INTRODUCTION
Diabetic foot ulcers (DFUs) are the main cause of hospitalization in diabetic patients and they are considered a worldwide health problem. In recent years, the improvement in diabetes therapy and the reinforcement of guidelines have reduced the amputation rate[1]. Furthermore the increased knowledge in the approach to DFUs has allowed the availability of several medical options to ensure the best local condition and wound healing. In this overview, negative pressure wound therapy (NPWT) plays a key role.
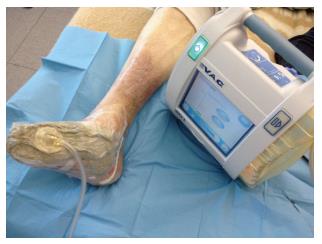
NPWT is a non-invasive therapy system that uses controlled negative pressure using a vacuum device to promote wound healing by removing fluid from open wounds through a sealed dressing or a foam dressing connected to a collection container using sub-atmospheric pressure[2,3] (Figure 1).
Figure 1 Diabetic foot ulcer treated by negative pressure wound therapy.
There are some differences between the various systems concerning the filler material used (foam vs nonadherent antimicrobial gauze), the connecting suction catheter (integrated with pressure sensor vs flat drain) and the intensity of negative pressure (ranging from 50 to 200 mmHg) continuous or intermittent[3].
Since the first publications, it has been documented that this tool has become a useful option for the treatment of acute and chronic wounds in diabetic foot (DF).
The aim of this review is to summarize the available literature on NPWT to describe mechanisms of action, clinical efficacy and when and how to use this device.
MECHANISM OF ACTION
The mode of action is not completely clear, but several levels of evidence are available. NPWT promotes a moist environment, reduces edema, creates a positive wound environment by removing healing inhibitors, increases blood flow, stimulates angiogenesis and granulation tissue and causes mechanical stress in the bed of the wound promoting cell proliferation.
MOIST ENVIRONMENT
As an occlusive wound dressing, NPWT provides a moist wound environment ideal for the process. A moist wound bed is favourable for reepithelialization, growth factor action, angiogenesis and granulation promotion. At the same time, a moist wound reduces local pain, protecting the nerve endings and improving quality of life[4,5].
EDEMA REDUCTION
The edema reduction decreases interstitial pressure and positively effects microvascular occlusion and lymphatic drainage, increasing the availability of nutrients, oxygen and antibiotic therapy in the wound area. A few studies describe this mechanism. One article by Kamolz et al[6] presents a clinical case study about seven patients with bilateral burns on the hand. The authors observed an increase of drained fluid and an evident edema reduction in the NPWT-treated side compared to the controlateral side treated with silver sulfadiazine cream[6]. A second study observed one pig with bilateral lesions on the back: the authors compared a wound covered with a split-skin graft treated with tie-over bolster and a wound treated by NPWT. Histological analysis described less wound edema in the side treated with NPWT[7].
POSITIVE WOUND ENVIRONMENT
Wound healing is the result of balance between promoting cytokines, growth factors and inhibiting proteases[8,9]. Wound exudate often contains high quantities of metalloproteinases (MMP) and low levels of their inhibitors; this condition is associated with an unsuitable environment for wound healing. MMP play a key role in the remodeling and turnover of the extracellular matrix (ECM) in several tissues. Each MMP has a defined role in tissue repair, and sometimes, its activity is involved in the same processes of wound healing. When MMP are present in high levels in the wound bed, they degrade proteins that are not their normal substrate: growth factors, receptors and ECM proteins; excessive degradation of ECM would deprive cells of attachment sites and signals for migration, differentiation and proliferation; this condition affects the different phases of tissue repair. Among the different proteases, MMP-9 is involved in the degradation of ECM, and it promotes angiogenesis and cell migration. Neutrophil gelatinase-associated lipocalin (NGAL) is a stabilizer of active enzymes in the degradation of ECM, and it creates a complex with MMP-9, playing a key role in the control of MMP-9 action. MMP-2 (latent and active form) has a central role in the regulation of vascularisation and inflammatory response. Its increase in the wound bed could alter the tissue remodeling by an excessive degradation of ECM. Tissue inhibitor of metalloproteinases 1 (TIMP-1) is a tissue inhibitor of MMP, balancing their activity, promoting cell proliferation and reducing apoptosis effect. Some studies showed that NPWT promotes an improvement of balance between proteases and their inhibitors and a greater expression of healing factors. Moues et al[10] described significantly lower levels of pro MMP-9 and a lower total MMP-9/TIMP-1 ratio in NPWT-treated wounds in comparison to wounds treated conventionally. Greene et al[11] observed a reduction in MMP-9/NGAL, MMP-9, latent MMP-2 and active MMP-2 by 15% to 76% in three patients treated with NPWT therapy. Although scientific evidences are few, several analyses suggest that NPWT influences cytokine modulation and promotes a positive wound environment.
BLOOD FLOW
Several studies showed an increase of blood flow in wounds treated with NPWT therapy. Morykwas et al[12] placed a laser Doppler probe inside the wounds of twenty five pigs and studied blood flow. NPWT was applied in increasing increments of 25 mmHg up to 400 mmHg and for fifteen minutes intervals, and they found that optimal pressure was 125 mmHg, which was a 4 times increase of blood flow. To maintain this result, they observed that pressure applied needed a pause of two minutes between each five minutes of application[12]. These data were use to establish the baseline setting for the treatment of different typologies of wounds. Wackenfors et al[13,14] measured blood flow by laser Doppler technique blood flow to an inguinal and sternal wound model using 50 to 200 mmHg. They marked an increase in microvascular flow a few centimetres from wound edges and a relative hypoperfusion in the immediate proximity of the wound edge. The study detected that negative pressure is distributed differently in the soft and dense tissue, and a low negative pressure (75 for soft tissue and 100 for muscle) reduces the risk of ischemic effects[13,14]. Chen et al[15] examinated the blood flow in wounds experimentally created in the ears of white rabbits treated with vacuum-assisted closure therapy using the microcirculation microscope and the image pattern analyses. They observed an increase of blood flow that was due to an increase of vascular diameter, blood flow velocity and blood flow volume[15]. Considering all these elements, the optimal pressure setting depends on the tissue treated and ideal pressure levels; the mode of action needs to be further investigated.
ANGIOGENESIS AND GRANULATION TISSUE
Fabian et al[16] showed a significant increase of granulation tissue in the treatment of ischemic full-thickness wounds in a rabbit model treated with NPWT with suction vs foam without suction. Still, Chen observed in his experimental study, in the rabbits treated with NPWT the increase of blood flow promotes the endothelial proliferation and angiogenesis more than that of the control group and a higher integrity of endothelial membrane[15]. In a prospective clinical non-randomized study of traumatic wounds treated with NPWT or Epigard (polyurethane foam), Labler et al[17] found higher levels of interleukin 8 and vascular endothelial growth factor (VEGF) in the group treated with TNP compared to the control group. Interleukin 8 has a significant role in the chemotaxis of granulocytes in the site of infections and, moreover, it is an effective stimulator of angiogenesis, promoting the migration of endothelial cells. VEGF is a family of growth factors involved in the angiogenesis that promote the development of blood vessels in the vasculature near the lesion. The same study showed a neovascularisation in histological analyses of wounds treated by NPWT documented by the finding of CD31 and the von Willerbrand factor (vWF)[17]. CD31 (cluster of differentiation 31) is a protein that allows the angiogenesis process, the leukocyte migration and the integrin activation. vWf is a glycoprotein that promotes platelet anchorage, playing a key role in the first step of wound healing. Nain et al[2] conducted a study on 30 patients divided in two groups, one treated with NPWT and a second with conventional saline gauze dressing. They observed a statistically significant difference in the rate of appearance of granulation tissue that appeared earlier in the study group[2]. A recent paper reported that NPWT activity induces a mobilization of endothelial progenitor cells (EPCs), allowing the healing of complex chronic wounds through the formation of new small blood vessels in lesion area[18]. Several studies showed that EPCs are retrieved from bone marrow in the case of ischemia to allow the neovascularisation of hypoperfused tissues[19,20]. A recent study conducted by Yang et al[21] showed that DFUs treated by NPWT developed granulated tissue with increased collaged deposition if compared to traditional gauze therapy. Further, immunohistochemical analysis revealed higher beta fibroblast growth factors (bFGF) in NPWT patients, highlighting the involvement of NPWT in the promoting of the healing phase. Therefore, an increase in the rate of angiogenesis and the promotion of granulation tissue are two mechanisms that may be attributed to NPWT.
MECHANICAL STRETCHING OF BED WOUND AND CELL PROLIFERATION
When NPWT is applied, the foam in the wound bed transmits a negative force to surrounding tissues. This force deforms the extracellular matrix, and the tension exerts a stress effect. These effects activate tyrosine kinases, transport genes, stimulate calcium release, and induce early growth response genes[22].
In a vitro study, Kremers et al[23] observed that, in fibroblast cultures exposed to mechanical stress with intermittent NPWT, there was a significant increase of p38 protein kinase and appended transcription factor, which represents a marker of cell proliferation. Scherer et al[24] treated full-thickness wounds in diabetic mouse models, and the group treated with NPWT revealed an increase of cell proliferation, a higher concentration of CD 31 and Ki-67 statistically significant compared to other group. In conclusion, these studies, and the evidence of granular tissue and angiogenesis promotion, imply that NPWT encourages cellular proliferation.
WOUND CHEMOTHERAPY WITH NPWT
New devices are now available, more comfortable and easier to use. Moreover, fluid instillation associated with NPWT is currently used as a new modality to treat diabetic and non diabetic ulcers. New devices allow infusion and fluid removal with the simultaneous application of local negative pressure[25]. Several kinds of fluids can be applied, mainly antiseptic, topical antibiotic, insulin saline solution[26-28]. This system could be useful, especially for contaminated or infected wounds.
INDICATIONS AND CONTRAINDICATION
The application of negative pressure therapy is indicated for acute and chronic wounds, and therefore to promote the healing of diabetic foot wounds, pressure ulcers, traumatic wounds, dehisced surgical wounds, partial thickness burns, flaps and grafts. NPWT can be used in any size wound, especially on deep, complicated, nonhealing wounds of mixed etiologies[29]. Several studies reported a positive effect of NPWT therapy in diabetic foot wounds. Authors observed a significant wound volume reduction[30-34] and a quicker healing time in wounds treated with NPWT vs the control group. NPWT in the treatment of diabetic foot ulcers is not indicated for ischemic and infected wounds. Wakenfors et al[13,14], using laser Doppler, observed a zone of relative hypoperfusion in the immediate proximity of the wound edges; Kairinos et al[35,36] showed that perfusion of intact skin decreases if NPWT is applied and hypoperfusion increases for increasing the suction pressure. In general, a TcpO2 > 40 mmHg is desirable, and several case studies reported failure in patients with inadequate flow[37,38]. Nevertheless, in some cases of revascularization failure, it was reported a therapeutic success with the use of NPWT[39,40]. In this case, it could be useful to apply a low pressure to avoid an impairment of local ischemia. Further, in a recent paper, Lavery reported a high rate of wound closure also with low pressure in the treatment of DF wounds[41].
In our experience, in the case of non-optimal peripheral perfusion, we use a low pressure (75 mmHg) if we have a TcPO2 < 40 mmHg and a higher pressure (100-125 mmHg) if TcPO2 is > 40 mmHg. In infected wounds, a surgical debridement and an appropriate antibiotic therapy is necessary before NPWT application[42,43], because of a high risk of worsening the infection using an occlusive dressing as NPWT. In our policy, we usually also treat the patients with NPWT in cases of infection that involve only the skin and subcutaneous tissue, without involvement of deeper tissues and without systemic signs of infection (grade 1-2 of PEDIS classification). In these circumstances, we perform a closer follow-up with a careful monitoring of the wound. Additional precautions are needed in patients on antiplatelet or anticoagulant therapy because they have a risk of bleeding, which is increased by topical suction.
REQUIREMENT FOR TNP
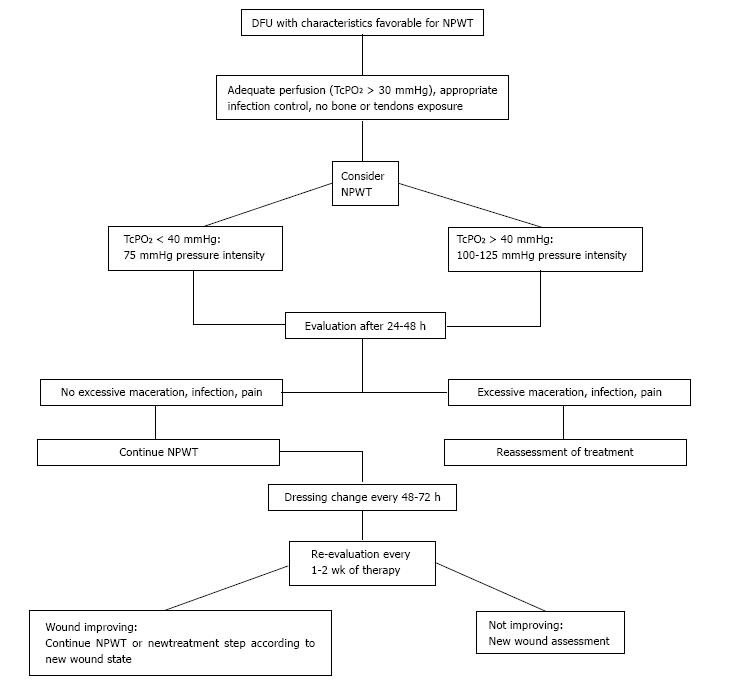
NPWT should not be applied to a critical ischemic wound. Before the application, it is necessary to have a sufficient perfusion in the wound side; infection should be treated. A radical debridement and excision of all infected and devitalised tissue must first be done[44,45]. After debridement, an adequate local haemostasis must be performed. If there is a potential for local bleeding, it is recommended to wait at least 24 h before applying NPWT[46]. Once healthy granulation tissue has been promoted, skin graft or flap could be performed. In Figure 2, we summarize the indications for management of NPWT in DFUs that represent the result of literature evidence and our experience. We retain that this flow chart could be an easy and immediate reference for clinicians who work with NPWT in the field of DF.
Figure 2 Proposed flow chart for management of negative pressure wound therapy in the treatment of diabetic foot ulcers.
DFU: Diabetic foot ulcer; NPWT: Negative pressure wound therapy; TcPO2: Transcutaneous oximetry.
CONCLUSION
Research focused on understanding healing process of DFUs allowed the availability of targeted treatment according to wound phase, and new options such as skin substitutes, extracellular matrix proteins, negative pressure and growth factors have emerged as adjunctive therapies.
Therefore NPWT has become a useful option in the management of DFUs. It can be applied with effectiveness to treat acute, chronic and complex wounds, and several studies have shown it to be more effective than traditional moist therapy in terms of healing and rate of wound closure. Before the application, it is mandatory to apply the standard care, to ensure an adequate blood flow in the wound area and to exclude an infectious process that could affect the deeper tissues. The mechanism of action, partly unclear, determines a favourable wound environment to promote and accelerate the healing process. NPWT reduces perilesional edema, allows the removal of infected fluid and exudate, increases blood flow and stimulates angiogenesis, granulation tissue and cell proliferation. Furthermore this medication creates an appropriate wound bed for a possible application of skin graft and flap surgery. Although excellent results are documented with the use of NPWT, further studies are needed to better define its use, the optimal pressure level, the use of intermittent or continuous pressure and the filler material covering the wound. In conclusion, we retain that currently, between advanced dressing, topical negative pressure plays a leading role in the field of diabetic foot, and physicians can use this medication to treat chronic and complex wounds with excellent benefits.
CLINICAL IMPLICATIONS, FUTURE DIRECTION AND LIMITATIONS
The availability of this device to treat inpatients and outpatients ensures clinicians a useful therapeutic option. DFUs managed with NPWT benefit from significant reduction in the ulcer size, increase in the granulation tissue, improvement with pain control and often a shorter treatment in comparison with ulcers treated with with traditional gauze dressing. Other advantages are lower time requirements and lower costs for nursing staff and hospitaliztion. However, this data is often reported by small pilot studies and Randomized Control Trials with larger samples and adequate randomization are required to reinforce the role of NPWT[47-52].
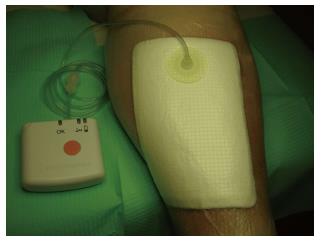
NPWT is also characterized by some limitations related both to the action and the management of the device. Pain and discomfort could be felt when suction is initially applied, a skin irritation from adhesive film can happen and the inadequate application of the foam can cause an abrasion. It is therefore mandatory that family members or caregivers are properly informed and educated on the use of this treatment because inappropriate use may result in an impairment of wound condition and discomfort for the patient. Nevertheless, new portable devices in smaller size and requiring reduced frequency of dressing changes that can be managed more easily and with good comfort are available for outpatients (Figure 3).
Figure 3 Model of lightweight and portable device that allows patients to perform daily activities.
P- Reviewer: Gorgey AS, Sebestyén A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ