patients with glenoid defects larger than 25% of the glenoid[17]. In this group they had a failure rate 67%, compared to the failure rate of patients without bony defects at 4%. As a result, they advocated for addressing the bony defects, as soft tissue repair alone did not provide adequate stability.
These glenoid cutoffs have been further supported by other biomechanical studies. Itoi et al[18] evaluated 10 cadaveric shoulders and performed four separate glenoid osteotomies each with increasingly larger deficits. They found a significant decrease in stability with glenoid defects above 21%. Greis et al[19], who had similar study methods, reported significant increases in dislocation risk and contact pressures at more than 31% loss of the glenoid arc. Overall, these studies support that isolated soft tissue repair is likely insufficient in preventing recurrent instability in patients with large glenoid deficiencies.
Humeral head defects
While humeral head defects can be found concomitantly with glenoid pathology, isolated depressions can significantly affect the stability of the shoulder. These lesions have been found in up to 70% of first time dislocators[7], and up to 100% of patients with recurrent instability or after failed primary stabilization[7,10,20,21].
Hill and Sachs[22] first classified these lesions in 1940; as such they are frequently referred to as “Hill-Sachs lesions”. In their landmark study they recognized these defects as markers for instability after an acute shoulder dislocation. These lesions were further defined by Boileau, who identified small to large Hill-Sachs lesions in up to 85% of their patients. They found significantly increased rates of recurrent instability in patients with these “Large” lesions[23]. In a retrospective case review by Burkhart and De Beers they explained that engagement into the glenoid rim was also needed for recurrent instability, and reported 100% recurrence in patients with an engaging Hill-Sachs[24]. As such this finding led them to suggest that if an engaging lesion is recognized, one must address not only the Bankart lesion but also take additional steps to treat the humeral head defect. In a follow up study, they further described this pattern of engagement, stating the Hill-Sachs lesion must be parallel to the arc of motion of the glenoid with abduction and external rotation to be truly engaging[17].
Despite previous clinical descriptions of size based on retrospective cases series, limited descriptions were available to define the percent of the humeral head defect necessary to cause recurrent instability. More recent biomechanical testing by Sekiya et al[25] demonstrated that humeral head lesions greater than 25% of the articular surface significantly increase the risk of recurrent instability. They recommended directly addressing the bony defect in these patients to prevent further instability. Additional studies have found ways to calculate this percent on MRI and CT scan to better define this distinct patient population[26,27].
Combined defects
While both Hill-Sachs lesions and glenoid defects each have an effect on the stability of the glenohumeral joint, combined lesion can add a level of complexity with regards to proper treatment selection. Indications for surgical management have been well described for isolated humeral head and glenoid defects. A recent study by Arceiro et al[28], evaluated the combined biomechanical effect of concomitant lesions. They developed their model using three-dimensional printing from CT scans of 142 patients, with varying degrees glenoid and Hill-Sachs lesions. After testing they found medium size Hill-Sachs lesion became clinically significant with greater than 2 mm of glenoid bone loss. Additionally with glenoid loss greater than 4 mm even small Hill-Sachs defects significantly increased instability despite a Bankart repair. As a result, they suggested bony augmentation with these combined defects. This understanding of the effects of these lesions on one another is essential, as soft tissue repair alone is likely not adequate in these clinical scenarios.
History
Clinical assessment of bony shoulder instability begins with a detailed history. Typically, an initial high-energy dislocation event occurs with the arm in an abducted, externally rotated, and extended position. These episodes often require reduction in the emergency room. Mechanisms involving an axial load on the glenoid predispose glenoid bone involvement[24]. Complaints of mechanical symptoms such as pain, crepitus, or catching when the arm is placed in the position of apprehension (abduction, external rotation) are suggestive of an engaging Hill-Sachs lesion. Subsequent instability in the midranges of motion (e.g., 20 to 60 degrees of abduction)[29] or after lower energy events and with daily activities of living may suggest loss of bony constraints of the glenohumeral joint such as a large glenoid or humeral head defect[30-32]. Additionally, failed arthroscopic capsulolabral reconstructions or multiple recurrences within a short timeframe are suggestive of significant bony defects.
PHYSICAL EXAM
Both shoulders should be examined for evidence of muscular atrophy, deformities, active and passive range of motion, and evidence of prior surgeries. A careful neurovascular exam, including an accurate assessment of the axillary nerve should be performed, as axillary nerve injuries are commonly observed in the acute setting[33]. Assessment of the rotator cuff, with special attention to subscapularis function, should be performed particularly in patients who have undergone prior open stabilization because of potential for subscapularis repair failure. When performing provocative maneuvers, such as the apprehension test and relocation test, comparison to the contralateral shoulder is necessary to quantify the direction and magnitude of laxity. The load and shift test can identify the direction of instability as well as the adequacy of the glenoid concavity. To perform this test, a load is placed on the humeral head to center it within the glenoid, and then a displacing force, either anterior or posterior, is applied to the humeral head. A decrease in resistance may be suggestive of a glenoid defect in the direction of displacement. The patient should also be asked to demonstrate the position of the shoulder at the time of initial dislocation or other subsequent events of instability or apprehension. Unlike patients with multidirectional instability, unidirectional and greater apprehension in the early and midrange of motion (e.g., 20 to 60 degrees) is also suggestive of more significant soft-tissue pathology and/or bony involvement[34,35].
IMAGING
While plain radiographs remain the mainstay of initial assessment, they are only moderately accurate at diagnosing bony defects[11,36]. Glenoid fragments may be visualized on standard AP or projects parallel to the glenoid such as an axillary or glenoid profile view[37]. Angled projects, such as the apical oblique[38] or Didiee[39], views have the highest yield in detecting glenoid defects on plain radiographs. The West Point view function similar to the Garth view but is designed to assess the anterior-inferior glenoid rim[40] and has demonstrated a high correlation with computed tomography (CT) in estimating glenoid bone loss[36]. Another view most commonly used in Europe is the Bergeneau view to assess anterior inferior bone loss. This view requires fluoroscopic imaging to get the perfect on fosse view as such its utility has been limited in the United States[41]. For humeral lesions, the Stryker notch or internal rotated AP views are more sensitive[39]. The Stryker notch, which can evaluate the size and orientation of a Hill-Sachs lesion[42], is obtained by placing the palm of the hand on top of the head, with fingers directed toward the back of the head. The beam is centered over the coracoid process and aimed 10 degrees cephalad.
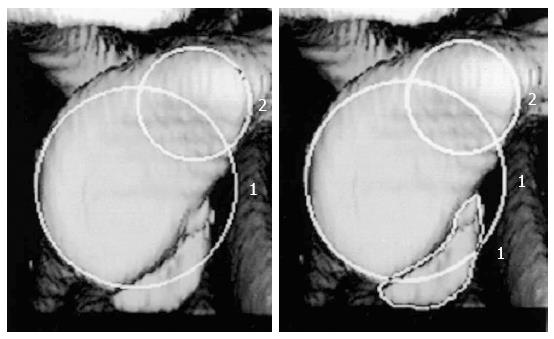
CT with 3D reconstruction, however, remains the gold standard in the evaluation of bone deficiency[11]. The sagittal 3D reconstruction with digital subtraction of the humeral head has been recommended for the evaluation of glenoid deficiency[32,43,44]. Using this modality, the glenoid defect can be quantified. A best-fit circle drawn on the inferior two thirds of the glenoid and the amount of bone missing is determined as a percentage of the total surface area of the circle. This is calculated directly by CT scan software[26,45] (Figure 2). To assess humeral lesions, the defect arc on coronal or axial cuts can be divided by the humeral head arc to quantify Hill-Sachs lesions[46]. Magnetic resonance imaging (MRI) may be useful in evaluating glenoid rim defects, soft tissue lesions, and for quantifying humeral impaction fractures, but are generally thought to be less accurate than CT for bony assessment[27,47].
Figure 2 Using 3D reconstruction computed tomography the size o f the defect is calculated as the percentage of the on fossa glenoid.
Using circle 2 as the reference selected by the radiologist, the CT software automatically calculates the deficit by using the equation (area of the deficit/circle 1 × 100%). Adapted with permission from JBJS Am 2003; 85-A: 878-884.
ARTHROSCOPY MEASUREMENTS
An evaluation and assessment of bony defects should be performed during the initial diagnostic arthroscopy. The bare area has been shown to reliable mark the center of the inferior glenoid[45,48,49]. Using the bare area as a landmark, a calibrated probe can used to measure the distance from the bare spot to the posterior rim and compare it to the distance from the anterior rim. Assuming that the normal inferior glenoid is shaped as a nearly perfect circle[45], anterior-inferior glenoid deficiencies can then be quantified by the following[50]:
Glenoid deficiency = (Distance from bare spot to posterior rim - Distance from bare spot to anterior rim)/(2 × Distance from bare area to posterior rim)
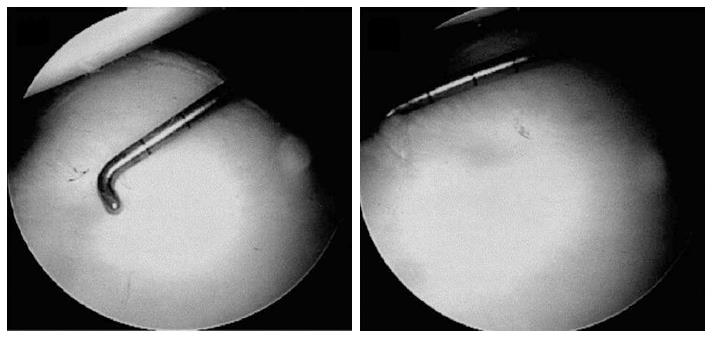
Quantification of glenoid bone loss should be routinely performed to determine the ideal anterior stabilization procedure (Figure 3).
Figure 3 Through a posterior portal a 3 mm calibrate probe is inserted and the distance from the center of the bare spot to the posterior glenoid rim is measured.
Following the distance from the bare sport to the anterior glenoid rim is measured. These values are used to preform the final glenoid deficit calculation. Adapted with permission from Arthroscopy 2004; 20: 169-174.
OPEN VS ARTHROSCOPIC TECHNIQUES
With advancing technology, arthroscopic techniques are becoming more popular. For small defects or soft tissues avulsions, the results are fairly definitive. Recent studies have demonstrated similar recurrence rate and outcomes for arthroscopic techniques compared to open procedures in most patient populations[51]. A larger systematic review by Harris et al[52] evaluated longer-term outcomes of Bankart repairs from 26 studies and also found no statistical difference between open vs arthroscopic approaches[52].
Despite this data most studies have failed to evaluate specific patient groups at higher risk. Burkhart et al[24] recommend open surgical management with younger patients, overhead or contact athletes. Another study by Rhee et al[4] found significantly higher recurrence rates after arthroscopic stabilization at 25% compared to open procedures at 13% in these contact athletes. As such they suggested open repair in these patients. In addition, a prospective study by Mohtadi et al[53] randomized 196 patients without identified bony lesions on radiographs to open vs arthroscopic repair. Additionally they matched patients by age (average age 27 years) and sex. They reported lower recurrence rates after open procedures at 11% compared to 23% for the arthroscopic stabilization group. While these are impressive results, they did have a trend towards more patients in the arthroscopic group who played a contact sport (P < 0.09). Finally a metanalysis by Chen et al[54] of 16 trials with 827 shoulders compared open to arthroscopic stabilization. They found arthroscopic approaches had significantly better post-operative range of motion, but reoperation rate (10.1% vs 3.5%; OR 2.63) and recurrence rate (13.1% vs 4.5%; OR 2.63) were significantly higher than open repair. While arthroscopic techniques are more commonly chosen for soft tissue instability, there has been a trend towards open stabilization for bony defects and certain high-risk groups.
SURGICAL MANAGEMENT
Glenoid defects
Large glenoid defects can be a difficult problem to manage (Figure 4). The initial consideration when determining the best treatment should include evaluation of the acuity of the glenoid injury. For acute lesions, Park et al[55] reported good results after direct repair of the fracture. For chronic injuries there is generally no fragment and bone loss must be reconstructed. We will review surgical techniques for these chronic glenoid defects.
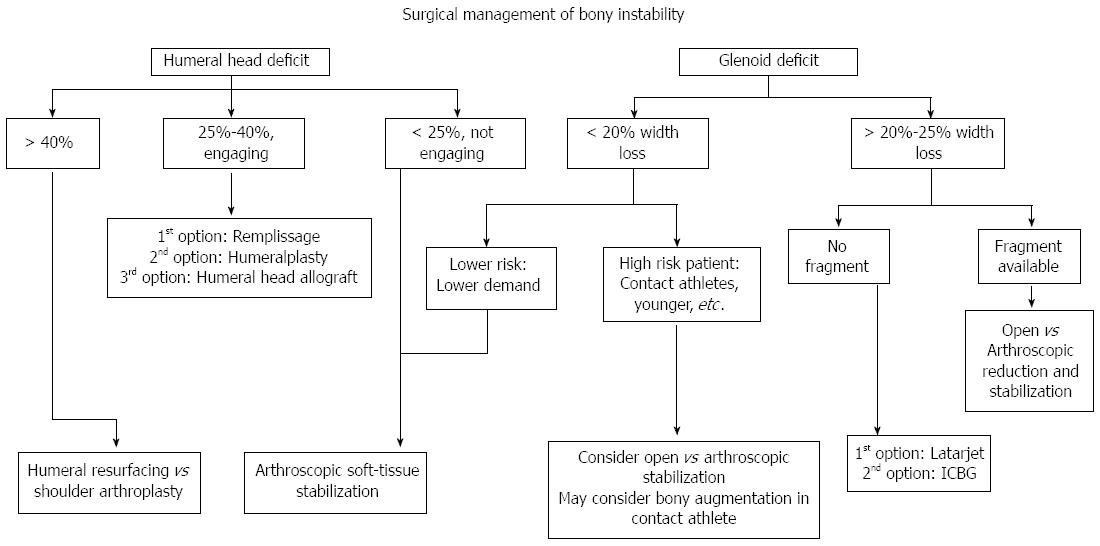
Figure 4 Our treatment algorithm of bony anterior shoulder stability.
First determination of the size of the defect is done, followed by evaluation of specific risk factors. For large glenoid defects the Latarjet procedure is preferred, while Hill-Sachs defects the remplissage is our recommended procedure.
The bristow procedure
Helfet first described the Bristow procedure in 1958. It involved transfer of the terminal 1 cm of the coracoid to the glenoid rim[56]. Usually the piece is secured with a single screw. The conjoined tendon is left intact to the transferred coracoid piece to act as a soft-tissue sling in abduction. Alternatively, detaching the tendinous attachments from the coracoid graft has been described, though we do not recommend this.
Hovelius et al[57] performed one of the largest studies of the Bristow procedure. He prospectively evaluated 319 patients with an average follow was 15.2 years and an overall satisfaction rate of 95%. For outcome scores, they reported 86% excellent to good Rowe scores and WOSI scores of 84.7%. Their recurrence rate was 20%, with 5% dislocation and 15% of patients with a postoperative subluxation. Additionally they found 14% of patients had mild arthropathy on radiographs, which directly correlated with lateral misplacement of the coracoid graft.
In the study by Schroder et al[58], the authors reported results of the Bristow procedure on 52 Navy midshipmen with 26 year follow up[58]. The failure rate was 15.4% with 9.6% dislocations and 5.8% subluxations. Sixty-nine percent of postoperative WOSI scores were good to excellent. They also found a significant loss in external rotation as well as an increased risk of glenohumeral arthritis in their cohort. Furthermore, 15% of the patients underwent a revision surgery on their shoulder.
Yamashita et al[59] evaluated 126 patients treated with concomitant Bankart repair and Bristow procedure. Their follow up was 41 mo, with a recurrence rate at 1.6%. For range of motion they reported an average loss of external rotation of 13 degrees.
While results have been promising for the Bristow procedure, longer-term studies have demonstrated increased risk of glenohumeral arthritis and external rotation loss as well as recurrence rates of up to 18%. These factors must be taken into account in treating this difficult patient population.
The latarjet procedure
Dr. Michel Latarjet described the Latarjet procedure four years before the Bristow procedure[60]. While studies have used term Latarjet-Bristow procedure synonymously, there are variable differences. Recently, the Latarjet has been the preferred technique because it uses a larger coracoid osteotomy of 2 to 3 cm. This increased length allows the surgeon to place the fragment more perpendicular to the base of the glenoid. Additionally, biomechanical evaluation has demonstrated improved stability with larger portions of the coracoid. Giles et al[61] evaluated 8 cadaveric shoulders comparing the stability of the Bristow to the Latarjet procedure. They found significantly more dislocations in the Bristow group with glenoid defects of 15% and 30% in comparison to the Latarjet procedure. As a result, they recommend the Latarjet procedure for these larger glenoid defects.
For surgical technique, a 1-cm cuff of coraco-acromial ligament (CAL) is left on the coracoid process (Figure 5). The coracoid is osteotomized at the “knee” (junction of horizontal and vertical parts), perpendicular to its base. All soft tissue is removed except the conjoined tendon and the CAL stump. Next the graft is molded with an oscillating saw to expose a broad flat cancellous bed to optimize healing. The coracoid is predrilled with 2 k-wire roughly 1 cm apart. The graft is passed through a split in the mid-portion of the subscapularis tendon and is then fixed 1-2 mm medial with the glenoid articular surface. This is done with two partially threaded screws, starting with the inferior screw. Following this the capsule is imbricated to the CAL stump with two sutures[62] (Figure 6).
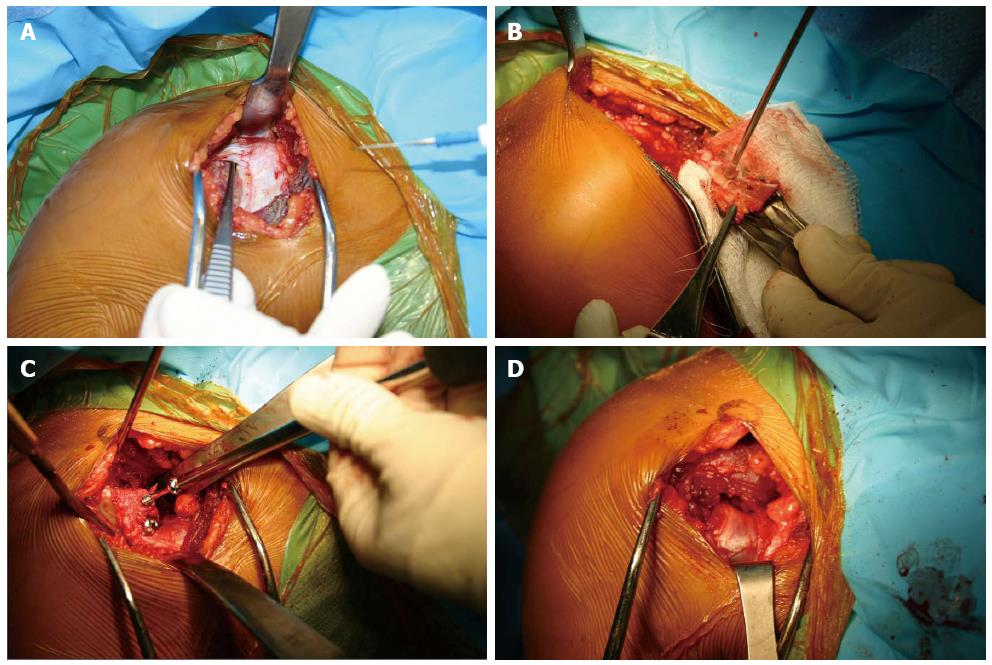
Figure 5 Intraoperative photos of the Latarjet technique.
(A) Though a deltopectoral approach the coracoid is identified (B) after osteotomizing the coracoid the entry points for the 2 screws are predrilled, and the soft tissue attachments are preserved (C) The coracoid fragment is secured with 2 partially thread screws on the anterior surface of the glenoid (D) The joint capsule is secured to the coracoid fragment with 2 sutures.
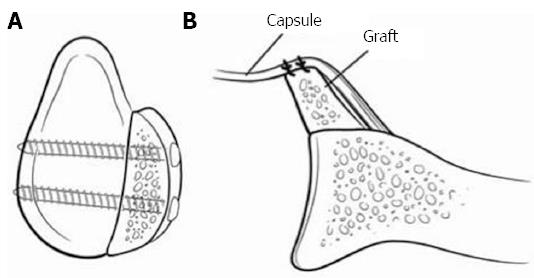
Figure 6 Represents a Latarjet procedure.
A: A sagittal view with 2 screws securing the coracoid fragment; B: The capsule is secured posterior to the graft making the construct extra-articular. Adapted with permission from J Am Acad Orthop Surg 2009; 17: 482-493.
A long-term study by Allain et al[63] evaluated 56 patients with an average follow up of 14.3 years who underwent the Latarjet procedure. For outcomes they reported 88% good to excellent Rowe scores. Their failure rate was 12% with no recurrent dislocations and 12% subluxations. As for range of motion, they had a significant loss of external rotation of 21 degrees. For longer-term evaluation, 65% of their patient developed glenohumeral arthritis. As a result they analyzed coracoid placement and deduced lateral overhang increased risk of arthritis while over medialization increased the risk of recurrent instability.
An additional study by Mizuno et al[64] evaluated 68 patients with an average follow up of 20 years. Their average postoperative Rowe scores were 89.0 with a documented failure rate of 5.9%. With regards to arthritis, 20% of the patients had signs of glenohumeral arthritis at most recent follow up. Their risk factors for arthritis included age, high demand sports and lateral placement of coracoid.
The largest combined series reported by Young et al[62] evaluates over 2000 patients treated with the open latarjet procedure. For outcomes, 76% of patients had good to excellent Rowe scores. Also, 83% of patients returned to their preinjury sports level after surgery. They reported a failure rate of 1%, with no significant loss of external rotation.
Burkhart et al[65] performed a modified latarjet procedure on 102 patients with an average follow up of 4.9 years. For outcomes scores, their average Constant scores were 94.4. They reported a failure rate of 4.9% with 4 dislocations and 1 subluxation. In addition, they did not have a significant loss of external rotation with an average loss of 5.1 degrees.
While most reported series of Latarjet are performed as an open procedure, LaFosse recently described an arthroscopic technique. Dumont et al[66] published these results on 62 patients who underwent arthroscopic Latarjet with an average follow up of 6.4 years. Their reported failure rate was 1.6%, with no dislocations and 1 subluxation. For outcome scores their average WOSI score was 90.6. While these results are promising the arthroscopic approach can be technically demanding.
An additional arthroscopic study by Boileau et al[67] performed an arthroscopic Bristow-Latarjet procedure on 79 patients with a mean follow up of 35 mo. At final follow up, their average Rowe scores were 89.7 with a recurrence rate of 2%. For return to sport, 83% of patients returned to pre-injury level. They reported an average loss of 9 degrees of external rotation, with 73% of grafts demonstrating full healing at final follow up. They determined risk factors for non-union included age higher than 35 years old, smoking, or misplacement of screws. As such age and smoking should be taken into consideration before performing this procedure.
The Latarjet procedure offers a good option for large glenoid defects. Concerns about external rotation loss and long term arthritis still exist, though these may be minor in comparison to the reduced recurrent instability rates for this complicated patient population. An advance in techniques such as the arthroscopic methods has promise; though the learning curve needs to be improved before the full clinical application can be evaluated.
Eden-hybinette procedure
Similar to the Latarjet, the Eden-Hybinette procedure directly addresses large glenoid lesions. Hindmarsh first described this in 1967 using tibia autograft to reconstruct the glenoid track[68]. Recently this technique has been broadened to the use of iliac crest, femoral head, or osteochondral allograft to re-approximate the glenoid track[29,69,70]. Of these the most commonly used today is the iliac crest graft.
In this procedure, the curve of iliac wing is matched to that of the glenoid, with the concave inner table facing laterally. The graft is fixed such that the iliac wing natural contour roughly matches that of the glenoid articular arc. The cancellous base of the graft is secured to the glenoid neck with two screws. As opposed to the Latarjet, the capsule is attached anterior to the bone block, making the graft intra-articular (Figure 7).
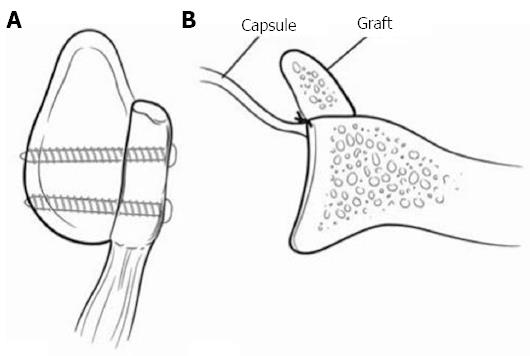
Figure 7 Represents an iliac crest autograft.
A: A sagittal view with 2 screws securing the iliac crest; B: The capsule is placed anteriorly making the construct intra-articular. The graphs natural wing is facing towards the joint to better match the glenoid previous contour. Adapted with permission from J Am Acad Orthop Surg 2009; 17: 482-493.
Warner et al[29] performed this procedure on 11 patients with an average follow up of 33 mo. They reported no failures and at six month CT evaluation, all grafts had fully incorporated into the glenoid.
More recently, Scheibel et al[71] reported on 10 patients who underwent tricortical grafting. Their average follow up was 37.9 mo and reported no cases of recurrent instability. Average Constant scores were 88.3 and WOSI scores were 82.6. On further CT imaging they had full incorporation of all grafts and calculated that the glenoid track increased by an average of 18.4%. After examining radiographs, 30% of patients had signs of mild osteoarthritis.
A larger cohort by Auffarth et al[72] reviewed 47 patients with an average Rowe score of 94.3 and no recurrent instability. Postoperatively, they had one traumatic graft failure and five iatrogenic nerve palsies at the donor site. In addition, long term data found 19.1% patients developed mild to moderate arthritis despite anatomic reduction.
Longer-term follow up Rahme et al[73] found more complications than previous studies. They reported results of 77 patients with a mean follow up of 29 years. Overall 83% of patients had good to excellent Rowe scores. Of concern they had a 20% recurrence rate. Furthermore, 50% of their patients developed glenohumeral arthritis and had a significant loss of external rotation. Additionally there were risks found associated with the use of autologous iliac crest graft, including hip pain and wound complications.
While these long-term results have limited the procedure’s overall clinical use, recent reports by Lunn et al[74] found it to be an adequate alternative after failed Latarjet procedure. They performed the procedure on 46 patients after recurrent instability with a previous Latarjet procedure. They reported good to excellent results in 70% of patients with a 13.0% failure rate.
As iliac crest bone graft has recently been the mainstay of allograft glenoid augmentation, additional studies have evaluated other sources for glenoid arc restoration. Provencher et al[69] used distal tibia allograft for glenoid deficiencies greater than 25%. In addition they reported biomechanical data stating constant pressure remained low in the implanted allograft with range of motion testing. In their cadavers, they showed the articular deformity reconstructed by the tibial allograft was nearly identical to the intact state. For patient results, they reported good results in a series of three patients with full incorporation of the graft on CT scan at final follow up. Despite good fusions, they did not report range of motion testing or recurrence rates.
Another source a graft used by Weng et al[70], was fresh frozen glenoid allograft. They performed the procedure on 9 patients with an average follow up of 4.5 years. All patients achieved bony union at 6 mo, with a mean loss of external rotation of 7 degrees. Despite some positive aspects of their study, they had a 22.2% recurrence rate. Given this high recurrence rate it’s likely further studies are need to determine the true clinical application of this procedure.
Overall since the introduction of the Eden-Hybinette procedure, many modifications of the technique have been described. While iliac crest bone grafting has become the predominant technique it is not without complications. These must be taken in consideration, and in many cases stabilization of the glenoid deficiency is based on surgeon preference as well as training.
HUMERAL HEAD LESIONS
While many patients with recurrent instability have elements of both glenoid and humeral bone loss, the amount of deficiency of each directly impacts surgical outcomes. Even in combined cases of both glenoid and humeral bone loss, patient with large Hill-Sachs lesions continued to have instability despite glenoid reconstruction[75]. These findings suggest the need to directly address these Hill-Sachs lesions. In most studies, humeral head procedures are usually reserved for patients with deficits of 25% to 40%[76]. Yet while size plays an important role, the position of the engagement with abduction and external rotation (generally posterior and superior on the humeral head) increases the risk of dislocation as well[77] (Figure 4).
The remplissage procedure
The remplissage technique has become more popular in recent years as one of the mainstay treatments for large engaging Hill-Sachs lesions. Originating from the French word “to fill”, it has gained further attention because it can be done arthroscopically and is technically reproducible. Purchase and Wolf originally described it in 2007. The procedure involves arthroscopic tenodesis of the infraspinatus into the humeral head defect and usually is accompanied by a Bankart repair[78] (Figure 8).
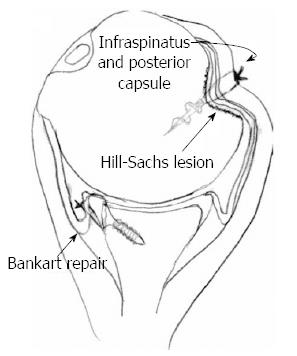
Figure 8 The remplissage technique with a suture anchor securing the infraspinatus and the posterior capsule into the Hill-Sachs defect.
In addition, a Bankart repair is performed during the procedure. Adapted with permission from Arthroscopy 2008; 24: 723-726.
Boileau et al[79] evaluated 47 patients treated with remplissage with a mean follow up of 24 mo. Overall they had a 2% recurrence rate and reported an average loss of external rotation of 9 degrees. As for return to sports, 90% of patients returned to their previous sport and 68% of patients returned to their previous level of sport.
An early study by Park et al[80] evaluated 20 patients at a mean follow up of 29.2 mo. Their average ASES scores were 92.5 and average WOSI scores were 72.7. They reported a recurrence rate of 15% but no range of motion testing was done. Interestingly, in their follow up study of MRIs on separate remplissage patients, they found infraspinatus integration into the humeral footprint at as early as 8 mo. They suggested this incorporation might increase the chances of longer-term success of the procedure[81]. In addition, they also reported range of motion testing with a mean external rotation loss of 5.2 degrees.
Wolf et al[82] published longer-term results on their original patient series[78]. They included 59 patients with up to 10-year follow-up. They found minimal complications and no significant loss of external rotation. Overall their recurrence rate at long term follow up was 4.4% and mean Rowe and Constant scores were 95.0. Despite long-term follow-up, no evaluation for signs of arthritis was done.
More recently, systematic reviews have further compiled recurrence risk after arthroscopic remplissage. Buza et al[83] demonstrated low recurrence rates of all eligible studies at 5.4%, with mean external rotation loss of 2.6 degrees. Additionally Rashid et al[84] found average remplissage recurrence rate at 4.2% though their overall average external rotation loss was higher at 11.3 degrees.
Overall most of the results demonstrate remplissage has a low recurrence rates, with minimal complications. Even though most studies found no significant loss in external rotation, the concerns are still present given previous case reports and cadaveric studies[85,86]. Additionally, in throwing athletes where less substantial loss of external rotation are tolerated, the implications of this procedure must be discussed extensively with the patient. Despite good short term results, longer term studies are needed to evaluate long term effects, with a focus on glenohumeral arthritis which has been found with the glenoid restoring procedures.
Osteochondral allograft transplantation
Osteochondral allograft has been used for many orthopedic articular procedures. While a majority of the focus has been knee literature, humeral head defects are another area it has proven beneficial. One of the first studies by Miniaci et al[87] treated 18 patients with Hill-Sachs lesions of greater than 25%. They used custom matched osteochondral allograft and reported good results with no recurrent instability. As a result they suggested the advantage of the technique is the anatomic reconstruction. Unfortunately there were other risks including: graft resportion, non-union and hardware failure.
Two further case reports by Chapovsky et al[88] and Nathan et al[89] reviewed two adolescent patients treated with osteochondral allograft reconstruction for large Hill-Sachs lesions. At final follow up, these patients had stable shoulders and no signs of recurrent instability.
A more recent article by Garcia et al[90], looked at outcomes of 19 patients treated with OATs for engaging large Hill-Sachs lesions with a mean follow up 32.1 mo. They reported average WOSI scores of 74.7 but a high recurrence rate of 31.5%. In addition to documenting results of osteochondral allograft, they matched 20 remplissage patients with similar preoperative Hill-Sachs lesions. They reported that remplissage patients had a 50% lower recurrence rate, and after controlling for confounding variables had significantly better WOSI scores. While they concluded OATs procedure is beneficial in this patient population they recommend performing the remplissage procedure for larger Hill-Sachs lesions.
Though limited studies are available osteochondral allograft transplantation is a reasonable alternative for large engaging Hill-Sachs lesions. Concern for graft-associated complications exist, as such further study is needed before true clinical success can be determined.
Humeralplasty
This procedure involves reducing the Hill-Sachs lesion through an anterior humeral window. In theory, by directly restoring the anatomy, this would obviate the need for potential failures such as lack of infraspinatus integration or osteochondral healing. With regards to biomechanics, two recent cadaveric studies have described such reduction techniques. The first study by Sandmann et al[91] described a method using balloon humeralplasty to reduce 80% of the lesions. More recently Stachowicz et al[92] used a similar method of balloon humeralplasty with 99.3% reduction of their Hill-Sachs lesions. Despite their biomechanical success, these studies were done with most of the soft tissue removed making the clinical application less relatable.
Re et al[93] did one of the few clinical studies; using a bone tamp and an ACL guide to reduce their Hill-Sachs lesions. They performed this technique in 4 patients and reported 12-mo follow up. They had good results with no recurrent instability and no postoperative complications. Despite good reductions, some of these patients did require concomitant Latarjet procedure, making it difficult to discern which procedure improved stability.
A second study by Hart et al[94] performed humeralplasty in 5 patients with humeral head defects of 30%. Their minimum follow up was 18 mo, with 100% satisfaction at final follow up. They reported no recurrent instability or postoperative complications.
While humeralplasty seems to have the most potential for anatomic reconstruction, limited cases series are available. In addition, this procedure is technically demanding and requires an open approach. Future studies are needed to evaluated longer-term results and possibly develop a minimally invasive method before true clinical application can be considered.
Larger hill-sachs lesions and humeral replacement
Techniques for humeral head defects from 25% to 40% have been discussed. When humeral head lesions approach greater than 40%, humeral resurfacing or traditional hemiarthroplasty has been suggested. Limited studies have evaluated these patients. Pritchett et al[95] described shoulder replacement results in 4 patients with humeral head defects up to 70% from chronic instability. All patients had good ROWE scores, but overall of range of motion improvement was poor. Despite these results arthroplasty techniques have improved significantly since this study and new implants have shown better longevity. Given the difficulty of dealing with these massive humeral head lesions, replacement still remains the best alternative at this point in time.
CONCLUSION
Anterior bony instability is a difficult pathology to manage and is multifactorial. As previously discussed, glenoid reconstruction is needed for defects greater than 20% to 25%. Multiple studies have shown improving the glenoid arc with a bony reconstructions is significantly better than soft tissues repair alone. Various surgical treatment options exist such as coracoid transfer, tibial autograft, iliac crest autograft, or osteochondral allograft. Each procedure has its own set of complications but has demonstrated improved recurrence rates in this patient population.
Humeral head lesions have also been identified as a source of instability. Studies have demonstrated that lesion greater than 25%-30% of the humeral head surface require reconstruction. To address these Hill-Sachs lesions, soft tissue, osteochondral allograft or anatomic reduction have been described and demonstrated significant improvement in stability of the shoulder. As bony deficiency of the glenohumeral joint is a common and difficult pathology to treat, surgeons must decide the best treatment based on the individual patient.