Published online May 18, 2015. doi: 10.5312/wjo.v6.i4.394
Peer-review started: October 20, 2014
First decision: January 8, 2015
Revised: January 14, 2015
Accepted: February 9, 2015
Article in press: February 12, 2015
Published online: May 18, 2015
Processing time: 215 Days and 14.5 Hours
AIM: To assess the clinical effects and the morphological grade of nerve compression.
METHODS: In a prospective single-center randomized, open study we assessed the clinical effects and the morphological grade of nerve compression during 20 min of either a silicon ring (group A) or pneumatic tourniquet (group B) placement variantly on the upper non-dominant limb in 14 healthy human volunteers. Before and during compression, the median and radial nerves were visualized in both groups by 3 Tesla MR imaging, using high resolutional (2.5 mm slice thickness) axial T2-weighted sequences.
RESULTS: In group A, Visual analog pain scale was 5.4 ± 2.2 compared to results of group B, 2.9 ± 2.5, showing a significant difference (P = 0.028). FPS levels in group A were 2.6 ± 0.9 compared to levels in group B 1.6 ± 1, showing a significant difference (P = 0.039). Results related to measureable effect on median and radial nerve function were equal in both groups. No undue pressure signs on the skin, redness or nerve damage occurred in either group. There was no significant difference in the diameters of the nerves without and under compression in either group on T2 weighted images.
CONCLUSION: Based on our results, no differences between narrow and wide tourniquets were identified. Silicon ring tourniquets can be regarded as safe for short time application.
Core tip: Nerve injury is a serious potential complication associated with clinical use of tourniquets in surgery. In a prospective single-center randomized, open study we assessed the clinical effects and the morphological grade of nerve compression during 20 min of either a silicon ring (group A) or pneumatic tourniquet (group B) placement variantly on the upper non-dominant limb, visualized by 3 Tesla magnetic resonance imaging, using high resolutional (2.5 mm slice thickness) axial T2-weighted sequences. Based on our results, no differences between narrow and wide tourniquets were identified. Silicon ring tourniquets can be regarded as safe for short time application.
-
Citation: Kovar FM, Jaindl M, Oberleitner G, Endler G, Breitenseher J, Prayer D, Kasprian G, Kutscha-Lissberg F. Nerve compression and pain in human volunteers with narrow
vs wide tourniquets. World J Orthop 2015; 6(4): 394-399 - URL: https://www.wjgnet.com/2218-5836/full/v6/i4/394.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i4.394
Nerve injury is a serious complication, associated with the clinical use of tourniquets, and influencing profoundly orthopedic surgery[1-3]. A bloodless operative field is considered mandatory for most surgical procedures on the upper and lower extremity, allowing surgical procedures to be performed with improved precision, safety and speed[1-7].
The invention by McEwen in 1981, a modern microcomputer-based tourniquet system can be seen as a modified version of this basic idea of Cushing[2]. Following a different approach, OHK Medical Devices Inc. launched an elastic rubber ring with a stockinet and gained common approval.
Several studies related to tourniquet use have investigated various complications, the most frequent one being nerve palsy[1-4,6,8,9]. In the current literature, the impact of the width of a tourniquet and as a consequence the pressure expansion, is discussed controversial[3,10-14].
To the best of our knowledge, none of them used magnetic resnane iamge (MRI) as a visualization model, in healthy human volunteers, wearing two different tourniquet devices. Therefore we conducted the present study, to investigate differences between HemaClear™ blood free device and a standard pneumatic tourniquet.
We investigated 16 upper extremities in 16 volunteers during an eight months period in an IRB approved (EK 1042/2011) single centre randomized prospective, controlled study, by the standards of International Conference of Harmonisation and Good Clinical Practice. (Registered: NCT02023476) All individuals gave written consent to participate in the study. Two individuals, one male and one female, had to be exclude after study Day 1, due to the fact of violating the inclusion criteria between Day 1 and Day 2. In the remaining group of 14 volunteers, mean age was 24.3 years (range 22 to 28), 9 (64%) were males and 5 (36%) were females. All remaining individuals finished the study without nerve impairment or skin lesion.
Volunteers who meet the inclusion criteria and provide written informed consent were included. Main criteria for inclusion were the following: self defined Caucasian, clinically healthy, body mass index (BMI) of ≤ 30, a systolic arterial blood pressure ≤ 190 mmHg, no rash or dermatologic condition or tattoos which may interfere with the placement site and no neurovascular impairment or previous surgery on the investigated limb. Self-defined Caucasian was implemented to guarantee an equal evaluation of possible skin lesions.
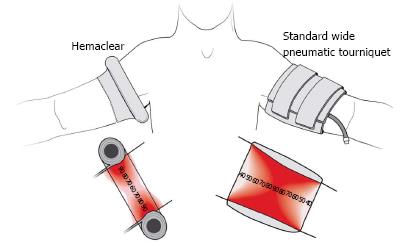
HemaClear™of OHK Medical Device (group A)
HemaClear™ consists of a silicon ring wrapped in a stockinet sleeve and pull straps (Figure 1). It performs three functions-blood removal (exsanguinations), arterial flow occlusion, and placement of sterile stockinet. The ring is placed on the extremity and then straps are pulled proximally. The silicone ring rolls up the limb and the stockinet sleeve unfolds onto the limb. During the rolling up process, the ring exerts pressure and squeezes the blood away from the limb. Pressure is exercised by only a single silicon ring, and therefore the profile is very small.
As standard pneumatic tourniquet system, we used the following setting: an inflatable cuff (Tourniquet Cuff REF 20-64-711, 35 cm/14 in., VBM Medical Technique), with a width of 8 cm/6.5 in. and an air compression unit (fine pressure actuator tube connector 645-1708.2, Synthes REF 520.95) using the inner hospital 5 bar pipeline system for inflating the tourniquet. Due to the containing metal of the air compression unit, we connected it with the tourniquet in the MRI room, using a flexible tube (PVC Extension Tubing, VBM Medical Technique) of 20 meter/187.4 in. length.
Defining the appropriate inflating pressure of the pneumatic tourniquet: In a similar approach like McEwen[2], we detected the Limb Occlusion Pressure (LOP) with a handheld dopplers device (MD2/SD2, Dopplex® High Sensitivity Pocket Dopplers, Huntleigh Healthcare Limited, Cardiff United Kingdom). RTP (Recommended tissue pressure) feature was calculated as following: LOP + 40 mmHg if LOP < 130 mmHg, LOP + 60 mmHg if LOP 131-190 mmHg, and LOP + 80 mmHg if LOP > 190 mmHg. Calculated RTP was the pressure, used for inflating the pneumatic tourniquet.
Subjects were examined by a clinical high field (3 Tesla) MR system (Philips Achieva, Best, The Netherlands) in supine position. A flex medium surface coil was consistently placed on the non-dominant upper arm, with the tourniquet centering the field of view. Before, 5 min after application of the tourniquet a T2-TSE (turbo spin-echo sequence: TR (repetition time) 4808 ms. TE (echo time) 90 ms, flip angle 90°, FOV 130 mm × 164 mm, acquisition data matrix 260 × 316, reconstruction image resolution 0.2 mm, slice thickness 2.5 mm, NEX 1; The total imaging was 6:25 min) was acquired in an axial plane, covering the region 3.7 proximally and 4.8 cm distally to the tourniquet and 6.7 proximally and 7 cm distally to the narrow tourniquet position.
The study was divided in three parts, screening visit (SV), study day 1 (Day 1) and study day 2 (Day 2). During SV, a physical examination, evaluation of the inclusion criteria and the device randomization (Group A-HemaClear™; Group B- standard pneumatic tourniquet) with a blinded envelope were performed. On Day 1, blood pressure, visual analog pain scale (VAS) and faces pain scale (FPS) baseline scores and pictures of the upper limb were performed. According to the randomization process to groups were formed for further proceedings.
In group A, the volunteer was placed in a supine position on the MRI, and the baseline MRI sequence was performed. Before starting the T2 sequence, the HemaClear™ device was placed on the non-dominant upper arm, using the same measurements criteria for exact placement. After finishing the T2 sequence, the tourniquet was removed immediately.
In group B, LOP and RTP detection were performed in a sitting position, and the volunteer was placed in a supine position on the MRI. Than, bating of three layers, and the standard pneumatic tourniquet were placed on the non-dominant upper arm. The exact position for the placement site was half the way of a drawn line between the greater tubercle and the lateral supercondylar ridge. A baseline MRI sequence was performed, and inflating to the calculated RTP was conducted, seconds before starting the T2 sequence, guaranteeing a full inflated tourniquet. After finishing the T2 sequence, the tourniquet was removed immediately.
Subsequently, the following procedures were performed in both groups: detecting the grade of muscle strength for the compressed upper extremity on a scale from 5 to 0, and evaluating VAS and FPS. Pictures of the device placement site were taken (iPhone 4, Apple Inc., Cupertiono, CA, United States) after the volunteer had left the MRI room. During a final check up, 30 min post removal, before the volunteer left the study site the following parameters were evaluated: blood pressure, VAS and FPS levels.
Day 2 was performed at least seven days after Day 1, but no longer than 2 wk after Day 1, with switched groups for each volunteer. At the end of Day 2 the volunteer was asked which device was more painful after all.
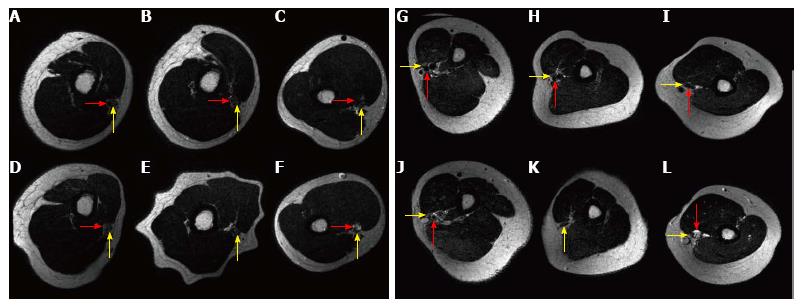
The maximum and minimum diameter of the median and the radial nerve and the brachial artery were measured on three axial planes in the T2 weighted sequences: the plane of the compression by the HemaClear™, 4 cm proximal and 4 cm distal to that point. Since the radial nerve divides in several fascicles at the spiral groove, the maximum diameter could not be measured at this point. The cross sectional area of the nerves was calculated assuming that the shape of the nerve resembles an ellipse.
For statistical analysis we used the SPSS 16.0 software package (SPSS, Chicago, Ill., United States). Mean values and standard error of the mean are given unless otherwise indicated for continuous variables. Discrete data are presented as counts and percentages. To compare the two study groups we used a dependent sample student’s t-test. A two-tailed P value less than 0.05 was considered statistically significant. Statistics was performed by GE, a biomedical statistican.
Fourteen subjects, nine males and five females with complete data participated in the present study. As a result we were able to acquire data from 14 placements of each device. For the HemaClear™, we used six Pink and eight Yellow devices. In the A group we used the same device in all patients, adapted to the circumference of the upper arm.
Levels for compression of the median and radial nerve where almost similar in both groups (Figure 2A and B, Table 1). The brachial artery was compressed in all individuals by both tourniquets as a sign of adequate vessel compression. In one patient the compression of the HemaClear™ was 2 cm proximal of the beginning of the spiral groove, in all other volunteers the radial nerve was passing the spiral groove at the point of compression.
We could not detect a significant difference concerning the diameters or of the calculated area of the nerves between no compression, compression by HemaClear™ and the standard pneumatic tourniquet.
VAS and FPS levels were evaluated at baseline, immediately after removal of the tourniquet device, and 30 min post removal. VAS and FPS levels at baseline were 0 in all volunteers. In group A, VAS was 5.4 ± 2.2 compared to results of group B, 2.9 ± 2.5, showing a significant difference (P = 0.028). FPS levels in group A were 2.6 ± 0.9 compared to levels in group B 1.6 ± 1, showing a significant difference (P = 0.039). VAS and FPS levels, post removal, were 1 and 1 in only two volunteers, both male and occurring after Day 1 with the HemaClear™ device.
Only two out of 14 volunteers described independent the pneumatic tourniquet as more painful. One volunteer was male, one female, both experienced the HemaClear™ device on Day 2. The reasons, given by the study subjects, why the HemaClear™ device was more painful were as following: the roll on process was described as uncomfortable, but the main pain was caused by the placement (silicon ring) at the upper arm, which was felt as a pulsing or throbbing sensation.
Levels (manual force grade) for both nerves were identical within the same group, but there was a slight difference between group A 4.5 ± 1.4 and group B 4.3 ± 1.1 (P = 0.098).
Placement of the silicon ring device was more practicable, because of the simple roll up whereas for the broad pneumatic tourniquet, placement of the bating, LOP/RTP detection, and finally inflating was mandatory.
No other data than mentioned are available.
The primary aim of the present study was to investigate the differences between HemaClear™ blood free device and standard pneumatic tourniquet, concerning possible nerve damage in healthy volunteers. We also investigated the pain scale during compression with both devices.
Our first hypothesis that a narrow silicone ring causes more nerve compression compared to a wide tourniquet was disapproved. Our second hypothesis that a narrow silicone ring causes more pain compared to a wide tourniquet was approved.
The most gravid article concerning this topic, by Noordin et al[14], defaming the use of a HemaClear™ similar device, caused some controversial response. The substituted opinion by McEwen and his study group is in sharp contrast to findings of other various trials, and may be influenced by commercial interests[1,2,4,13-16].
A study of female baboons suggests that the damage to the nerve fibers is a direct result of the applied pressure, and not a consequence of secondary ischemia[17]. The same paper also showed that the pressure gradient was higher at the edges rather than in the middle of the tourniquet, a finding that also supports the idea of a narrow tourniquet[17]. Another trial concluded that a wider cuff would not be intrinsically safer than a regular cuff, a result that is contrary to Crenshaw’s findings[18].
The relationship between tourniquet cuff width and the pressure that, last on the surface and the layers underneath it, is the core point in the current discussion. The fundamental difference is the technique, attaining the pressure and as a direct consequence fulfill the goal of exsanguination. In a narrow cuff the pressure is substantially diminished towards the middle of the limb, with a drop of 45%-55%, leading to a small gradients at the cuff’s end and a short length of vessels and nerves under compression[18]. In contrast, in a wider cuff the nerves and vessels are exposed to a relatively high compression stress, because the high pressure is transmitted across the limb at the same level as in the cuff and leads to high shear forces at the edges[18]. The wide tourniquet applies the pressure over a wide surface, resulting in shear forces at both edges, squeezing the nerve at two points in an unnatural way, and not as suggested by many users over the whole length of the tourniquet[18].
Behind the HemaClear™ device, is a different model of pressure application, which is at the beginning confusing and controversial discussed in the literature[14] (Figure 3).
Contrary to Noordin’s suggestions, narrow tourniquets can look back on a broad use in surgical settings in civilian hospitals and their safe use should not be reduced to military indications only[1,3,19]. Depending on the placement of the cuff, the occurrence of nerve related injuries has been experienced by 21%-28% of surgeons[5]. An experimental study in 20 healthy volunteers concluded that wider cuffs result in more severe changes in the nerve[3].
The experience of a novel elastic tourniquet in 43 pediatric patients was published, concluding that it is safe and valuable in clinical practice[1]. Another trial reports, that application of a silicon ring device is practical, provides bloodless field for a certain time, and does not increase the complication rate related with the pressure applied to underlying tissues, but is not appropriate for long surgical procedures[20].
First is the small number of volunteers (n = 16), due to the fact of limited financial and logistical feasibility. The limited number of tourniquet time is accidental by the local ethics commission, due to their concerns of pain and soft tissue damage. For ethical reasons, we were not allowed to use anesthesia. The time interval of 20 min of compression in this study can not be compared to a clinical setting with compression times over 60 min and longer. We also have to admit that we did not investigated the possible influence of secondary ischemic factors on our reported results.
The HemaClear™ occupies only 2 cm on the limb after application and enables a wider limb surface compared to regular pneumatic tourniquets. But there are also disadvantages like the constant pressure, performed by the silicon ring, which cannot be changed during surgery. The use in open or dislocated fractures has to be seen limited because of the roll up mechanism and in limbs with applied external fixation devices it cannot be used.
In contrast to those findings, the broad pneumatic tourniquet can be used in open and dislocated fractures because of its different application technique. Inflation and deflation during surgery are possible and enable longer surgical procedures, because reperfusion is possible after 2 h. Despite the mentioned advantages, the main disadvantages are the large surface the tourniquet occupies on the limb, the additional console to operate the tourniquet and to detect LOP and RTP.
To the best of our knowledge we are the first to have visualized nerve compression with MRI using two different devices of surgical tourniquets in vivo in healthy human volunteers. Application of both devices resulted in a similar degree of vascular- and nerve compression. There were no indirect MR imaging signs of nerve compression (change in nerve cross sectional area, increased T2-weighted signal intensity) noted. No patient related complications where observed.
I want to give eminent credit for my parents and friends for their unlimited support for my research and when the sun was not shining. I also want to say thank you to the staff of the Department of Radiology for their easygoing collaboration and workflow during the study period. Special thanks to MJ and GK for their support, and to all volunteers, making this study possible.
Nerve injury is a serious potential complication associated with clinical use of tourniquets in surgery. Several studies related to tourniquet use have investigated various complications, the most frequent one being nerve palsy. In the current literature, the impact of the width of a tourniquet and as a consequence the pressure expansion, is discussed controversial.
The invention by McEwen in 1981, a modern microcomputer-based tourniquet system can be seen as a modified version of this basic idea of Cushing. Following a different approach, OHK Medical Devices Inc. launched an elastic rubber ring with a stockinet and gained common approval.
Based on these results, no differences between narrow and wide tourniquets were identified.
Silicon ring tourniquets can be regarded as safe for short time application.
HemaClear™ consists of a silicon ring wrapped in a stockinet sleeve and pull straps.
The authors describe a valuable study which is well conducted and gives important clinical conclusions which may influence surgical and ethical conduct of interested surgical specialties.
P- Reviewer: Alimehmeti R, Nikolopoulos D, Sano H, Teli MGA S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Eidelman M, Katzman A, Bialik V. A novel elastic exsanguination tourniquet as an alternative to the pneumatic cuff in pediatric orthopedic limb surgery. J Pediatr Orthop B. 2006;15:379-384. [PubMed] |
| 2. | McEwen JA. Complications of and improvements in pneumatic tourniquets used in surgery. Med Instrum. 1981;15:253-257. [PubMed] |
| 3. | Mittal P, Shenoy S, Sandhu JS. Effect of different cuff widths on the motor nerve conduction of the median nerve: an experimental study. J Orthop Surg Res. 2008;3:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Nogueira MP, Paley D, Bhave A, Herbert A, Nocente C, Herzenberg JE. Nerve lesions associated with limb-lengthening. J Bone Joint Surg Am. 2003;85-A:1502-1510. [PubMed] |
| 5. | Kalla TP, Younger A, McEwen JA, Inkpen K. Survey of tourniquet use in podiatric surgery. J Foot Ankle Surg. 2003;42:68-76. [PubMed] |
| 6. | Klenerman L. The tourniquet in surgery. J Bone Joint Surg Br. 1962;44-B:937-943. [PubMed] |
| 7. | Lister JB. Collected papers. Vol 1. Oxford: Clarenton Press 1909; 176. |
| 8. | Ochoa J, Danta G, Fowler TJ, Gilliatt RW. Nature of the nerve lesion caused by a pneumatic tourniquet. Nature. 1971;233:265-266. [PubMed] |
| 9. | Weingarden SI, Louis DL, Waylonis GW. Electromyographic changes in postmeniscectomy patients. Role of the pneumatic tourniquet. JAMA. 1979;241:1248-1250. [PubMed] |
| 10. | Odinsson A, Finsen V. Tourniquet use and its complications in Norway. J Bone Joint Surg Br. 2006;88:1090-1092. [PubMed] |
| 11. | Fukuda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee arthroplasty. Arch Orthop Trauma Surg. 2007;127:671-675. [PubMed] |
| 12. | Lewis T, Pickering GW, Rotschild P. Centripetal paralysis arising out of arrested blood flow to the limb, including notes on a form of tingling. Heart. 1931;16:1-32. |
| 13. | Graham B, Breault MJ, McEwen JA, McGraw RW. Occlusion of arterial flow in the extremities at subsystolic pressures through the use of wide tourniquet cuffs. Clin Orthop Relat Res. 1993;286:257-261. [PubMed] |
| 14. | Noordin S, McEwen JA, Kragh JF, Eisen A, Masri BA. Surgical tourniquets in orthopaedics. J Bone Joint Surg Am. 2009;91:2958-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Younger AS, McEwen JA, Inkpen K. Wide contoured thigh cuffs and automated limb occlusion measurement allow lower tourniquet pressures. Clin Orthop Relat Res. 2004;286-293. [PubMed] |
| 16. | Younger AS, Kalla TP, McEwen JA, Inkpen K. Survey of tourniquet use in orthopaedic foot and ankle surgery. Foot Ankle Int. 2005;26:208-217. [PubMed] |
| 17. | Ochoa J, Fowler TJ, Gilliatt RW. Anatomical changes in peripheral nerves compressed by a pneumatic tourniquet. J Anat. 1972;113:433-455. [PubMed] |
| 18. | Crenshaw AG, Hargens AR, Gershuni DH, Rydevik B. Wide tourniquet cuffs more effective at lower inflation pressures. Acta Orthop Scand. 1988;59:447-451. [PubMed] |
| 19. | Kragh JF, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, Holcomb JB. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Ann Surg. 2009;249:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Orbay H, Unlü RE, Kerem M, Sensöz O. Clinical experiences with a new tourniquet device. Ann Plast Surg. 2006;56:618-621. [PubMed] |