Published online Mar 18, 2015. doi: 10.5312/wjo.v6.i2.290
Peer-review started: February 27, 2014
First decision: April 28, 2014
Revised: August 15, 2014
Accepted: September 4, 2014
Article in press: September 10, 2014
Published online: March 18, 2015
Processing time: 385 Days and 17.7 Hours
AIM: To summarise and compare currently available evidence regarding accuracy of pre-operative imaging, which is one of the key choices for surgeons contemplating patient-specific instrumentation (PSI) surgery.
METHODS: The MEDLINE and EMBASE medical literature databases were searched, from January 1990 to December 2013, to identify relevant studies. The data from several clinical studies was assimilated to allow appreciation and comparison of the accuracy of each modality. The overall accuracy of each modality was calculated as proportion of outliers > 3% in the coronal plane of both computerised tomography (CT) or magnetic resonance imaging (MRI).
RESULTS: Seven clinical studies matched our inclusion criteria for comparison and were included in our study for statistical analysis. Three of these reported series using MRI and four with CT. Overall percentage of outliers > 3% in patients with CT-based PSI systems was 12.5% vs 16.9% for MRI-based systems. These results were not statistically significant.
CONCLUSION: Although many studies have been undertaken to determine the ideal pre-operative imaging modality, conclusions remain speculative in the absence of long term data. Ultimately, information regarding accuracy of CT and MRI will be the main determining factor. Increased accuracy of pre-operative imaging could result in longer-term savings, and reduced accumulated dose of radiation by eliminating the need for post-operative imaging and revision surgery.
Core tip: At present there is not enough published data to convincingly conclude in favour of computerised tomography (CT) or magnetic resonance imaging for accuracy of pre-operative imaging in patient-specific instrumentation. We recommend CT as a more favourable option at present due to reduced scanning times, increased availability, and relatively cheaper cost.
-
Citation: Stirling P, Valsalan Mannambeth R, Soler A, Batta V, Malhotra RK, Kalairajah Y. Computerised tomography
vs magnetic resonance imaging for modeling of patient-specific instrumentation in total knee arthroplasty. World J Orthop 2015; 6(2): 290-297 - URL: https://www.wjgnet.com/2218-5836/full/v6/i2/290.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i2.290
Patient-specific instrumentation (PSI) was developed to simultaneously optimize patient outcomes and surgical efficiency in total knee arthroplasty (TKA), and PSI evolved with the aims to improve component placement accuracy. Improving accuracy of placement of the tibial component can reduce the incidence of malalignment and rotation, errors which are associated with patient dissatisfaction after arthroplasty[1] - reported in up to 19% of cases[2] - and are believed to reduce implant survival[3,4]. The current technique combines pre-operative imaging using computerised tomography (CT) or magnetic resonance imaging (MRI) and full-length radiograph in combination with rapid-prototyping technology to create bespoke guides or jigs which direct cutting tools during bony resection. Pre-operative computer-assisted planning allows determination of resection margins, implant size and position, the overall aim being to improve component alignment and operative efficiency[5,6] whilst avoiding violation of the intramedullary canal. Pre-operative imaging and planned guides are approved by the surgeon, thus shifting the navigational aspects of the procedure to the pre-operative stage, which may improve operative efficiency[6].
Proponents of the technique argue that PSI can improve component alignment accuracy, post-operative functional outcome[7], whilst reducing intraoperative blood loss, operative time, number of surgical steps[7], and time between cases, ultimately resulting in cost savings associated with reduced inventory and sterilization costs. Evidence in this area is still however conflicting. Many studies report good alignment achieved with PSI[8-11] in both coronal and frontal planes, yet separate studies have demonstrated no significant difference in component alignment using PSI[12-14], with some even reporting an increase in outlier incidence[7,15]. In addition, there are conflicting reports of the proposed reduction in operative time and technicality: whilst some studies report a significant reduction in operating time[7,16], others report increased intra-operative changes to implant size[17] due to mismatching of the specific prosthesis and the pre-operative plan[18].
Many current opinions regarding PSI remain speculative in the absence of medium and long-term data. Proposed benefits regarding patient functional outcomes, complication rates, alignment, and cost-effectiveness are summarized comprehensively in review articles authored by Ast et al[6], Nam et al [19], and Lachiewicz el al[20].
The use of pre-operative CT vs MRI varies depending on the PSI system used, and is a source of major ongoing debate. CT is relatively inexpensive and imaging times are short. It is therefore financially appealing. However, exposure to radiation is a concern and there have been recent reports of increase in cancer attributed to unnecessary CTs[21,22]. MRI, on the other hand, does not use ionising radiation and is deemed a safer and more appealing imaging modality. Some pre-operative MRI imaging for PSI also requires a whole leg plain film and so may not wholly be without additional radiation exposure. Moreover, most CT-based PSI systems utilise focused scans of the hip, knee, and ankle to reduce unnecessary exposure: the equivalent dose has been calculated at 5 mSv, comparable to a yearly background radiation dose, or roughly 70 chest X-rays[23].
Although MRI avoids radiation exposure, the cost and time of the investigation is greater than for CT. For PSI, there is no need to report the scans once performed as the image data is sent directly to the company, and the only cost of imaging is in performing the scan itself. In our trust, the cost of pre-operative imaging using MRI is almost double that of CT (£171 vs£97). Importantly, the comparatively longer length of time for an MRI scan may result in movement artefacts worsening the quality of MRI images.
Ultimately, information regarding accuracy of CT and MRI will be the main determining factor. This review article summarises and compares currently available evidence regarding accuracy of pre-operative imaging, which is one of the key choices for surgeons contemplating PSI surgery.
The MEDLINE and EMBASE medical literature databases were searched, from January 1990 to December 2013, to identify relevant studies. The Keywords used were (1) Patient Specific templates in total knee replacement (TKR); (2) Patient specific instrumentation in TKR; and (3) Customised Patient Jigs in TKR. Studies were eligible for review if they met the following criteria: (1) the language was English and one of the following; (2) had a comparison between conventional TKR and PSI; (3) comparison between CT and MRI for PSI; and (4) reported cadaveric or clinical analysis of accuracy of component placement using PSI. Due to scarcity of clinical studies available, studies were stratified for inclusion, with animal studies considered lowest in the hierarchy, followed by human cadaveric studies, and finally, human clinical studies.
As most studies available focus on validation of a single technique rather than a direct comparison, the data from several clinical studies was assimilated to allow appreciation and comparison of the accuracy of each modality. The overall accuracy of each modality was calculated as proportion of outliers > 3% in the coronal plane of both CT and MRI. A test for assumption of homogeneity between studies was conducted using Cochrane Q statistics and ratio of heterogeneity to total variance was calculated (I2 statistic). A random-effects method was performed with single stage proportion meta-analysis using R-software. The metaprop command available in meta library was applied with Freeman-Tukey Double arcsine transformation to calculate overall proportion and DeSimonian-Laird method for estimation of variance between studies[24].
Only six studies were identified which directly compared CT and MRI. These studies are summarised in Table 1.
| Ref. | Article type | Sample size | Comparison of accuracy | Dimensional accuracy |
| Ensini et al[25] | Prospective randomized trial | 25 CT PSI and 25 MRI PSI | Intra-operative navigation system and post-operative radiographic alignment | Comparable outcome |
| Cenni et al[9] | Prospective randomised trial | 23 CT and 21 MRI PSI | Post-operative radiograph | Comparable outcome |
| Fritschy et al[26] | Prospective controlled trial | 10 PSI patient, 10 standard TKAs (control) | Intra-operative navigation and post-operative long standing X-ray | Comparable outcome |
| Van den Broeck et al[27] | Human cadaveric study | 9 cadaveric tibia | Comparison with bone dimensions using optical white-light scanner | Comparable outcome |
| White et al[28] | Animal study | 10 ovine knees | Direct comparison with bone dimensions | CT > MRI |
| Rathnayaka et al[29] | Animal study | 5 ovine limbs | Direct comparison with bone dimensions | Comparable outcome |
Aside from the study by Ensini et al[25] and Cenni et al[9], all studies focus on 3D reproduction of bone models, rather than surgical outcomes. White et al[28] undertook an animal study in 2008, comparing CT and MRI based 3D reproductions using PSI systems and compared the reproduced bone dimensions with the actual bony anatomy of 10 ovine knees. They found that bony dimensions of the MRI-based models were significantly less accurate than those created by CT, reporting an average accuracy of 0.61 mm ± 0.41 mm for CT, and 2.15 mm ± 2.44 mm with MRI. This study also found increased bony landmark resolution in CT-based systems compared with MRI. This contradicts previous theories that CT may be less accurate due to its reduced ability to delineate articular cartilage from bone[30]. Rathnayaka et al[29] repeated the study in 2012 using five ovine femora but found comparable outcomes with both imaging modalities. The cadaveric study undertaken by Van den Broeck et al[27] and presented at the European Society of Biomechanics in 2013 corroborated these results, using clinical scanning protocols and human tibia, and finding comparable accuracy of 0.42 mm ± 0.38 mm for MRI and 0.53 mm ± 0.38 mm for CT[27]. Fritschy et al[26] prospectively compared accuracy of CT and MRI in ten patients undergoing computer-navigated TKA, concluding that either technique may be used effectively for PSI synthesis.
There were only two clinical studies which directly compared post-operative alignment in two separate patient groups randomised to CT or MRI. Ensini et al[25] undertook a prospective, randomized study comparing 25 patients randomized to TKA with MRI-based PSI, with 25 patients randomized to TKA with CT. Outcomes measured were intra-operative accuracy and resection thickness, and post-operative axis alignment as defined by post-operative plain radiograph. The authors found acceptable alignment and intra- and post-operative measurements with both systems in the coronal, sagittal and frontal planes. Importantly, the authors reported a higher incidence of mechanical axis outliers of 37% in the pre-operative CT group, compared with 18% in the MRI group. This result however did not reach statistical significance. Cenni et al[9] report a similar study with 23 patients randomised to pre-operative CT and 21 to MRI. Similar mean post-operative mechanical axes were found in both groups (-0.9 ± 2.3 for CT, 0.7 ± 2.4 for MRI) with three outliers in each group.
Seven clinical studies matched our inclusion criteria (3) for comparison and were included in our study for statistical analysis. Three of these reported series using MRI and four with CT. The data from these studies is summarised in Table 2.
| Ref. | Comparison | Type of study | System used | Imaging used | No. of patients | % outliers > 3% |
| Boonen et al[8] | PSI with disposable guides vs conventional intramedullary guides | Case control | Signature | MRI | 40 | 29 |
| Barrett et al[12] | PSI vs conventional - absolute mechanical axis measure | Prospective cohort study | Trumatch | CT | 66 | 19 |
| Bugbee et al[33] | PSI vs conventional | Retrospective cohort | Trumatch | CT | 25 | 4 |
| Chareancholvanich et al[13] | PSI vs conventional instrumentation | RCT | Zimmer | MRI | 80 | 2.5 |
| Koch et al[31] | PSI vs computer-navigated | My knee | CT | 301 | 12.4 | |
| Chen et al[15] | PSI vs conventional TKA | Randomised control study | Zimmer PSI | MRI | 30 | 31 |
| Roh et al[32] | PSI vs conventional | RCT | Signature | CT | 50 | 12 |
All studies reported alignment in the coronal plane. Several studies reported alignment and percentage of outliers with reference to the sagittal plane, and rotational alignment. As a result we were only able to compare percentage of outliers in the coronal plane between studies.
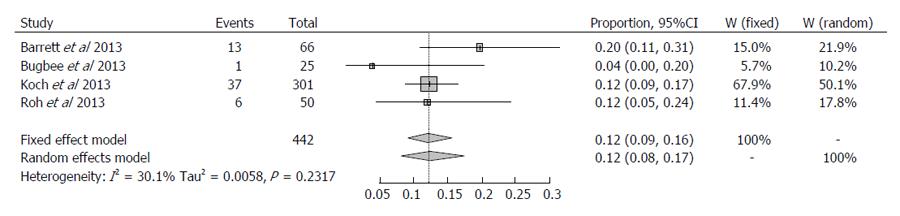
Table 2 shows consistent outlier percentage in all studies investigating accuracy of pre-operative CT. The largest study was reported by Koch et al[31] reporting outlier incidence of 12.4% in a cohort of 301 patients. Accuracy of component placement was found to be equal to computer-navigated surgery. Comparable outcomes were reported by Roh et al[32] in their randomised controlled trial of 50 patients treated with PSI and 50 with conventional TKA, reporting outlier incidence of 12%. Barrett et al[12] reported a slightly higher outlier incidence of 19% from their study of 66 patients using the CT-based Trumatch system. Bugbee et al[33] reported the most accurate results in a retrospective cohort study with 25 patients treated with conventional TKA and 25 with the CT-based Trumatch PSI system, with an outlier incidence of 4%. These result show little variation and all studies report comparable accuracy between CT-based PSI and computer-navigated or conventional instrumentation.
For the four studies using CT, I2 was 30.1% (0%; 74.6%) and this was not significant (P = 0.2317). To maintain the similarity with MRI a random-effects method was applied. The overall proportion of outlier > 3% was 0.1249 (95%CI: 0.0827-0.1737). Therefore we conclude that percentage of outliers > 3% is 12.50% and with 95% confidence at least 9.27% and at most 17.4% for the cohort using pre-operative CT.
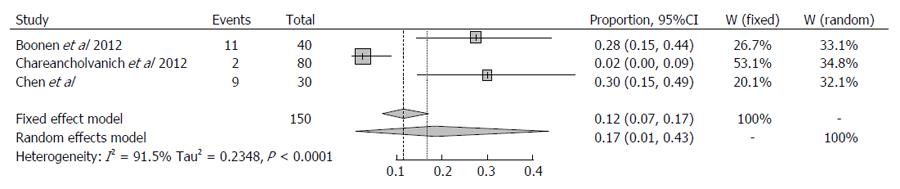
Three studies were identified which investigate post-operative outcomes with MRI-based PSI. Although Chareancholvanich et al[13] reported excellent post-operative alignment and an outlier incidence of 2.5% in their randomised controlled trial of 80 patients, results from the studies by Boonen et al[8], and Chen et al[15] reported much higher outlier incidence of 29% and 31% respectively. It is worth noting that the highest incidence of outliers in the MRI-based systems occurred in the sagittal plane with 24% for both femoral and tibial component as reported by Chen et al[15] , and 41% and 36% for sagittal femoral and tibial respectively as reported by Boonen et al[8].
For the three studies evaluating MRI, I2 was 91.5% (95%CI: 0.782-0.967) suggesting a high degree of variance between the studies (Q statistic, P < 0.0001). Sensitivity analysis revealed that the study reported by Chareancholvanich et al[13] dramatically influenced on the heterogeneity of the analysis. The overall proportion of outliers > 3% was 0.1696 (95%CI: 0.0117-0.4349). Therefore the percentage of outliers > 3% was 16.96% and with 95% confidence at least 1.2% and at most 44% for the cohort using pre-operative MRI. This suggests a higher level of variability between studies, and that overall outlier percentage may in fact be higher than the 16.96% reported.
It was not possible to directly compare the two cohorts, however due to the overlapping confidence intervals, it can be concluded that the difference in outlier incidence appears to be slightly lower using pre-operative CT. No statistically significant conclusions can be drawn from this analysis, however. These results are presented in Figures 1 and 2.
Increasing costs in healthcare together with financial restraints are forcing further rationalisation of available resources. There is a year-on-year increase in the number of primary knee arthroplasties and all national joint registries are reporting increasing numbers of revision arthroplasties[34,35]. A great percentage of these revisions are due to mechanical failure as a result of malalignment[36,37]. The cost of revision TKR is high and depending on the bone loss, implant required, and hospital stay, it can exceed many times over the cost of primary TKR[38-40].
If through a primary procedure we can accurately reproduce a patient’s mechanical and anatomical alignment, then we may reduce the burden of revision surgery. PSI was introduced to improve implant positioning. Component positioning with traditional instrumentation uses coronal plane alignment with reference to the femoral head and ankle joint[41], or anterior or posterior referencing in the sagittal plane. Rotational alignment of the femoral component is also a point of debate as it may affect patella tracking.
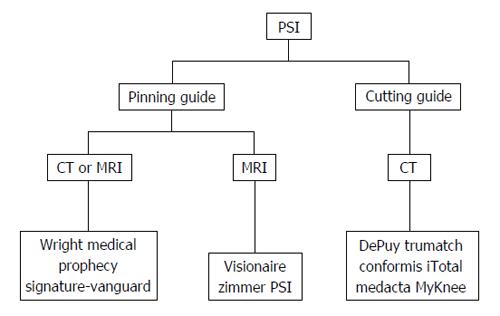
Despite the rapid evolution and growing body of evidence around PSI in recent years, Joint Registry Data shows that conventional arthroplasty using standard instrumentation remains in more widespread use[42]. Current commercial systems differ from each other in three key ways. Firstly, pre-operative imaging modality, whether CT or MRI, is always required to collate the 3-dimensional data required for creation of the patient-specific guide. Secondly, two types of PSI are currently in use: pinning guides and cutting guides. The third and final difference is the method of alignment used, whether mechanical axis or pre-arthritic knee anatomy matching. All currently available systems favour mechanical axis alignment.
Table 3 and Figure 3 summarise the different pre-operative imaging modalities and types of guide employed for currently available PSI systems.
| Manufacturer | Product | Imaging | Type of guide | Launched |
| Biomet | Signature-vanguard | CT or MRI | Pinning | MRI-2007 CT-2010 |
| DePuy | Trumatch | CT | Cutting | 2009 |
| Smith and nephew | Visionaire | MRI | Pinning | 2008 |
| Wright medical | Prophecy | CT or MRI | Pinning | 2009 |
| Zimmer | PSI | MRI | Pinning | 2009 |
| Conformis | Conformis iTotal | CT | Cutting | 2011 |
| Medacta | My knee | CT | Cutting | 2009 |
Choice of pre-operative imaging modality will be affected by availability and cost, but will ultimately be determined by accuracy. Comparison of the accuracy of 3D modeling of knee anatomy using CT and MRI was first reported by Smith et al[43] in 1989, who found equally high quality of reconstruction using the two techniques. Subsequent development of PSI means that the emphasis has shifted to accuracy of the reciprocal guides created. Due to the subsequent rapid-prototyping stages of manufacturing, there will result a small degree of acceptable variation between the final models and the native anatomy[23]. This inherent variation may be a source of incremental error between imaging modalities. We were not able to account for this and thus it is a potential limitation to this study.
The non-clinical cadaveric and animal studies summarised in Table 1 show similar accuracies for both modalities. Due to the heterogeneous nature of these studies, it is impossible to perform statistical analysis on this subset of data. Hypothetically, CT would be expected as a more accurate imaging modality, due to improved imaging of osteophytes over MRI as landmarks for PSI guides. The small-sample comparison studies in Table 1 did not demonstrate this. The two direct clinical comparison undertaken by Cenni et al[9] and Ensini et al[25] also demonstrated comparable results both in terms of proportion of outliers and in post-operative alignment.
Assimilation of currently available clinical reports showed good post-operative mechanical alignment using CT-based PSI systems. Outlier incidence, although high at 19% in the study by Barrett et al[12] was not found to be significantly higher than conventional or computer-assisted TKA in any of the studies.
Examination of studies using MRI-based PSI systems revealed a range of post-operative results. Chareancholvanich et al[13] found a low outlier incidence of 2.5% with MRI-based systems. This study only performed post-operative imaging in the coronal plane, which may partially explain their lower incidence of outliers. Comparison of all studies evaluating MRI showed comparable outlier percentage, but a wider range of outlier percentages when compared to CT.
In our study we were only able to directly compare outlier incidence with CT and MRI in the coronal plane. We were unable to analyse component alignment in the sagittal or rotational axes and this is a further limitation of our study. We found the overall percentage of outliers > 3% in patients with CT-based PSI systems to be 12.5% in the current literature. For MRI-based PSI, outlier percentage was higher at 16.9%. Therefore outlier incidence appears to be slightly lower using pre-operative CT. There is also lower variability between studies in the CT group, however no statistically significant conclusions can be drawn from this analysis.
Current evidence shows comparable accuracy with both imaging modalities. Increased accuracy of pre-operative imaging could result in longer-term savings, and reduced accumulated dose of radiation by eliminating the need for post-operative imaging or revision surgery. Concern regarding radiation exposure with CT, and increased cost of MRI could both be accepted if one modality had been proven superior to the other. The lack of convincing evidence towards one imaging modality creates difficulty for the clinician. Our review has been unable to demonstrate a significant difference in accuracy between the two systems, primarily due to a lack of published evidence. As such, imaging selection will depend on surgeon preference, PSI system used, and local facilities available to the surgeon. At present there is no difference in waiting times for manufacture of the PSI components from MRI or CT-based models once the images are acquired. It is important to note that many district general hospitals will have more than one CT scanner, but usually only one MRI scanner, and these must be shared with other elective specialties as well as emergency work. This will be of logistical concern to the surgeon and may increase waiting times to PSI arthroplasty.
At present there is not enough published data to convincingly conclude in favour of CT or MRI for accuracy of pre-operative imaging in PSI. Large-number randomised controlled trials would be required to determine the ideal modality. Given the developing nature of PSI, this seems unlikely in the near future. It is our conclusion, therefore, that CT would be a more favourable option at present due to reduced scanning times, increased availability, and relatively cheaper cost.
Patient-specific instrumentation (PSI) was developed to improve the accuracy of component placement in total knee arthroplasty (TKA). The technique utilizes pre-operative imaging using computed tomography (CT) or magnetic resonance imaging (MRI) to create bespoke cutting or pinning guides for bony resection customized to the patients’ own anatomy.
The benefits and drawbacks of CT vs MRI for use in PSI is a source of ongoing debate. CT is widely available, relatively inexpensive, and imaging times are short when compared with MRI, yet MRI avoids radiation exposure and so is deemed a safer method of imaging. Although many studies have been undertaken to determine the ideal pre-operative imaging modality, conclusions remain speculative in the absence of long term data.
Ultimately, information regarding accuracy of CT and MRI will be the main determining factor. Increased accuracy of pre-operative imaging could result in longer-term savings, and reduced accumulated dose of radiation by eliminating the need for post-operative imaging and revision surgery.
This could improve patient satisfaction following TKA, and reduce the rate of implant failure and revision arthroplasty.
PSI: Patient-specific instrumentation; TKA: Total knee arthroplasty; CT: Computed tomography; MRI: Magnetic resonance imaging.
Well researched and presented paper on a very relevant topic. Adds to the current knowledge. The statistics are well presented.
P- Reviewer: Kamat YD S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Choong PF, Dowsey MM, Stoney JD. Does accurate anatomical alignment result in better function and quality of life? Comparing conventional and computer-assisted total knee arthroplasty. J Arthroplasty. 2009;24:560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 337] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 2. | Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 298] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73:709-714. [PubMed] |
| 5. | Hafez MA, Chelule KL, Seedhom BB, Sherman KP. Computer-assisted total knee arthroplasty using patient-specific templating. Clin Orthop Relat Res. 2006;444:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Ast MP, Nam D, Haas SB. Patient-specific instrumentation for total knee arthroplasty: a review. Orthop Clin North Am. 2012;43:e17-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Noble JW, Moore CA, Liu N. The value of patient-matched instrumentation in total knee arthroplasty. J Arthroplasty. 2012;27:153-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Boonen B, Schotanus MG, Kort NP. Preliminary experience with the patient-specific templating total knee arthroplasty. Acta Orthop. 2012;83:387-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Cenni F, Timoncini A, Ensini A, Tamarri S, Belvedere C, D’Angeli V, Giannini S, Leardini A. Three-dimensional implant position and orientation after total knee replacement performed with patient-specific instrumentation systems. J Orthop Res. 2014;32:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Daniilidis K, Tibesku CO. A comparison of conventional and patient-specific instruments in total knee arthroplasty. Int Orthop. 2014;38:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Daniilidis K, Tibesku CO. Frontal plane alignment after total knee arthroplasty using patient-specific instruments. Int Orthop. 2013;37:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Barrett W, Hoeffel D, Dalury D, Mason JB, Murphy J, Himden S. In-vivo alignment comparing patient specific instrumentation with both conventional and computer assisted surgery (CAS) instrumentation in total knee arthroplasty. J Arthroplasty. 2014;29:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Chareancholvanich K, Narkbunnam R, Pornrattanamaneewong C. A prospective randomised controlled study of patient-specific cutting guides compared with conventional instrumentation in total knee replacement. Bone Joint J. 2013;95-B:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Hamilton WG, Parks NL, Saxena A. Patient-specific instrumentation does not shorten surgical time: a prospective, randomized trial. J Arthroplasty. 2013;28:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Chen JY, Yeo SJ, Yew AK, Tay DK, Chia SL, Lo NN, Chin PL. The radiological outcomes of patient-specific instrumentation versus conventional total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Stronach BM, Pelt CE, Erickson J, Peters CL. Patient-specific total knee arthroplasty required frequent surgeon-directed changes. Clin Orthop Relat Res. 2013;471:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Scholes C, Sahni V, Lustig S, Parker DA, Coolican MR. Patient-specific instrumentation for total knee arthroplasty does not match the pre-operative plan as assessed by intra-operative computer-assisted navigation. Knee Surg Sports Traumatol Arthrosc. 2014;22:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Nam D, McArthur BA, Cross MB, Pearle AD, Mayman DJ, Haas SB. Patient-specific instrumentation in total knee arthroplasty: a review. J Knee Surg. 2012;25:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Lachiewicz PF, Henderson RA. Patient-specific instruments for total knee arthroplasty. J Am Acad Orthop Surg. 2013;21:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Sodickson A, Baeyens PF, Andriole KP, Prevedello LM, Nawfel RD, Hanson R, Khorasani R. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 710] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 22. | Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2366] [Cited by in RCA: 2180] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 23. | Koch P. MyKnee system: a new vision in total knee replacement. Maitrise Orthopédique. 2011;2:32-35. |
| 24. | Schwarzer G. Meta-analysis with R package 3.1.2. 2013; Available from: http: //CRAN R-project.org/package=meta. |
| 25. | Ensini A, Timoncini A, Cenni F, Belvedere C, Fusai F, Leardini A, Giannini S. Intra- and post-operative accuracy assessments of two different patient-specific instrumentation systems for total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2014;22:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Fritschy D, Messerli G. Patient-specific cutting block in TKR: comparison between CT and MRI 3D planning. Arthroscopy. 2011;27:e70-e71. [DOI] [Full Text] |
| 27. | Van den Broeck J, Vereecke E, Wirix-Speetjens R, Vander Sloten J. Comparing CT and MRI segmentation accuracy of long bones. 2013; Available from: http://www.esbiomech.org/papers/ESB_congress_2013/oral/S27.2-132.pdf. |
| 28. | White D, Chelule KL, Seedhom BB. Accuracy of MRI vs CT imaging with particular reference to patient specific templates for total knee replacement surgery. Int J Med Robot. 2008;4:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Rathnayaka K, Momot KI, Noser H, Volp A, Schuetz MA, Sahama T, Schmutz B. Quantification of the accuracy of MRI generated 3D models of long bones compared to CT generated 3D models. Med Eng Phys. 2012;34:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Winder J, Bibb R. Medical rapid prototyping technologies: state of the art and current limitations for application in oral and maxillofacial surgery. J Oral Maxillofac Surg. 2005;63:1006-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 31. | Koch PP, Müller D, Pisan M, Fucentese SF. Radiographic accuracy in TKA with a CT-based patient-specific cutting block technique. Knee Surg Sports Traumatol Arthrosc. 2013;21:2200-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Roh YW, Kim TW, Lee S, Seong SC, Lee MC. Is TKA using patient-specific instruments comparable to conventional TKA? A randomized controlled study of one system. Clin Orthop Relat Res. 2013;471:3988-3995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Bugbee WD, Mizu-Uchi H, Patil S, D’Lima D. Accuracy of implant placement utilizing customized patient instrumentation in total knee arthroplasty. Adv Orthop. 2013;2013:891210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2079] [Cited by in RCA: 3257] [Article Influence: 180.9] [Reference Citation Analysis (0)] |
| 35. | Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 639] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 36. | Rousseau MA, Lazennec JY, Catonné Y. Early mechanical failure in total knee arthroplasty. Int Orthop. 2008;32:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Hofmann S, Romero J, Roth-Schiffl E, Albrecht T. [Rotational malalignment of the components may cause chronic pain or early failure in total knee arthroplasty]. Orthopade. 2003;32:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Dreghorn CR, Hamblen DL. Revision arthroplasty: a high price to pay. BMJ. 1989;298:648-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Iorio R, Healy WL, Richards JA. Comparison of the hospital cost of primary and revision total knee arthroplasty after cost containment. Orthopedics. 1999;22:195-199. [PubMed] |
| 40. | Burns AW, Bourne RB, Chesworth BM, MacDonald SJ, Rorabeck CH. Cost effectiveness of revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 42. | National Joint Registry for England and Wales: 8th Annual report. 2011. Available from: http: //www.njrcentre.org.uk/. |
| 43. | Smith DK, Berquist TH, An KN, Robb RA, Chao EY. Validation of three-dimensional reconstructions of knee anatomy: CT vs MR imaging. J Comput Assist Tomogr. 1989;13:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |