Published online Mar 18, 2015. doi: 10.5312/wjo.v6.i2.298
Peer-review started: February 8, 2014
First decision: April 4, 2014
Revised: August 15, 2014
Accepted: September 4, 2014
Article in press: September 10, 2014
Published online: March 18, 2015
Processing time: 413 Days and 21.9 Hours
AIM: To review the published literature reporting bone loss in patients with axial spondyloarthritis (SpA) particularly those studies using dual X-ray absorptiometry (DXA) methods.
METHODS: This literature review examines the reported bone mass in patients with ax-SpA, particularly those using the DXA methods. The MEDLINE, Web of Science and Scopus databases were searched for relevant articles published between September 1992 and November 2013. Some of used search terms were ankylosing spondylitis (AS), SpA, spondyloarthropathy, bone loss, bone mass, osteopenia, bone mineral density, osteoporosis (OP), densitometry. Studies in which bone loss was investigated by using DXA in patients with SpA were eligible. Each article was reviewed and the key elements were noted.
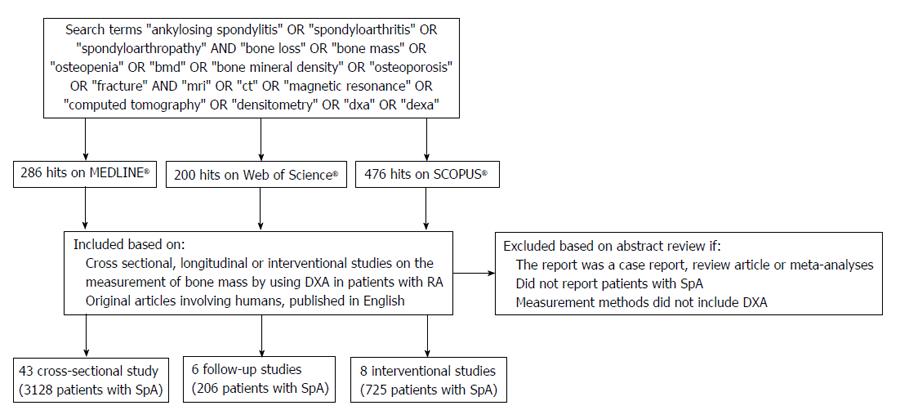
RESULTS: There were 286 hits on MEDLINE, 200 on Web of Science and 476 on Scopus. After applying inclusion and exclusion criteria, we identified 55 articles in our systematic search. The sample size of the studies varied from 14 to 332 patients with SpA. The reported age range varied from 25 to 56 years in the reviewed studies. The symptom duration of patients with axSpA varied from 1.6 to 49 years. There were more males than females in these studies. Most of the recruited females were premenopausal women. Reported HLA-B27 positivity changed between 19% to 95%. The prevalence of OP and osteopenia in patients with SpA varied from 3%-47% to 5%-88%, respectively, in the included studies. In particular, the prevalence of OP and osteopenia ranged from 2.0%-47.0% and 5.0%-78.3%, respectively, in patients with AS. There are conflicting results regarding the relationship among disease activity, acute phase response and bone mass. Some studies suggest good correlation of bone mass with disease activity and acute phase reactants.
CONCLUSION: Bone loss may be determined in patients with axSpA at the lumbar spine or proximal femur even in the early phase of the disease and may be associated with inflammation (bone marrow edema) at the vertebral colon.
Core tip: Osteoporosis is a well-known problem in patients with ankylosing spondylitis and other forms of spondyloarthritis. It may begin even in the early stages of the disease and inevitably causes vertebral fractures. Bone loss can be prevented with tumor necrosis factor blocking therapy by reducing inflammation at skeletal sites. Dual X-ray absorptiometry (DXA) is the preferred method to assess bone mass in the early stages of the disease or in patients without aberrant ossification of the spine. In advanced cases DXA measurements with lateral spinal projections or quantitative computed tomography may be referred.
- Citation: Kilic E, Ozgocmen S. Bone mass in axial spondyloarthritis: A literature review. World J Orthop 2015; 6(2): 298-310
- URL: https://www.wjgnet.com/2218-5836/full/v6/i2/298.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i2.298
Spondyloarthritis (SpA) is a chronic inflammatory disease characterized by predominant involvement of the spine and/or sacroiliac joints. It consists of ankylosing spondylitis (AS), psoriatic arthritis, reactive arthritis, arthritis associated with inflammatory bowel disease and undifferentiated type[1]. Axial (SpA) comprises a heterogeneous group of diseases which predominantly involve the axial skeleton and have many overlapping clinical features. The axial SpA spectrum ranges from non-radiographic axial SpA (nr-axSpA) at one end to AS at the other. Nr-axSpA comprises SpA patients without definite sacroiliitis on pelvic X-ray[2]. The most important clinical and laboratory features of this group are inflammatory back pain, enthesitis, dactylitis, extra-articular manifestations (acute anterior uveitis, psoriasis and inflammatory bowel disease) and association with HLA-B27.
Low bone mass [osteopenia or osteoporosis (OP)] and osteoporotic vertebral fractures are well known complications of SpA, especially in AS[3]. The pathogenesis and onset of OP in SpA is not clear. The prevalence of low bone mineral density (BMD) has been reported to be as high as 47% at the hip and lumbar spine even in patients with early SpA[4]. Patients with SpA may have increased risk of bone loss as a result of high disease activity, pro-inflammatory cytokines, mechanical factors (i.e., rigidity of the spine, vertebral deformities) and decrease in physical activity or mineralization defects due to subclinical gut involvement[5,6].
Several techniques have been used to measure bone mineral density in SpA including quantitative ultrasound (QUS), quantitative computed tomography (QCT), high-resolution peripheral QCT (HRpQCT), single-photon absorptiometry, dual photon absorptiometry, dual-energy X-ray absorptiometry (DXA), and morphometric X-ray absorptiometry[7-11]. Among these techniques DXA can be considered as an accurate, repeatable and quantitative method to assess BMD at the spine and hip[12]. Several studies have indicated that DXA may be a misleading method to assess BMD in advanced AS. New bone formation and aberrant hyperostosis inevitably cause a pseudo increase in bone density. However the most appropriate and valid method to assess BMD in patients with advanced AS is still unclear. A systematic evaluation of DXA methods used to assess BMD in SpA is strongly needed. Therefore this comprehensive review will examine the published literature assessing bone density in patients with axial SpA particularly those studies using DXA as the measurement method.
The MEDLINE, Web of Science and Scopus databases were searched for relevant articles published between September 1992 and November 2013. The following search terms (synonyms and combinations) were used: “ankylosing spondylitis” OR “spondyloarthritis” OR “spondyloarthropathy” AND “bone loss” OR “bone mass” OR “osteopenia” OR “bmd” OR “bone mineral density” OR “osteoporosis” OR “fracture” AND “mri” OR “ct” OR “resonance” OR “computed tomography” OR “densitometry” OR “dxa” OR “dexa”. The references of the reviewed articles were manually scanned for other relevant studies. Studies in which bone loss was investigated by using DXA in patients with SpA were eligible. The selection criteria consisted of original articles involving humans and published in English. Articles were excluded if they were case reports, review articles or meta-analyses and did not measure bone density by using DXA. Each article was reviewed and the key elements are summarized in Tables 1, 2, 3.
| Ref. | Sample size(M/F) | Mean age(yr) | Menopausal status pre:post | Diseaseduration (yr) | DXAmachine | Dexa site (coefficientvariation %, if available) | Outcome | Conclusion |
| Devogelaer et al[19] | AS: 70 (60/10) | 39 | 10:0 | 15.4 | Novo | SPA: non dominant radius DXA: L2-4 QCT: 10 patients LS | DXA values at LS was decreased in the male VF: 2.9% | In patients with severe AS, DXA demonstrates normal values due to new bone formation |
| Donnelly et al[42] | SpA: 87 (62/25) AS: 82.5% PsA: 8% | M: 43.5 F: 44.8 | NM | M: 16.3 F: 16.6 | Hologic | L1-4 (0.7), FN (1.5), whole body | AS: in early disease LS-BMD decreased, in advanced AS increased Lumbar Spine density lower in M than F VF 10.3% | DXA is doubtful to truly reflect the state of demineralization in the spine and more emphasis should be placed on measures on FN-BMD |
| Mullaji et al[43] | AS: 33 (27/6): Mild: 22 (16/6) Adv: 11 (11/0) | 32.3 | 0:6 | M: Mild: 8.7 Adv: 11.7 F: Mild: 6.8 | Norland | Whole body | LS BMD lower in mild and higher in advanced AS than C In Adv. AS, LS BMD higher than mild AS and C HLA-B27: 100% LS, FN and leg BMD decreased in mild AS compared with C in men | The relation between BMD and severity of disease in the axial skeleton may help to explain the etiology and pathogenesis of the spinal deformities and complications of this disabling condition |
| Singh et al[44] | AS: 14 (14/0) | 50 | NA | NM | Hologic | AP L1-4, non dominant hip | FT BMD lower than LS Osteopenia at FN: 64%, LS: 36% | Femoral measurements of BMD are superior to lumbar measurements in the detection of osteopenia in patients with AS |
| Acebes et al[51] | AS: 18 (16/2) | 44.7 | NM | 10.3 | Hologic | L2-4, FN | M: OP 0% osteopenia: 53.8% F: OP and Osteopenia 0% HLA-B27: 100% | Osteopenia in AS occurs as a result of high resorption of bone with normal formation |
| Meirelles et al[50] | AS: 30 (27/3) | 37 | 3:0 | 17 | Hologic | L1-4, PF | LS openia: 23% OP: 27% FT: openia: 55% OP: 31% AS has lower BMD at LS and proximal femur than C | Bone mass loss in AS is better evaluated in the proximal femur, because of almost free of artifacts |
| Juanola et al[52] | AS: 18 (0/18) | 36.7 | 18:0 | 15.1 | Hologic | L2-4 (0.5), FN (1) | HLA-B27: 94.4% OP: 5.6%, Osteopenia: 11.1% VF: 5.6% | Slight reduction in BMD in premenopausal women with early AS, but the difference was not statistically significant |
| Mitra et al[3] | AS: 66 (66/0) | 37.8 | NA | 9.9 | Hologic | L1-4 (1.4), FN (2.9) | In patients with AS, BMD and T scores were reduced in both LS and FN VF: 16.7% in AS, 2.6% in C | AS patients with mild disease had higher risk of VF compared with the normal population and this increased with the duration of disease |
| Borman et al[53] | AS: 32 (32/0) | 39.1 | NA | 14.8 | Hologic | Lat L1-4 (2.7) | L1-4 T score and BMD similar among AS and C BMD was similar among active and inactive AS VF: 31.2% Osteopenia: 34.3% in AS, 21.8% in C OP: 34.3% in AS, 6.2% in C | The incidence of osteoporosis is high in AS and patients with active disease are have risk for developing osteoporosis |
| Dos Santos et al[54] | AS: 39 (39/0) | 37.6 | NA | 8.4 | Hologic | Whole body | HLA-B27 79.5% AS had bone loss at spine compared with control group 46% of patients with AS had Z score < –1.5 SD | AS is associated with bone loss, mainly concerning the lumbar spine, in patients whose disease is biologically most active |
| Toussirot et al[13] | AS: 71 (49/22) | 39.1 | 22:0 | 10.6 | Lunar | L2-4 (1), left FN (1.5) | HLA-B27: 84.5 AS: Lumbar osteopenia: 32.4%, OP: 14.1% higher than C Femur: osteopenia: 22.5%, OP: 14.1% higher than C Good correlation between lumbar, femur, total BMD with QUS | AS has decreased lumbar, hip and total body BMD but soft tissue composition was not involved in disease process |
| Grisar et al[55] | AS: 30 (22/8) PsA: 23 (17/6) ReA: 10 (5/5) | AS: 44.2 PsA: 45.2 ReA: 47.8 | NM | AS: 9.2 PsA: 10.4 ReA: 1.3 | Hologic | LS and non dominant hip | AS; OP 47% | |
| Speden et al[7] | AS: 66 (0/66) | 43.4 | 50:16 | 21.1 | Hologic | PA L1-4 (1), non-dominant hip (1.8) and Whole body (0.82) | Hip and whole body BMD reduced in AS Femoral neck OP: 6%, osteopenia: 52% in AS and higher than control Lumbar OP: 8%, osteopenia: 18% in AS | Women with AS have lower hip BMD without correlation with disease duration suggesting that low BMD is an early feature of disease |
| Capaci et al[56] | AS: 73 (49/24) | 37.3 | NM | 11.8 | Hologic | L1-4, FT | L BMD similar in mild and advanced AS, F BMD lower in advanced AS In advanced AS osteopenia or OP higher in the total hip than mild AS VF: 5.5% LS Osteopenia or OP: 68.4%-54.3% PF osteopenia or OP: 51.9-91.7 (mild-advanced) | Syndesmophytes and ligament calcification may mask bone loss in LS therefore hip BMD more convenient to asses OP in AS |
| Jansen et al[14] | AS: 50 (35/15) | 52 | NM | 21 | Hologic or Lunar | AP LS, FN | HLA-B27: 88% VF LS: 6% LS openia: 54% OP: 15% FN openia: 72% OP: 20% and 70% of them correctly diagnosed with QUS | The performance of QUS is similar to DEXA in finding patients with osteoporosis-associated fractures Both osteoporosis and fractures are common sequel in AS |
| Obermayer-Pietsch et al[16] | AS: 104 (71/33) | 41 | 33:0 | 15 | Hologic or Lunar | LS (2.2-0.9), PF (2-1.6) QCT (1) | HLA-B27: 19%-93% OP: 25% In male AS patients FokI genotypes were independent predictors of low BMD | Vitamin D receptor gene may be involved in BMD differences, bone metabolism and inflammatory processes in ankylosing spondylitis |
| Baek et al[47] | AS: 76 (76/0) mild AS: 59.2% severe AS: 40.8% | 28.1 | NA | 9.4 | Lunar | L2-4, PF | BMD and T score at FN and FT lower in severe AS than mild AS but not at LS Osteopenia: 48% in mild AS (more frequently at LS than proximal Femur) and 31% in severe AS | Osteopenia is frequently observed in both severe and mild AS with little mobility limitation Both BMD in severe disease are lower than in mild disease at the FT but not in the lumbar spine, probably due in part to progressing paravertebral calcification during the course of AS |
| Gilgil et al[48] | AS: 20 (20/0) | 25-63 | NA | 16.7 | Norland | PA L2-4 (1), lat L3 (2.7), left FN (1.2) | PA L2-4 BMD similar between groups but lateral L3 and FN BMD reduced in AS No VF Syndesmophytes: 60% PA LS OP: 20% in AS, 15% in C | Lateral L3 DXA is superior to PA DXA in detecting a decrease in BMD in patients with AS |
| Karberg et al[20] | AS: 103 (66/37) I: < 5 yr (n:27) II: 5-10 yr (48) III: > 10 yr (28) | I: 34.2 II: 38.1 III: 49.1 | NM | I: 2.5 II: 7.0 III: 19.7 | Hologic | L1-4, FN, radius | HLA-B27: 92.2% Disease duration < 5 yr OP: 11%, 15% (hip, spine) > 10 yr OP: 29%, 4% (hip, spine) DEXA: OP: 24%, 14% and osteopenia: 52%, 31% (hip, spine) DEQCT OP: 11% and openia: 44% (L) pQCT OP: 1% openia: 16% (radius) | Patients with AS already have reduced BMD at the lumbar spine and the femoral neck early in the disease process. In later stage, OP ratio at hip increased but at LS did not increase |
| Lange et al[8] | AS: 84 (53/31) I: (10/17) II: (12/10) III: (12/3) IV: (19/1) | I: 32 II: 47 III: 45 IV: 56 | NM | I: 9 II: 20 III: 21 IV: 32 | Lunar | LS (0.9-1), total hip (1.6) | A high decrease in axial bone density could be verified in both initial and advanced stages of the disease (SE-QCT is better) DXA: osteopenia in 5% and OP in 9.2% SE-QCT: osteopenia in 11.8% and osteoporosis in 30.3% HLA-B27: 81.5%-95% VF: 10.7% | In stages of advanced ankylosis in the vertebral region, priority should be given to SE-QCT to detect bone loss, due to the selective measurement of trabecular and cortical bone |
| Incel et al[45] | AS: 53 (46/7) | 39.5 | 7:0 | 10.6 | Lunar | L2-4, FN | AS patients have lower BMD in LS and FN in both inactive and especially active patients. Osteopenia is 78.3% in early AS Osteopenia or OP is 63.3% in advanced AS | Severe disease and concomitant urolithiasis may increase bone loss and fracture risk especially at the femur neck |
| Jun et al[28] | AS: 68 (68/0) | 30.7 | NA | 7.2 | Hologic | PA L2-4, left Prox Femur | BMD of LS and FN significantly lower than C VF correlated with BMD femur. VF: 16.2% | Measurement of femur BMD may provide useful information to predict the risk of vertebral fractures in patients with AS |
| Kim et al[24] | AS: 60 (51/9) | 31.2 | NM | 5.5 | Hologic | AP L1-4 (1), right FN (1.2) | HLA-B27 83% OP: LS 19%, FN 33% Osteopenia: LS 37%, FN 41% The patients with AS presented reduced BMD and T score at spine | About 74% of AS patients have reduced BMD The imbalance between RANKL and OPG might be involved in the pathogenesis and clinical courses of osteoporosis in AS |
| Sarikaya et al[57] | AS: 26 (21/5) | 44.3 | 5:0 | NM | Hologic | Non dominant hip (1), forearm (1) | Hip BMD values are lower in AS whereas radius BMD values are similar between 2 group Hip Osteopenia or OP: 76.9% | OP at hip region may be due to localized effects of inflammatory activity or immobility rather than a systemic effect |
| Altindag et al[58] | AS: 62 (36/26) | 33.4 | NM | 5.7 | Hologic | AP L2-4, left FN | Lumbar and femoral neck BMD scores are significantly lower in AS OP: 32% osteopenia: 17.7% | Lumbar BMD scores negatively correlated with the length of disease duration in AS patients |
| Stupphann et al[15] | AS: 21 (10/11) | 51 | NM | 25.4 | Lunar | L1-4, total hip | TH: Osteopenia or OP 45% by DXA LS: Osteopenia or OP 48% by QCT QCT and DXA at proximal femur show a significant correlation but not at LS | Activated CD4+ and CD8+ T cells contribute to the production of RANKL in the inflammatory bone-resorption |
| Ghozlani et al[23] | AS: 80 (67/13) | 38.9 | 13:0 | 10.8 | Lunar | AP L1-4, proximal F | OP: 25% VF: 18.8% OP is common in patients with AS and seems to be related to disease activity | Measuring BMD in early disease should include DXA in the spine and hip. In advanced disease, BMD evaluation should rely on hip DXA |
| Mermerci Başkan et al[25] | AS: 100 (75/25) | 39.9 | 25:0 | 10.5 | Hologic | AP L1-4 and Lat L2-3, FN | Thoracic VF: 16% Lumbar VF: 3% OP: 32% Acute phase reactant levels of the AS patients with OP are higher than the patients without OP | Vitamin D deficiency in AS may indirectly lead to osteoporosis by causing an increase in the inflammatory activity |
| Arends et al[22] | AS: 128 (93/35) | 41 | 14 | Hologic | AP L1-4, PF | BMD of the lumbar spine, measured by DXA, may be overestimated due to osteoproliferation in patients with advanced AS HLA-B27: 84% VF: 39% Osteopenia or OP: 57% | Bone turnover, inflammation, and low vitamin D levels are important in the pathophysiology of AS-related osteoporosis | |
| Korczowska et al[59] | AS: 66 (66/0) | AS: 51.6 | NA | 17.4 | DTX-200 or ECLIPSE | Forearm and hip | Forearm: Osteopenia: 54% and OP: 14% Hip: Osteopenia: 51% and OP: 5% | Accelerated loss of bone tissue is observed in patients with AS |
| Vasdev et al[29] | AS: 80 (80/0) C: 160 (160/0) | 32.9 | 8.1 | Hologic | LS (1), hip (1) | In active and inactive patients, BMD is similar OP: 28.8% at LS and 11.5% at FN VF: 1.25% HLA-B27: 86% | OP is a significant complication in AS even in early disease, and more prevalent in the spine compared to femur Spinal BMD is the most sensitive site for defining OP in AS | |
| van der Weijden et al[4] | SpA: 130 (86/44) AS: 72% uSpA: 12% PsA: 8%; ReA: 4% | 38 | 42:2 | 6.3 | Lunar | L2-4, left PF | Osteopenia: 38%, OP: 9% HLA-B27: 74% No differences between group for distribution of the osteopenia and OP at hip or LS BMD | A high frequency of low BMD is found in patients with early SpA and it is associated with male gender and decreased functional capacity |
| Grazio et al[26] | AS: 80 (46/34) | 52.3 | NM | 21.8 | Hologic | L2-4, left PF | HLA-B27 86% at LS: OP: 25% and osteopenia: 20% at FN OP: 22.5 and osteopenia: 47.4% More patients with osteopenia at the lumbar spine had lower BASDAI score | Hip BMD seems to be more associated with disease activity and functional ability than BMD at the lumbar spine |
| Klingberg et al[27] | AS: 204 (117/87) | 50 | 42:45 | 24 | Hologic | AP L1-4 (0.4), lateral L2-4 (0.6), left hip, non-dominant radius | HLA-B27: 87% ≥ 50 yr osteopenia: 43.6 and OP: 20.8% < 50 yr low BMD 4.9% BMD at lateral LS was lower than AP and revealed more OP | OP and osteopenia is common in AS and associated with high disease burden. Lateral and volumetric lumbar DXA are more sensitive than AP DXA in detecting OP |
| Klingberg et al[60] | 204 (117/87) | 50 | 42:45 | 24 | Hologic | AP L1-4, Lat L2-4, non dominant PF and forearm | BMD was significantly lower in the patients with VF HLA-B27: 87% VF: 11.8% | BMD in the femoral neck, total hip, and estimated vertebral BMD show the strongest association with VF |
| Taylan et al[61] | AS: 55 (48/7) | AS: 36 | 10 | Hologic | PA L2-4, Left femur | BMD at proximal femur is lower but at lumbar spine was similar HLA-B27: 64.9% | ||
| van der Weijden et al[62] | SpA: 113 (75/38) AS: 71% | 37 | 38:0 | 5.7 | Lunar | L2-4, left PF | In patients with VF, BMD at LS is lower than patients without VF HLA-B27: 75% VF: 15% | The VFs are associated with low BMD of the lumbar spine and with axial PsA |
| Akgöl et al[30] | nr-axSpA: 46 (32/14) | 31.4 | 14:0 | < 3 | Hologic | LS (1), PF (3) | Patients with nr-axSpA have significant bone loss at the lumbar spine compared with patients with mLBP Comparison of BMD in the nr-axSpA subgroups reveal that patients with inflammation had lower BMD at the LS and PF HLA-B27: 60.8%; no VF | Inflammation on MRI is closely associated with low bone mass in patients who are in the very early stage of the disease |
| Briot et al[21] | SpA: 332 (174/158) | 33.8 | 151:7 | 1.6 | Hologic or Lunar | L1-4, FN, FT | Low BMD associated with presence of inflammatory lesions on MRI, ESR or CRP HLA-B27 62.1% Low BMD: 13% (M: 88%) | Patients with early SpA had 13.0% low BMD and the main risk factor associated with low BMD was inflammation on MRI |
| Klingberg et al[9] | AS: 69 (69/0) | 49 | NA | 23 | Hologic | AP L1-4, lat L2-4, non dominant forearm and hip HRpQCT: radius (0.3-3.9) and tibia (0.1-1.6) QCT: L1-4 | The AS patients have lower vBMD in peripheral bone Syndesmophytes are significantly associated with decreasing trabecular vBMD in lumbar spine Estimated lumbar vBMD by DXA correlate with trabecular vBMD measured by QCT HLA-B27 94% | Male patients with AS have axial osteopenia. New bone formation cause false normal BMD at LS by DXA |
| Ulu et al[46] | AS: 86 (69/17) | AS: 34.5 | NM | 11.7 | Hologic | PA L1-4, lat L2-4, femur | HLA-B27: 66.3% Syndesmophytes: 37.2% VF: 28% PA spine BMD similar with C Lateral spine, hip BMD lower in AS PA BMD higher in late stage AS than early stage FN, FT BMD lat spine BMD similar in two stage | Bone loss increase in AS The BMD measurement at the lateral lumbar spine reflects bone loss and fracture risk better than PA spine and femoral measurements |
| Ref. | Sample size(M/F) | Mean age(yr) | Menopausal status(pre:post) | Diseaseduration (yr) | Dexamachine | Dexa site (coefficientvariation %) | Follow-up(mo) | Outcome | Conclusion |
| Lee et al[17] | AS: 14 (14/0) 7 early AS 7 advanced AS | 33.3 54.6 | NA | 5.4 27 | Hologic | LS (1), FN (1) | 15 | Baseline LS BMD measured by QCT decrease in both early (also by DXA) and advanced diseases and do not change significantly over 15 mo HLA-B27 92.9% | AP LS DXA in late AS is less useful than QCT in determining the degree of osteopenia in late AS |
| Gratacós et al[6] | AS: 34 (27/7) Active 14 (12/2) Inactive 20 (15/5) | Active: 33 Inactive: 31 | 7:0 | 7.5 5.3 | Lunar | LS (0.8), FN (2.3) | 19 | At the end of the follow-up period, patients with active AS show a significant reduction in bone mass in the LS (5%) and FN (3%) | Loss of bone mass only in patients with persistent active AS suggests that inflammatory activity plays a major role in the pathophysiology of the early bone loss |
| Maillefert et al[32] | AS: 54 (35/19) | 37.3 | 16:3 | 12.4 | Hologic | PA L2-4 (2.8), left FN (4) | 24 | After 2 yr, BMD did not change at the LS and decreased at the FN The change in BMD at FN was related to persistent systemic inflammation HLA-B27 88.9% VF: 3.7% after 24 mo | Persistent inflammation may be an etiologic factor of bone loss in AS |
| Kaya et al[31] | AS: 55 (42/13) Active: 22 Inactive: 33 | 35.8 | 13:0 | 11.1 | Lunar | AP L2-4 (2.1), PF (2.3) | 24 | Active AS have lower BMD at PF than inactive ones but LS BMD was similar 0.9% decrease in BMD at FN and increase at LS after follow-up, this change not different in active and inactive AS Active AS OP: PF: 22.7%, LS: 27.3% Osteopenia: PF: 40.9%, LS: 31.8 inactive AS OP; PF: 3%, LS: 21,2% Osteopenia; PF 45.5%, LS: 33.3% | PF measurements seem to be less affected from disease-related new bone formation |
| Haugeberg et al[33] | SpA: 30 (15/15) | 31.1 | 15:0 | 6 | Lunar | AP L2-4 (2.3), both hip (2.8) and hand (1.1) | 12 | No significant reduction in BMD at hip, spine and hand is seen after 12 mo follow-up Bone loss at PF is found to be associated with raised baseline CRP levels, baseline BMO of the SIJs on MRI HLA-B27 56.7 | Bone loss in patients with SpA is a result of systemic inflammation and starts early in the disease process |
| Korkosz et al[18] | AS: 19 (19/0) | 45.6 | NA | 16.5 | Lunar | L2-4 (1.6-2.2), left hip QCT: L1-5 | 120 | During the follow-up VF: 15.8% In spine, trabecular BMC decrease by QCT whereas BMD increase by DXA | In AS patients, spinal trabecular bone density evaluated by QCT decrease over 10-yr follow-up and it is not related to baseline radiological severity of spinal involvement |
| Ref. | Sample size(M/F) | Mean age | Menopausal statuspre:post | Disease duration(yr) | Dexa machine | Dexa site (coefficientvariation %) | Follow-upduration | Outcome | Conclusion |
| Allali et al[39] | SpA: 29 (23/6) | 35 | 6:1 | 13 | Hologic | AP L2-4, left PF | 6 | A significant increase in BMD at the LS, total hip and trochanter is observed in patients with SpA treated with anti-TNF | Benefit of anti-TNFα therapy on BMD in patients with SpA may be through an uncoupling effect on bone cells |
| Briot et al[37] | SpA: 19 (17/2) | 40 | NM | 16.5 | Hologic | L2-4, left FT | 12 | After 1 yr of treatment BMD increase at the spine and femur total | Treatment with anti-TNFα in SpA is associated with an increase of BMD, which results from a decrease of bone resorption |
| Biriot et al[41] | SpA: 106 (80/26) AS: 87.8% PsA: 6.6% | 38 | NM | 16.5 | Hologic | L2-4, left PF | 24 | At 1 and 2 yr of treatment, there is a significant gain in BMD at both lumbar spine and PF HLA-B27: 89% Baseline: OP: 28%, osteopenia: 23% | This 2-yr prospective study show a significant increase in BMD, in patients with SpA receiving anti-TNFα treatment |
| Visvanathan et al[40] | AS: 279 (225/54) | 40.3 | NM | 11.9 | NM | L1-4, PF | 24 | BMD at the spine and hip increase after anti-TNF therapy compared with placebo HLA-B27: 86.7% | Infliximab have positive effect on BMD over 2 yr |
| Kang et al[34] | AS: 90 (72/18) | 29.9 (onset age) | 18:0 | 8.2 | Lunar | AP L1-4, right PF | 36 | The most increase in BMD is observed at the spine and hip in the group treated with concurrent bisphosphonate and anti-TNF HLA-B27: 97% OP: 36.7% | BMD increases more with the combination treatment (bisphosphonate and anti-TNF) and gain of bone mass is associated with the decrease in inflammation |
| Arends et al[35] | AS: 111 (78/33) | 42.2 | NM | 16 | Hologic | AP L1-4, PF | 36 | LS and hip BMD significiantly increase compared to baseline after anti-TNFα theraphy HLA-B27: 81% LS OP: 9%, openia: 34% TF OP: 2%, openia: 37% | Three years of anti-TNF therapy results increase in bone formation in accordance with the continuous improvement in lumbar spinal BMD |
| Dischereit et al[38] | RA: 18 (3/15) AS: 16 (9/7) | RA: 62 AS: 48 | NM | - | Lunar | AP L2-4 (1.5), FN (2) | 24 | At baseline in AS, osteopenia: 50% and OP: 6.3% A stable peripheral BMD, significant increases in axial BMD, could be observed after 24 mo of anti-TNFα therapy compared with baseline | Anti-TNF therapy has favorable effects over osteoprotective pathways in patients with AS and RA |
| Kang et al[36] | AS: 63 (52/11) | 36.8 | 11:2 | 8.6 | Prodigy | L1-4, right PF | 24 | BMD at LS and FT of patients receiving anti-TNF increase regularly over 2 yr TNF blocking therapy and the increase in SASSS are independently associated with increased BMD at lumbar spine HLA-B27: 87% | TNF inhibitors appear to be associated with increased SASSS scores and improvements in BMD |
Figure 1 shows the flow chart and the selection process. There were 286 hits on MEDLINE, 200 on Web of Science and 476 on Scopus. Using the above-mentioned inclusion and exclusion criteria, we identified 55 articles (Cross sectional studies: 41, follow-up studies: 6 and interventional studies: 8) in our systematic search.
Fifty five articles are summarized in Tables 1-3. The sample size of the studies varied from 14 to 332 patients with SpA including AS, ReA, PsA, undifferentiated SpA and nr-axSpA. The reported age range varied from 25 to 56 years in the reviewed studies. The reported symptom duration of patients with axSpA ranged between 1.6 to 49 years. As expected, there were more males than females in these studies. Most of the recruited females were premenopausal women. Reported HLA-B27 positivity changed between 19% to 95% and vertebral fractures were reported with a prevalence of up to 39% in the reviewed studies. The prevalence of OP and osteopenia in patients with SpA varied from 3%-47% to 5%-88%, respectively, in the included studies. In particular, the prevalence of OP and osteopenia ranged from 2.0%-47.0% and 5.0%-78.3%, respectively, in patients with AS.
We included studies which used DXA as the technique of BMD assessment in patients with axSpA. Eleven of the 55 studies were comparative studies in which DXA techniques were compared with QUS[7,13,14], single energy QCT[8], QCT[9,15-19], dual-energy QCT[20], peripheral QCT[20] and HRpQCT[9].
Regarding the comparative studies, one study demonstrated that QUS correlated with DXA[13] but this result was not confirmed in any other study[7]. On the other hand, Jansen et al[14] demonstrated similar performance with QUS compared to DXA in detecting OP-associated fracture risk.
Numerous QCT studies demonstrated higher prevalence of OP compared to those studies which used DXA as the assessment tool[8,9,18], whereas only one study revealed no difference between QCT and DXA[20]. Two studies revealed a good correlation between QCT and DXA[9,15], however lumbar spine DXA was shown to be less useful than QCT to detect the degree of osteopenia in late stage AS[15,17].
The change in bone formation and resorption markers including bone alkaline phosphatase (bALP), osteocalcin (OC), C-terminal cross-linking telopeptide of type I collagen (CTX), and deoxypyridinoline is presented in Table 4. There are conflicting results regarding the levels of bone formation and resorption markers in patients with AS and other forms of SpA.
| Ref. | Bone formation markers | Bone resorption markers | ||
| bALP | OC | CTX | DPD | |
| Borman et al[53] | Increased | |||
| Grisar et al[55] | Increased | Increased | Increased | Increased |
| Speden et al[7] | Decreased | Decreased | Increased | |
| Sarikaya et al[57] | Decreased | Increased | ||
| Lee et al[17] | Normal | Normal | ||
| Altindag et al[58] | Increased | Decreased | Increased | |
| Mermerci Başkan et al[25] | Normal | |||
| Acebes et al[51] | Normal | Increased | ||
There are conflicting results regarding the relationship between disease activity, acute phase response markers and bone mass. Some studies suggested a good correlation between bone mass with disease activity[16,21-26] and acute phase reactants[4,16,21,23-28], whereas others[7,13,20,29] did not report a significant relationship.
A recent study revealed the close association between bone mineral density and magnetic resonance (MR) defined acute inflammatory changes in the lumbar spine[30]. The results of this study, as well as the results obtained in patients with early inflammatory back pain, clearly defined the inflammation induced bone loss in patients with axial SpA[21,30].
Patients with active disease (BASDAI > 4) had significantly lower proximal femur BMD compared to patients with inactive disease, whereas spinal BMD was similar in the study by Kaya et al[31]. After 24-mo of follow-up lumbar spinal BMD increased in both groups; however hip BMD decreased in the inactive group[31]. On the other hand, Gratacós et al[6] reported that BMD at the lumbar spine and at the femoral neck decreased in patients with active disease but no change was observed in patients with inactive disease after 19 mo of follow-up. There are conflicting results in follow-up studies. For examples, Maillefert et al[32] reported unchanged lumbar BMD but decreased femoral neck after 12 mo of follow-up, whereas Haugeberg et al[33] failed to demonstrate significant reduction in hip, spine or hand BMD.
C-reactive proteine (CRP) levels have been suggested as an independent predictor of BMD change in patients with AS[6]. Additionally, femoral neck BMD has been found to be associated with persistent systemic inflammation which was defined by elevated erythrocyte sedimentation rate (ESR)[32]. On the other hand, another study failed to show significant interactions among spinal or hip BMD measurements and age, body mass index, disease duration, lumbar Schober, BASDAI, ESR or CRP[31].
Although bath ankylosing spondylitis functional index had a significant negative effect on hip BMD[31]. An 8 year follow-up study revealed that hip bone loss was associated with raised baseline CRP levels, MR defined bone marrow edema of the SIJs and the presence of radiographic sacroiliitis[33].
In all interventional studies BMD at the lumbar spine[34-41] increased in patients treated with anti-tumor necrosis factor (TNF) therapy. Additionally, hip BMD also increased[34-37,39-41] except for one study in which hip BMD remained unchanged[38].
Baseline bALP, OC and CTX levels significantly correlated with the increase in spinal BMD at weeks 24 and 102 after anti-TNF therapy[40]. Changes in acute phase reactants as well as disease activity scores have been demonstrated to correlate with the changes in BMD measurements[36,39,41]. Spinal BMD changes were shown to be associated with changes in ESR and newly formed syndesmophytes under anti-TNF therapy[36].
OP is a well-known problem in patients with AS which begins in the early stages of the disease and inevitably causes vertebral fractures[42-44]. The reported prevalence of OP in AS varies from 3% to 47% according to the measurement techniques and patient selection criteria used. Osteopenia has been reported in up to 88% of patients with SpA. An increased prevalence of spinal bone loss may occur even in early and mild forms of SpA[8,42-46].
Systemic inflammation may play a critical role in the pathogenesis of OP in patients with systemic inflammatory disorders including SpA. This notion is supported with data from studies revealing reduced spinal BMD in patients with early or mild disease without advanced structural damage at the spine[20,21,30,43,47]. In advanced cases, spinal ossifications may mislead normal or artificially increased BMD at the lumbar spine. In such cases DXA measurements of the spine with lateral projections have been suggested to improve sensitivity[27,48]. On the other hand, the precision of DXA measurements on the lateral spine is reasonably lower than on the AP spine or proximal femur[27,48].
As an alternative method QCT, which selectively measures trabecular and cortical bone density, can be used to determine spinal BMD in cases with advanced structural changes[8,9,19,20].
Dual-energy X-ray absorptiometry is known as the reference method to measure BMD. It is an accurate, reproducible, and non-invasive method with good short or long-term precision. Multiple skeletal sites can be safely and precisely assessed by DXA[49]. Direct radiography is still a valid method for assessing structural damage in patients with axial SpA; however it gives little information about bone density since demineralization needs to reach 50% in order to confirm a reliable bone loss on radiographs. Higher incidence of bone loss at the hip compared to the lumbar spine has been suggested in various studies conducted in patients with AS[7,14,15,20,24,44,46-48,50].
There are inconclusive results regarding the association between DXA measurements with clinical and laboratory findings. Bone mineral density at the lumbar spine and hip has been shown to correlate with BASDAI[16,24-26], ESR[16,24-26,28] and CRP[16,24-26,28]. However conflicting results have also been reported[7,13,20,29].
The follow-up studies included in this review revealed that BMD measurements at the proximal hip usually decreased but lumbar spinal measurements increased or were unchanged after a reasonable follow-up.
Regarding the interventional studies, we identified 8 studies which assessed the influence of TNF blocking therapy on BMD in patients with SpA. In 7 out of 8 studies, BMD at the lumbar spine and proximal hip increased after treatment with anti-TNF drugs[34-37,39-41]. The positive effects of these potent anti-inflammatory treatments (TNF blockers) on BMD indirectly support the role of systemic or local inflammation in bone metabolism.
In patients with SpA, bone loss starts in the early stages of the disease and can be prevented with TNF blocking treatments that have been shown to reduce inflammation at the skeletal sites. DXA is the most suitable technique to determine bone mass at both the lumbar spine and proximal femur in early or non-advanced cases. However it may cause misleading results particularly at the AP lumbar spine due to the aberrant ossification or degenerative changes. Despite its limitations, DXA measurements with lateral spinal projections or QCT may be a solution to this problem in patients with advanced disease.
Spondyloarthritis (SpA) is a chronic inflammatory disease characterized by predominant involvement of the spine and/or sacroiliac joints. Low bone mass [osteopenia or osteoporosis (OP)] and osteoporotic vertebral fractures are well known complications of SpA, especially in ankylosing spondylitis (AS). The pathogenesis and onset of OP in SpA is not clear.
Low bone mass and osteoporotic vertebral fractures are common complications of SpA, especially in AS. The prevalence of low BMD has been reported to be as high as 47% at the hip and lumbar spine even in patients with early SpA. Patients with SpA may have increased risk of bone loss as a result of high disease activity, pro-inflammatory cytokines and decrease in physical activity or mineralization defects due to subclinical gut involvement.
This review includes studies, which used dual X-ray absorptiometry (DXA) as the technique of BMD assessment in patients with axSpA. In twenty percent of studies, DXA techniques were compared with quantitative ultrasound or different type of quantitative computed tomography. Among these techniques DXA can be considered as an accurate, repeatable and quantitative method to assess BMD at the spine and hip but new bone formation and aberrant hyperostosis inevitably cause a pseudo increase in bone density.
The most appropriate and valid method to assess BMD in patients with advanced AS is still unclear. A systematic evaluation of DXA or alternative methods used to assess BMD in SpA is strongly needed.
Overall the paper is well written and the subject is certainly of interest.
P- Reviewer: Daoussis D S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, Braun J, Chou CT, Collantes-Estevez E, Dougados M. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2694] [Cited by in RCA: 2471] [Article Influence: 154.4] [Reference Citation Analysis (0)] |
| 2. | Ozgocmen S, Khan MA. Current concept of spondyloarthritis: special emphasis on early referral and diagnosis. Curr Rheumatol Rep. 2012;14:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Mitra D, Elvins DM, Speden DJ, Collins AJ. The prevalence of vertebral fractures in mild ankylosing spondylitis and their relationship to bone mineral density. Rheumatology (Oxford). 2000;39:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | van der Weijden MA, van Denderen JC, Lems WF, Heymans MW, Dijkmans BA, van der Horst-Bruinsma IE. Low bone mineral density is related to male gender and decreased functional capacity in early spondylarthropathies. Clin Rheumatol. 2011;30:497-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Lange U, Teichmann J, Stracke H. Correlation between plasma TNF-alpha, IGF-1, biochemical markers of bone metabolism, markers of inflammation/disease activity, and clinical manifestations in ankylosing spondylitis. Eur J Med Res. 2000;5:507-511. [PubMed] |
| 6. | Gratacós J, Collado A, Pons F, Osaba M, Sanmartí R, Roqué M, Larrosa M, Múñoz-Gómez J. Significant loss of bone mass in patients with early, active ankylosing spondylitis: a followup study. Arthritis Rheum. 1999;42:2319-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Speden DJ, Calin AI, Ring FJ, Bhalla AK. Bone mineral density, calcaneal ultrasound, and bone turnover markers in women with ankylosing spondylitis. J Rheumatol. 2002;29:516-521. [PubMed] |
| 8. | Lange U, Kluge A, Strunk J, Teichmann J, Bachmann G. Ankylosing spondylitis and bone mineral density--what is the ideal tool for measurement? Rheumatol Int. 2005;26:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Klingberg E, Lorentzon M, Göthlin J, Mellström D, Geijer M, Ohlsson C, Atkinson EJ, Khosla S, Carlsten H, Forsblad-d’Elia H. Bone microarchitecture in ankylosing spondylitis and the association with bone mineral density, fractures and syndesmophytes. Arthritis Res Ther. 2013;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Vosse D, Heijckmann C, Landewé R, van der Heijde D, van der Linden S, Geusens P. Comparing morphometric X-ray absorptiometry and radiography in defining vertebral wedge fractures in patients with ankylosing spondylitis. Rheumatology (Oxford). 2007;46:1667-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | El Maghraoui A, Tellal S, Chaouir S, Lebbar K, Bezza A, Nouijai A, Achemlal L, Bouhssain S, Derouiche el M. Bone turnover markers, anterior pituitary and gonadal hormones, and bone mass evaluation using quantitative computed tomography in ankylosing spondylitis. Clin Rheumatol. 2005;24:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | El Maghraoui A, Do Santos Zounon AA, Jroundi I, Nouijai A, Ghazi M, Achemlal L, Bezza A, Tazi MA, Abouqual R. Reproducibility of bone mineral density measurements using dual X-ray absorptiometry in daily clinical practice. Osteoporos Int. 2005;16:1742-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Toussirot E, Michel F, Wendling D. Bone density, ultrasound measurements and body composition in early ankylosing spondylitis. Rheumatology (Oxford). 2001;40:882-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Jansen TL, Aarts MH, Zanen S, Bruyn GA. Risk assessment for osteoporosis by quantitative ultrasound of the heel in ankylosing spondylitis. Clin Exp Rheumatol. 2003;21:599-604. [PubMed] |
| 15. | Stupphann D, Rauner M, Krenbek D, Patsch J, Pirker T, Muschitz C, Resch H, Pietschmann P. Intracellular and surface RANKL are differentially regulated in patients with ankylosing spondylitis. Rheumatol Int. 2008;28:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Obermayer-Pietsch BM, Lange U, Tauber G, Frühauf G, Fahrleitner A, Dobnig H, Hermann J, Aglas F, Teichmann J, Neeck G. Vitamin D receptor initiation codon polymorphism, bone density and inflammatory activity of patients with ankylosing spondylitis. Osteoporos Int. 2003;14:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Lee YS, Schlotzhauer T, Ott SM, van Vollenhoven RF, Hunter J, Shapiro J, Marcus R, McGuire JL. Skeletal status of men with early and late ankylosing spondylitis. Am J Med. 1997;103:233-241. [PubMed] |
| 18. | Korkosz M, Gąsowski J, Grzanka P, Gorczowski J, Pluskiewicz W, Jeka S, Grodzicki T. Baseline new bone formation does not predict bone loss in ankylosing spondylitis as assessed by quantitative computed tomography (QCT): 10-year follow-up. BMC Musculoskelet Disord. 2011;12:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Devogelaer JP, Maldague B, Malghem J, Nagant de Deuxchaisnes C. Appendicular and vertebral bone mass in ankylosing spondylitis. A comparison of plain radiographs with single- and dual-photon absorptiometry and with quantitative computed tomography. Arthritis Rheum. 1992;35:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Karberg K, Zochling J, Sieper J, Felsenberg D, Braun J. Bone loss is detected more frequently in patients with ankylosing spondylitis with syndesmophytes. J Rheumatol. 2005;32:1290-1298. [PubMed] |
| 21. | Briot K, Durnez A, Paternotte S, Miceli-Richard C, Dougados M, Roux C. Bone oedema on MRI is highly associated with low bone mineral density in patients with early inflammatory back pain: results from the DESIR cohort. Ann Rheum Dis. 2013;72:1914-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Arends S, Spoorenberg A, Bruyn GA, Houtman PM, Leijsma MK, Kallenberg CG, Brouwer E, van der Veer E. The relation between bone mineral density, bone turnover markers, and vitamin D status in ankylosing spondylitis patients with active disease: a cross-sectional analysis. Osteoporos Int. 2011;22:1431-1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Ghozlani I, Ghazi M, Nouijai A, Mounach A, Rezqi A, Achemlal L, Bezza A, El Maghraoui A. Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone. 2009;44:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Kim HR, Lee SH, Kim HY. Elevated serum levels of soluble receptor activator of nuclear factors-kappaB ligand (sRANKL) and reduced bone mineral density in patients with ankylosing spondylitis (AS). Rheumatology (Oxford). 2006;45:1197-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Mermerci Başkan B, Pekin Doğan Y, Sivas F, Bodur H, Ozoran K. The relation between osteoporosis and vitamin D levels and disease activity in ankylosing spondylitis. Rheumatol Int. 2010;30:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Grazio S, Kusić Z, Cvijetić S, Grubišić F, Balenović A, Nemčić T, Matijević-Mikelić V, Punda M, Sieper J. Relationship of bone mineral density with disease activity and functional ability in patients with ankylosing spondylitis: a cross-sectional study. Rheumatol Int. 2012;32:2801-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Klingberg E, Lorentzon M, Mellström D, Geijer M, Göthlin J, Hilme E, Hedberg M, Carlsten H, Forsblad-d’Elia H. Osteoporosis in ankylosing spondylitis - prevalence, risk factors and methods of assessment. Arthritis Res Ther. 2012;14:R108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Jun JB, Joo KB, Her MY, Kim TH, Bae SC, Yoo DH, Kim SK. Femoral bone mineral density is associated with vertebral fractures in patients with ankylosing spondylitis: a cross-sectional study. J Rheumatol. 2006;33:1637-1641. [PubMed] |
| 29. | Vasdev V, Bhakuni D, Garg MK, Narayanan K, Jain R, Chadha D. Bone mineral density in young males with ankylosing spondylitis. Int J Rheum Dis. 2011;14:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Akgöl G, Kamanlı A, Ozgocmen S. Evidence for inflammation-induced bone loss in non-radiographic axial spondyloarthritis. Rheumatology (Oxford). 2014;53:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Kaya A, Ozgocmen S, Kamanli A, Ardicoglu O. Bone loss in ankylosing spondylitis: does syndesmophyte formation have an influence on bone density changes? Med Princ Pract. 2009;18:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Maillefert JF, Aho LS, El Maghraoui A, Dougados M, Roux C. Changes in bone density in patients with ankylosing spondylitis: a two-year follow-up study. Osteoporos Int. 2001;12:605-609. [PubMed] |
| 33. | Haugeberg G, Bennett AN, McGonagle D, Emery P, Marzo-Ortega H. Bone loss in very early inflammatory back pain in undifferentiated spondyloarthropathy: a 1-year observational study. Ann Rheum Dis. 2010;69:1364-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Kang KY, Lee KY, Kwok SK, Ju JH, Park KS, Hong YS, Kim HY, Park SH. The change of bone mineral density according to treatment agents in patients with ankylosing spondylitis. Joint Bone Spine. 2011;78:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Arends S, Spoorenberg A, Houtman PM, Leijsma MK, Bos R, Kallenberg CG, Groen H, Brouwer E, van der Veer E. The effect of three years of TNFα blocking therapy on markers of bone turnover and their predictive value for treatment discontinuation in patients with ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther. 2012;14:R98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Kang KY, Ju JH, Park SH, Kim HY. The paradoxical effects of TNF inhibitors on bone mineral density and radiographic progression in patients with ankylosing spondylitis. Rheumatology (Oxford). 2013;52:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Briot K, Garnero P, Le Henanff A, Dougados M, Roux C. Body weight, body composition, and bone turnover changes in patients with spondyloarthropathy receiving anti-tumour necrosis factor {alpha} treatment. Ann Rheum Dis. 2005;64:1137-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Dischereit G, Tarner IH, Müller-Ladner U, Lange U. Infliximab improves bone metabolism and bone mineral density in rheumatoid arthritis and ankylosing spondylitis: a prospective 2-year study. Clin Rheumatol. 2013;32:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Allali F, Breban M, Porcher R, Maillefert JF, Dougados M, Roux C. Increase in bone mineral density of patients with spondyloarthropathy treated with anti-tumour necrosis factor alpha. Ann Rheum Dis. 2003;62:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Visvanathan S, van der Heijde D, Deodhar A, Wagner C, Baker DG, Han J, Braun J. Effects of infliximab on markers of inflammation and bone turnover and associations with bone mineral density in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68:175-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Briot K, Gossec L, Kolta S, Dougados M, Roux C. Prospective assessment of body weight, body composition, and bone density changes in patients with spondyloarthropathy receiving anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2008;35:855-861. [PubMed] |
| 42. | Donnelly S, Doyle DV, Denton A, Rolfe I, McCloskey EV, Spector TD. Bone mineral density and vertebral compression fracture rates in ankylosing spondylitis. Ann Rheum Dis. 1994;53:117-121. [PubMed] |
| 43. | Mullaji AB, Upadhyay SS, Ho EK. Bone mineral density in ankylosing spondylitis. DEXA comparison of control subjects with mild and advanced cases. J Bone Joint Surg Br. 1994;76:660-665. [PubMed] |
| 44. | Singh A, Bronson W, Walker SE, Allen SH. Relative value of femoral and lumbar bone mineral density assessments in patients with ankylosing spondylitis. South Med J. 1995;88:939-943. [PubMed] |
| 45. | Incel NA, Gökoğlu F, Nacir B, Incel N. Bone and stone in ankylosing spondylitis: osteoporosis and urolithiasis. Clin Rheumatol. 2006;25:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Ulu MA, Çevik R, Dilek B. Comparison of PA spine, lateral spine, and femoral BMD measurements to determine bone loss in ankylosing spondylitis. Rheumatol Int. 2013;33:1705-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Baek HJ, Kang SW, Lee YJ, Shin KC, Lee EB, Yoo CD, Song YW. Osteopenia in men with mild and severe ankylosing spondylitis. Rheumatol Int. 2005;26:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Gilgil E, Kaçar C, Tuncer T, Bütün B. The association of syndesmophytes with vertebral bone mineral density in patients with ankylosing spondylitis. J Rheumatol. 2005;32:292-294. [PubMed] |
| 49. | Hans D, Downs RW, Duboeuf F, Greenspan S, Jankowski LG, Kiebzak GM, Petak SM; International Society for Clinical D. Skeletal sites for osteoporosis diagnosis: the 2005 ISCD Official Positions. J Clin Densitom. 2006;9:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 50. | Meirelles ES, Borelli A, Camargo OP. Influence of disease activity and chronicity on ankylosing spondylitis bone mass loss. Clin Rheumatol. 1999;18:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Acebes C, de la Piedra C, Traba ML, Seibel MJ, García Martín C, Armas J, Herrero-Beaumont G. Biochemical markers of bone remodeling and bone sialoprotein in ankylosing spondylitis. Clin Chim Acta. 1999;289:99-110. [PubMed] |
| 52. | Juanola X, Mateo L, Nolla JM, Roig-Vilaseca D, Campoy E, Roig-Escofet D. Bone mineral density in women with ankylosing spondylitis. J Rheumatol. 2000;27:1028-1031. [PubMed] |
| 53. | Borman P, Bodur H, Bingöl N, Bingöl S, Bostan EE. Bone mineral density and bone turnover markers in a group of male ankylosing spondylitis patients: relationship to disease activity. J Clin Rheumatol. 2001;7:315-321. [PubMed] |
| 54. | Dos Santos FP, Constantin A, Laroche M, Destombes F, Bernard J, Mazières B, Cantagrel A. Whole body and regional bone mineral density in ankylosing spondylitis. J Rheumatol. 2001;28:547-549. [PubMed] |
| 55. | Grisar J, Bernecker PM, Aringer M, Redlich K, Sedlak M, Wolozcszuk W, Spitzauer S, Grampp S, Kainberger F, Ebner W. Ankylosing spondylitis, psoriatic arthritis, and reactive arthritis show increased bone resorption, but differ with regard to bone formation. J Rheumatol. 2002;29:1430-1436. [PubMed] |
| 56. | Capaci K, Hepguler S, Argin M, Tas I. Bone mineral density in mild and advanced ankylosing spondylitis. Yonsei Med J. 2003;44:379-384. [PubMed] |
| 57. | Sarikaya S, Basaran A, Tekin Y, Ozdolap S, Ortancil O. Is osteoporosis generalized or localized to central skele-ton in ankylosing spondylitis? J Clin Rheumatol. 2007;13:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Altindag O, Karakoc M, Soran N, Tabur H, Demirkol A. Bone mineral density in patients with ankylosing spondylitis. Romatizma-Rheumatism. 2008;23:42-45. |
| 59. | Korczowska I, Przepiera-Bedzak H, Brzosko M, Lacki JK, Trefler J, Hrycaj P. Bone tissue metabolism in men with ankylosing spondylitis. Adv Med Sci. 2011;56:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Klingberg E, Geijer M, Göthlin J, Mellström D, Lorentzon M, Hilme E, Hedberg M, Carlsten H, Forsblad-D’Elia H. Vertebral fractures in ankylosing spondylitis are associated with lower bone mineral density in both central and peripheral skeleton. J Rheumatol. 2012;39:1987-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 61. | Taylan A, Sari I, Akinci B, Bilge S, Kozaci D, Akar S, Colak A, Yalcin H, Gunay N, Akkoc N. Biomarkers and cytokines of bone turnover: extensive evaluation in a cohort of patients with ankylosing spondylitis. BMC Musculoskelet Disord. 2012;13:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | van der Weijden MA, van der Horst-Bruinsma IE, van Denderen JC, Dijkmans BA, Heymans MW, Lems WF. High frequency of vertebral fractures in early spondylarthropathies. Osteoporos Int. 2012;23:1683-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |