Published online Nov 18, 2015. doi: 10.5312/wjo.v6.i10.829
Peer-review started: May 27, 2015
First decision: June 18, 2015
Revised: July 15, 2015
Accepted: September 7, 2015
Article in press: September 8, 2015
Published online: November 18, 2015
Processing time: 170 Days and 14.7 Hours
AIM: To study a modified porous tantalum technique for the treatment of osteonecrosis of the femoral head.
METHODS: The porous tantalum rod was combined with endoscopy, curettage, autologous bone grafting and use of bone marrow aspirates from iliac crest aspiration in 49 patients (58 hips) with a mean age of 38 years. The majority of the patients had idiopathic osteonecrosis, followed by corticosteroid-induced osteonecrosis. Thirty-eight hips were of Steinberg stage II disease and 20 hips were of stage III disease. Patients were followed for 5 years and were evaluated clinically with the Merle D’Aubigne and Postel score and radiologically. The primary outcome of the study was survival based on the conversion to total hip arthroplasty (THA). Secondary outcomes included deterioration of the osteonecrosis to a higher disease stage at 5 years compared to the preoperative period and identification of factors that were associated with survival. The Kaplan-Meier survival analysis was performed to evaluate the survivorship of the prosthesis, and the Fisher exact test was performed to test associations between various parameters with survival.
RESULTS: No patient developed any serious intraoperative or postoperative complication including implant loosening or migration and donor site morbidity. During the 5-year follow up, 1 patient died, 7 patients had disease progression and 4 hips were converted to THA. The 5-year survival based on conversion to THA was 93.1% and the respective rate based on disease progression was 87.9%. Stage II disease was associated with statistically significant better survival rates compared to stage III disease (P = 0.04). The comparison between idiopathic and non-idiopathic osteonecrosis and between steroid-induced and non-steroid-induced osteonecrosis did not showed any statistically significant difference in survival rates. The clinical evaluation revealed statistically significantly improved Merle d’Aubigne scores at 12 mo postoperatively compared to the preoperative period (P < 0.001). The mean preoperative Merle d’Aubigne score was 13.0 (SD: 1.8). The respective score at 12 mo improved to 17.0 (SD: 2.0). The 12-mo mean score was retained at 5 years.
CONCLUSION: The modified porous tantalum rod technique presented here showed encouraging outcomes. The survival rates based on conversion to THA are the lowest reported in the published literature.
Core tip: In the present study, we present the results of a modified porous tantalum technique for the treatment of femoral head avascular necrosis. The porous tantalum rod was combined with endoscopy, curettage, autologous bone grafting and use of bone marrow aspirates from iliac crest aspiration in 58 hips. The 5-year survival based on conversion to total hip arthroplasty was 93.1% and the respective rate based on disease progression was 87.9%. Stage II disease was associated with statistically significantly better survival rates compared to stage III disease, while no other factor was found to be associated with outcomes.
- Citation: Pakos EE, Megas P, Paschos NK, Syggelos SA, Kouzelis A, Georgiadis G, Xenakis TA. Modified porous tantalum rod technique for the treatment of femoral head osteonecrosis. World J Orthop 2015; 6(10): 829-837
- URL: https://www.wjgnet.com/2218-5836/full/v6/i10/829.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i10.829
Osteonecrosis of the femoral head is an increasing cause of musculoskeletal disability, and it poses a major diagnostic and therapeutic challenge. It is a progressive disease that may lead to deterioration of the hip joint even with optimal treatment, and mostly occurs in the young and previously fit population (usually between the third and the fifth decade of life). It is estimated that approximately 10% of the performed total hip arthroplasties (THA) are due to osteonecrosis[1].
Investigators have not yet agreed on the exact pathogenetic mechanisms that contribute to the disturbance of blood supply to the bone. However, various factors such as trauma, alcoholism, blood cell diseases, corticosteroid administration, pregnancy, collagen disorders (especially lupus), adipogenesis of bone marrow, colon disorders and factors or diseases, which cause immune deficiency, have been reported as etiologic factors for the disease[2,3]. It is also proposed that hypercoagulability may be a major pathway by which the above-mentioned abnormalities lead to impairment of the vascular supply[4]. In many cases, osteonecrosis can be characterized as idiopathic since the patients do not have any risk factor.
While efforts are being made to find ways to prevent and reverse the course of the disease, the main goal of current treatment is to preserve the integrity of the femoral head, before it collapses and leads to hip joint degeneration. In late stages of osteonecrosis in which the subchondral bone is fractured and the collapse of the articular surface leads to arthritic alterations, surface replacement[5] or THA[6] is inevitable. It is therefore critical to treat osteonecrosis in early stages, to delay or possibly prevent the finality of total hip replacement, especially in the young population where poorer long-term outcomes have been reported[7]. Various therapeutic procedures depending on the degenerative stage of the necrosis have been implemented and include restricted weight bearing, osteotomies, core decompression, non-vascularized and vascularized bone grafting[8,9]. However, most of these methods did not show satisfactory clinical outcomes and were associated with either prolonged surgical times due to the complex surgical procedure, or high donor-site morbidity and prolonged rehabilitation.
The need for an easy, effective and safe way to support the subchondral bone has led to the use of porous tantalum rods, firstly described by Pedersen et al[10] in 1997. Tantalum rods have high volumetric porosity, providing excellent osteoconductive properties, while their elastic modulus is similar to bone and they have exceptional biocompatibility[11,12]. They provide structural support like that of bone graft and avoid the risks associated with bone grafting. The surgical technique is simple and safe, with short operative time and minimal blood loss. Short and mid-term results can be satisfactory, especially in the early stages of the disease (pre-collapse and early post-collapse), but it is essential that particular attention is given to careful patient selection and exact positioning of the rod into the necrotic lesion and the subchondral bone.
The present retrospective study represents a bi-centre trial that reports on the clinical outcomes of porous tantalum rod for the treatment of femoral head osteonecrosis. The technique used in our study is a variation of the classical technique, since the tantalum rod is combined with endoscopy, lesion curettage, autologous bone grafting and use of bone marrow aspirates.
Porous tantalum rods were designed to support the subchondral plate of a necrotic human femoral head. The implant is entirely made of porous tantalum metal which is currently used in a variety of hip, knee and spine components[11]. Tantalum biomechanical characteristics such as porosity and mean pore size have been earlier reported[13]. The rod has a 10-mm-diameter cylindrical shape with length options ranging from 70 to 130 mm (in 5-mm increments). There is also a threaded section which engages the lateral cortex of the femur and a hemispherical tip which supports the subchondral plate. Through mechanical experimentation, Tsao et al[12] identified the ability of this implant to support the articular surface. It is also possible that the tantalum induces bone formation without bone grafting, which has been reported in animal models by Bobyn et al[11,13].
Between 2001 and 2010, 49 patients with osteonecrosis of the hip were treated with a porous tantalum rod in the Department of Orthopaedic Surgery of the University Hospital of Ioannina (24 patients) and the Department of Orthopaedic Surgery of the University Hospital of Patras (25 patients). The study was approved by the Institutional Review Board of both participating institutions. Nine patients had bilateral osteonecrosis of the hip that was treated with tantalum rod at separate stages. The classification of osteonecrosis was performed using the Steinberg classification system[14]. Patients with serious subchondral collapse and/or destruction of the hip joint (Steinberg stage IV or higher) were excluded from the present study. We have also excluded patients with previous surgical management of osteonecrosis (i.e., core decompression, osteotomy, bone grafting). Overall, 21 female patients and 28 male patients with 58 affected hips and a median age of 38 years (range 22-55) were included in the study. Thirty-nine patients had the right hip affected.
Twenty-two patients (45%) were diagnosed with idiopathic osteonecrosis, 16 patients had corticosteroid-induced osteonecrosis, 3 patients had primary diagnoses of leukemia and developed osteonecrosis due to a combination of chemotherapy and corticosteroid treatment, 2 patients had osteonecrosis secondary to bowel disease, 2 patients had alcohol-induced osteonecrosis, 2 patients had dyslipidemia, 1 patient developed osteonecrosis after labor and 1 patient had polycythemia vera. The grade of osteonecrosis was determined with a combination of plain anteroposterior (AP) and lateral radiographs as well as magnetic resonance imaging (MRI) imaging. Among the 58 hips included in the study, 38 hips were of Steinberg stage 2 (6 hips 2a, 22 hips 2b, 10 hips 2c) and 20 hips of Steinberg stage 3 (20 hips 3a).
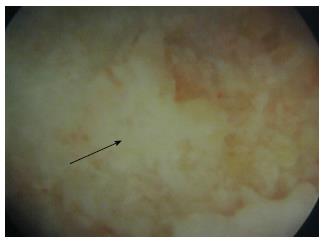
All operations were performed by the senior authors (Megas P, Xenakis TA). Under general anesthesia, the patient was positioned supine on the fracture table and internal rotation was applied to the affected lower limb. Under fluoroscopic control, a guide pin was placed over the skin directed to the exact place of the necrotic area in order to mark the skin incision at the greater trochanter. A lateral lengthwise 1.5-cm incision was performed and the subcutaneous soft tissue, the fascia lata and the vastus lateralis muscle were blindly dissected. Under fluoroscopic control, a 3.2-mm guide pin was inserted at the level of the upper margin of the lesser trochanter with the goal to place the guide pin exactly within the necrotic region and 5 mm from the subchondral surface of the femoral head. The core was progressively reamed with cannulated reamers up to 10 mm in diameter. At a second stage, an endoscopy was performed through the canal in order to evaluate the posterior aspect of the osteonecrosis (Figure 1). The posterior aspect of the osteonecrotic lesion was the only visible area, since the endoscopy was performed through a narrow canal. A partial curettage of the osteonecrotic lesion was then performed, with meticulous care in order not to invade the cartilage. The curettage was performed with small drills into the necrotic lesion using a threaded flexible Ø3.2 × 450 mm Kirschner wire through the canal.
The next step involved bone marrow aspiration from the ipsilateral anterior iliac crest. A 5-mm incision was performed approximately 3-4 cm posterior to the anterior superior iliac spine directly on the crest. A 16-gauge aspiration needle was advanced into the bone marrow with an angle of 15 degrees cephalad by rotating it in an alternating clockwise/counter clock-wise motion. The needle was advanced approximately 4–6 cm into the cancellous bone. The stylet/trocar was removed and a heparin-coated 30-mL syringe was adjusted onto the needle. Aspirates of 30 mL of bone marrow were obtained. If no bone marrow was obtained, the needle was reoriented within the ilium, and the aspiration was repeated.
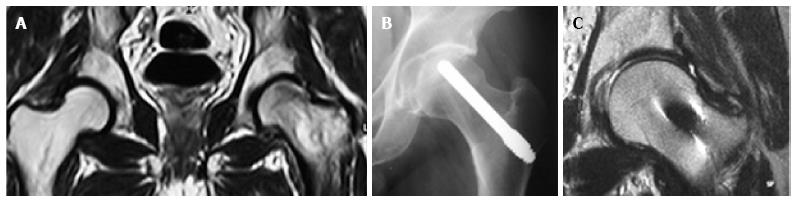
The aspirates were divided in 2 equal parts. The first part was mixed with the morselized bone graft from the canal reaming and was then placed into the canal and was pushed to reach the necrotic area. Finally, the tantalum rod (Zimmer, Inc., Warsaw, IN, United States) was impregnated with the second part of bone marrow aspirates (Figure 2). The impregnation was performed by immersing the rod into the aspirates for 5 min.
Although the initially described technique involved the measurement of the canal and the placement of the lateral part of the rod at the lateral femoral cortex, we used shorter rods in the majority of the patients so that the rod ended distally at the cancellous bone of the trochanteric area. This method enabled the easier removal of the rod (the lateral edge of the rod was at the level of femoral head osteotomy) and the filling of the cortical defect with bone in case of future arthroplasty.
Prophylactic antibiotics (second generation cephalosporins) were administered intravenously for 1 d post-operatively, while thromboprophylaxis with low molecular weight heparin according to the body mass index was administered subcutaneously for 4 wk. Postoperative pain was treated with paracetamol and pethidine. All patients followed the same routine rehabilitation program that included mobilization on the 2nd postoperative day with the help of a specialized physiotherapist, partial weight bearing on the operative side with 2 crutches for 6 wk, and then full weight bearing with 1 crutch for another 6 wk. No patient required intensive physiotherapy.
Patients were evaluated clinically and radiologically in the postoperative period. The clinical evaluation was based on the Merle D’Aubigne and Postel score[15] and was performed at 6 mo, 1 year and 5 years postoperatively. The Merle d’Aubigne and Postel score evaluates pain, gait and mobility, on a scale of 1 to 6 for each item, where 1 indicates the worst and 6 the best state of the patient. The total minimum score reached is 3, and the maximum is 18. Scores were compared with those from the immediate preoperative period. All intra-operative and postoperative complications were recorded. The radiological evaluation was performed at 1.5, 3, 6 and 12 mo postoperatively and annually thereafter till the 5th year. The postoperative radiological evaluation included the restaging according to the Steinberg staging system[15] based on plain X-rays and MRI on indication. Post-operative follow-up examinations were performed by two independent investigators who reached consensus on all patients.
The follow-up time was calculated from the day of operation. Primary endpoint of the study was the 5-year survival of the hip joint defined as hips that did not undergo total hip arthroplasty. Secondary outcomes included deterioration of the osteonecrosis to a higher disease stage at 5 years compared to the preoperative period. Finally, we tested for associations between disease stage and etiology of osteonecrosis and survival.
A Kaplan-Meier survival analysis was performed to evaluate the survivorship of the prosthesis. A Fisher exact test was performed to test associations between various parameters with survival. A t test for independent samples was used to determine whether there was a significant difference in mean values of the clinical scores or not. P values < 0.05 were considered statistically significant; all P values were two-tailed. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS 22.0, Chicago, IL, United States).
The median duration of operation was 40 min (range 35-50 min). One patient had a superficial wound infection at 5 d postoperatively and was treated with oral antibiotic therapy for 1 wk without further evidence of infection. No other patient developed any intraoperative (i.e., fat embolism) or postoperative complication. No patient developed any loosening or migration of the tantalum rod. No patient reported any donor site morbidity from the ipsilateral iliac crest. The mild pain or irritation at the iliac crest was not retained for more than 6 wk.
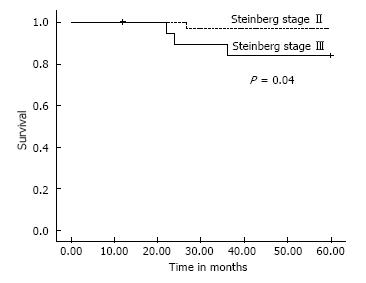
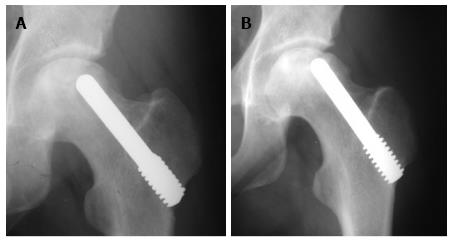
All patients except one were available for follow up for up to 5 years (Figures 3 and 4). One 38-year-old patient with unilateral osteonecrosis of stage 3b due to leukaemia died 12 mo postoperatively with no apparent sign of disease deterioration. During the 5-year follow up, the radiographic evaluation showed increase of the Steinberg stage in 7 patients. Four hips developed advanced osteoarthritic changes of Steinberg stage V and were converted to THA within a mean time of 27.5 mo (SD: ± 6.2 mo). The 5-year survival was 93.1% (95%CI: 83.3%-98.1%). Patients with stage III disease had increased risk to undergo THA and the difference was statistically significant (P = 0.04) (Figure 5).
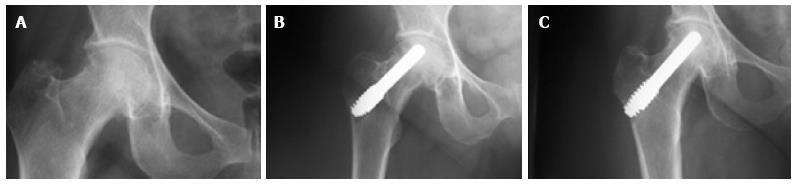
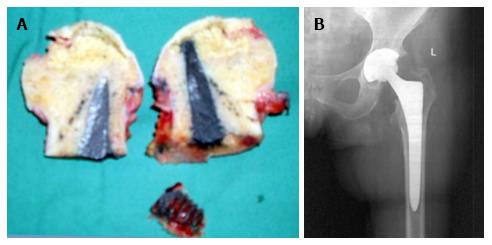
In 2 of the hips that were converted to THA, the removal of the tantalum rod was easily performed due to the fact that the rod was shorter, ending distally at the cancellous bone of the trochanteric area at the level of femoral head osteotomy. In two patients where the lateral part of the rod was placed at the lateral femoral cortex, the conversion was performed with the osteotomy cut through the rod (Figure 6A), and the lateral part of the rod was carefully removed with the use of a lexer chisel. No trochanteric fracture was noticed. The trochanteric hole was filled with morselized bone graft from the femoral head and the 6-wk postoperative X-rays showed callous formation (Figure 6B). The 12 and 24 mo postoperative X-rays in these patients did not show any apparent change.
Among the hips that underwent THA, 1 was of stage IIa and 3 were of stage IIIa. Three hips had disease progression with no conversion to THA. One of these hips was initially of stage IIc and was restaged as stage IIIb at 24 mo postoperatively. Two hips of initial stage IIIa were restaged as stage IV at 12 and 60 mo, respectively (Figure 7). The 5-year survival defined as disease deterioration and increase of osteonecrosis stage was 87.9% (95%CI: 76.7%-95.0%). Again, patients with stage III disease had increased risk to deteriorate (P = 0.02).
Among the hips that were converted to THA 2 that had idiopathic osteonecrosis, in one case the osteonecrosis was due to corticosteroid use and 1 patient had combined chemotherapy and steroid treatment for leukaemia. No significant association was observed between the etiology of osteonecrosis and the 5-year survival (P = 0.31 for the comparison between idiopathic osteonecrosis and non-idiopathic osteonecrosis). Similarly, no significant difference was observed in survival rates between steroid-induced and non-steroid-induced osteonecrosis (P = 0.28).
All patients had available clinical evaluation preoperatively, at 12 mo postoperatively and at 60 mo postoperatively. Patients that had THA were excluded from the 60-mo clinical evaluation. The postoperative clinical evaluation of the patients showed significant improvement compared to the preoperative period. All but 1 patient reported apparent pain relief at 6 wk postoperatively. The mean preoperative Merle d’Aubigne score was 13.0 (SD: 1.8). The respective score at 12 mo improved to 17.0 (SD: 2.0). The difference was highly statistically significant (P < 0.001). The improved clinical scores were retained till the final follow up at 5 years where the mean score was 16.8 (SD: 1.5).
Management of femoral head osteonecrosis in young adults where THA does not represent the ideal solution is challenging. Indeed, the survival duration of a THA in a young patient and the need for future revision favours joint-preserving techniques for femoral head osteonecrosis. In our study, we report on the clinical and radiological outcomes of a novel variation of the classical porous tantalum rod technique that was combined with canal endoscopy, curettage of the lesion, impaction of autologous bone graft mixed with bone marrow aspirates from the iliac crest and impregnation of the rod with these aspirates. This technique showed encouraging outcomes for the treatment of osteonecrosis of the femoral head. The 5-year survival defined as conversion of the tantalum rod to THA was 93.1%. The 5-year survival defined as disease deterioration and increase of disease stage was 87.9%. Both survivals were statistically significantly associated with the disease stage, since patients with stage II disease had increased survival rates compared to patients with stage III disease.
One treatment option for osteonecrosis of the femoral head is free vascularized fibula grafting (FVFG). This technique was introduced approximately 40 years ago and has shown efficiency in preserving the hip joint[16,17]. The popularization of the technique highlighted that there are certain indications for its application and its efficiency is dependent on proper patient selection[18,19]. Several disadvantages of FVFG have been also highlighted, such as long surgical time, demanding operative technique and donor morbidity[20-22]. These limitations make tantalum core decompression an attractive option for femoral head osteonecrosis.
Several studies have evaluated the efficacy of the porous tantalum rod in the treatment of femoral head osteonecrosis, with controversial outcomes. In the majority of these studies the porous tantalum rod has proven an effective and safe management for osteonecrosis of femoral head[23-25]. However, other studies showed poor outcomes with high rates of conversion to THA[26]. A recent meta-analysis showed that porous tantalum rod application was associated with higher improvement in Harris Hip Score as well as better survivorship and complication rate in comparison to bone grafting techniques[25,27]. Specifically, femoral collapse occurred in approximately 6% of the patients, which was significantly lower when compared to 28% in the bone grafting group[27]. Tantalum rod results in less bleeding, fewer transfusions, and shorter hospitalization[27]. Our findings confirm the effectiveness and safety for the porous tantalum rod in these patients. Our series, utilizing a modified technique of porous tantalum rod, shows improved effectiveness compared to the published literature. Conversion to arthroplasty within the 5-year follow up period was performed in 7% of our patients, which represents the lowest rate up to date in such a long term period.
The effectiveness of the porous tantalum rod in osteonecrosis treatment may be correlated with the material properties of the tantalum. Porous tantalum has a porosity of 75%-80% and allows more than 40% of bone ingrowth within the first 4 wk of implantation and up to 80% at a later stage[13]. Recently, reports of new bone ingrowth with associated small blood vessels at the bone/tantalum interface highlighted the biocompatibility of the material[28]. At the same time, it is less stiff than the traditional tantalum, with a stiffness that approximates that of the subchondral bone[13]. Thus, it provides an environment that favours bone remodelling, which is critical in femoral head osteonecrosis.
Porous tantalum insertion at the femoral head removes the necrotic lesion, forms a decompression zone, and provides support to the subchondral bone. Core decompression has been reported to be an effective method in preventing the progression of osteonecrosis[29,30]. However, it was proven that it offers more symptomatic relief rather than delay in the natural history of femoral head osteonecrosis and subsequent collapse and degeneration[31]. Furthermore, it has been suggested that decompression alone may weaken the cancellous bone in an area adjacent to the necrotic area with obvious disadvantages[8]. Therefore, porous tantalum offers mechanical support to the subchondral bone, which not only addresses the concern of bone weakening, but also may represent a key intervention for osteonecrosis progression[12,32].
In our series, the porous tantalum rod has been combined with other techniques in an attempt to increase its efficiency. An endoscopy of the lesion through the canal was performed in order to evaluate the situation of the subchondral bone. Consequently, curettage of the lesion was performed in order to remove the necrotic bone and to create sockets which the morselized bone graft and the new bone would fill. Finally, in order to enhance new bone formation from the tantalum rod, the rod was impregnated with bone marrow aspirates before implantation. This the first time in the published literature that such a combination was used. Moreover, the outcomes of the present study confirm all previous published studies that improved survival rates are associated with an earlier disease stage. Finally, associations of factors such as corticosteroid use or chronic systemic disease that were previously reported to correlate with higher failure rates[24] were not found in the present study. Previously reported complications from the use of the tantalum rod such as fat embolism[33] and subtrochanteric fractures[34] were not seen in our series.
Porous tantalum implantation appears to play a prominent role in the treatment of osteonecrosis of the femoral head, especially when combined with other techniques that can increase its effectiveness and applicability. The combination of tantalum rod insertion with vascularized iliac grafting has shown a survival rate of more than 90% for stage II and III osteonecrosis[35]. Recently, in a randomized control trial, Mao et al[36] have reported the combination of a tantalum rod with targeted intraarterial infusion of peripheral blood stem cells, with improved survival compared to the control group (6.5% conversion to THA in the combined group). Another recent study where a tantalum rod was combined with curettage of the sequestrum and insertion of nano-hydroxyapatite/polyamide 66 material showed 19.3% conversion to THA in 45 mo[37]. Finally, the porous tantalum rod may be used in combination with low intensity ultrasound in order to promote bone ingrowth into the porous implant that could enhance the effectiveness of this technique[38].
In conclusion, the modified porous tantalum rod technique that was applied to our series is a safe and quick method that showed encouraging outcomes and improved survival rates compared to previous reported techniques. The limitations of the present study include the retrospective design, the limited sample size, the inhomogeneous group of eligible patients, the absence of control groups and the absence of long term survival outcomes exceeding 10 years. These limitations should be considered in future study designs.
Osteonecrosis of the femoral head is an increasing cause of musculoskeletal disability, and it poses a major diagnostic and therapeutic challenge. Although various factors have been reported as etiologic factors, the exact pathogenetic mechanism of the disease is unknown. The goal of current treatment modalities is to preserve the integrity of the femoral head and prevent hip joint degeneration and eventually total hip arthroplasty (THA). Various therapeutic procedures depending on the degenerative stage of the necrosis have been implemented and include restricted weight bearing, osteotomies, core decompression, bone grafting (either non-vascularized or vascularized) and the use of porous tantalum rods. In this study the authors evaluated the efficacy of a modified porous tantalum rod technique where the tantalum rod was combined with endoscopy, lesion curettage, autologous bone grafting and use of bone marrow aspirates.
The surgical technique of porous tantalum is simple and safe, with short operative time and minimal blood loss. The majority of the published literature has shown promising clinical outcomes with low rates of conversion to THA, especially in the early stages of the disease. The results of the present study where a modified technique is used contribute to further improvement of clinical outcomes, by lowering the rates of conversion to THA.
In this study, the modified porous tantalum technique showed increased 5-year survival rates compared to the published literature. The 5-year survival defined as conversion of the tantalum rod to THA was 93.1%. The 5-year survival defined as disease deterioration and increase of disease stage was 87.9%. Patients with stage II disease had increased survival rates compared to patients with stage III disease, in concordance with the published literature.
The modified porous tantalum rod technique that was applied to the authors series is a safe and quick method that showed encouraging outcomes and improved survival rates compared to previous reported techniques. The use of the authors technique can lower the conversion to THA.
The authors of this paper evaluated the use of a modified porous tantalum technique in the treatment of femoral head osteonecrosis of 58 hips that were followed for 5 years. They describe a novel technique with good results which has potential to be adopted by other surgeons.
P- Reviewer: Cui Q, Saithna A S- Editor: Tian YL L- Editor: Logan S E- Editor: Lu YJ
| 1. | Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459-474. [PubMed] |
| 2. | Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med. 1992;326:1473-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 617] [Article Influence: 18.7] [Reference Citation Analysis (1)] |
| 3. | Seamon J, Keller T, Saleh J, Cui Q. The pathogenesis of nontraumatic osteonecrosis. Arthritis. 2012;2012:601763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Korompilias AV, Ortel TL, Urbaniak JR. Coagulation abnormalities in patients with hip osteonecrosis. Orthop Clin North Am. 2004;35:265-271, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Sayeed SA, Johnson AJ, Stroh DA, Gross TP, Mont MA. Hip resurfacing in patients who have osteonecrosis and are 25 years or under. Clin Orthop Relat Res. 2011;469:1582-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Kim SM, Lim SJ, Moon YW, Kim YT, Ko KR, Park YS. Cementless modular total hip arthroplasty in patients younger than fifty with femoral head osteonecrosis: minimum fifteen-year follow-up. J Arthroplasty. 2013;28:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Adelani MA, Keeney JA, Palisch A, Fowler SA, Clohisy JC. Has total hip arthroplasty in patients 30 years or younger improved? A systematic review. Clin Orthop Relat Res. 2013;471:2595-2601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Scully SP, Aaron RK, Urbaniak JR. Survival analysis of hips treated with core decompression or vascularized fibular grafting because of avascular necrosis. J Bone Joint Surg Am. 1998;80:1270-1275. [PubMed] |
| 9. | Hamanishi M, Yasunaga Y, Yamasaki T, Mori R, Shoji T, Ochi M. The clinical and radiographic results of intertrochanteric curved varus osteotomy for idiopathic osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2014;134:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Pedersen DR, Brown TD, Poggie RA. Finite element characterization of a po-rous tantalum material for treatment of avascular necrosis. Trans Orthop Res Soc. 1997;22:598. |
| 11. | Bobyn JD, Poggie RA, Krygier JJ, Lewallen DG, Hanssen AD, Lewis RJ, Unger AS, O’Keefe TJ, Christie MJ, Nasser S. Clinical validation of a structural porous tantalum biomaterial for adult reconstruction. J Bone Joint Surg Am. 2004;86-A Suppl 2:123-129. [PubMed] |
| 12. | Tsao AK, Roberson JR, Christie MJ, Dore DD, Heck DA, Robertson DD, Poggie RA. Biomechanical and clinical evaluations of a porous tantalum implant for the treatment of early-stage osteonecrosis. J Bone Joint Surg Am. 2005;87 Suppl 2:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br. 1999;81:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 453] [Reference Citation Analysis (0)] |
| 14. | Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34-41. [PubMed] |
| 15. | D’aubigne RM, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36-A:451-475. [PubMed] |
| 16. | Malizos KN, Soucacos PN, Beris AE. Osteonecrosis of the femoral head. Hip salvaging with implantation of a vascularized fibular graft. Clin Orthop Relat Res. 1995;67-75. [PubMed] |
| 17. | Urbaniak JR, Coogan PG, Gunneson EB, Nunley JA. Treatment of osteonecrosis of the femoral head with free vascularized fibular grafting. A long-term follow-up study of one hundred and three hips. J Bone Joint Surg Am. 1995;77:681-694. [PubMed] |
| 18. | Aldridge JM, Berend KR, Gunneson EE, Urbaniak JR. Free vascularized fibular grafting for the treatment of postcollapse osteonecrosis of the femoral head. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:87-101. [PubMed] |
| 19. | Malizos KN, Quarles LD, Dailiana ZH, Rizk WS, Seaber AV, Urbaniak JR. Analysis of failures after vascularized fibular grafting in femoral head necrosis. Orthop Clin North Am. 2004;35:305-314, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Vail TP, Urbaniak JR. Donor-site morbidity with use of vascularized autogenous fibular grafts. J Bone Joint Surg Am. 1996;78:204-211. [PubMed] |
| 21. | Gaskill TR, Urbaniak JR, Aldridge JM. Free vascularized fibular transfer for femoral head osteonecrosis: donor and graft site morbidity. J Bone Joint Surg Am. 2009;91:1861-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Korompilias AV, Paschos NK, Lykissas MG, Kostas-Agnantis I, Vekris MD, Beris AE. Recent updates of surgical techniques and applications of free vascularized fibular graft in extremity and trunk reconstruction. Microsurgery. 2011;31:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Varitimidis SE, Dimitroulias AP, Karachalios TS, Dailiana ZH, Malizos KN. Outcome after tantalum rod implantation for treatment of femoral head osteonecrosis: 26 hips followed for an average of 3 years. Acta Orthop. 2009;80:20-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Veillette CJ, Mehdian H, Schemitsch EH, McKee MD. Survivorship analysis and radiographic outcome following tantalum rod insertion for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88 Suppl 3:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Shuler MS, Rooks MD, Roberson JR. Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplasty. 2007;22:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Floerkemeier T, Thorey F, Daentzer D, Lerch M, Klages P, Windhagen H, von Lewinski G. Clinical and radiological outcome of the treatment of osteonecrosis of the femoral head using the osteonecrosis intervention implant. Int Orthop. 2011;35:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Li L, Shi ZJ, Wang J, Li ZH. Porous tantalum rod implant is an effective and safe choice for early-stage femoral head necrosis: a meta-analysis of clinical trials. Eur J Orthop Surg Traumatol. 2013;23:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Wang Q, Zhang H, Li Q, Ye L, Gan H, Liu Y, Wang H, Wang Z. Biocompatibility and osteogenic properties of porous tantalum. Exp Ther Med. 2015;9:780-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res. 1996;169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 333] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, Hartman KE. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Koo KH, Kim R, Ko GH, Song HR, Jeong ST, Cho SH. Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. J Bone Joint Surg Br. 1995;77:870-874. [PubMed] |
| 32. | Buckley PD, Gearen PF, Petty RW. Structural bone-grafting for early atraumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1991;73:1357-1364. [PubMed] |
| 33. | Schaffer JC, Adib F, Cui Q. Intraoperative fat embolism during core decompression and bone grafting for osteonecrosis of the hip: report of 3 cases and literature review. Am J Orthop (Belle Mead NJ). 2014;43:275-279. [PubMed] |
| 34. | Stronach BM, Duke JN, Rozensweig SD, Stewart RL. Subtrochanteric femur fracture after core decompression and placement of a tantalum strut for osteonecrosis of the femoral head. J Arthroplasty. 2010;25:1168.e5-1168.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Zhao D, Zhang Y, Wang W, Liu Y, Li Z, Wang B, Yu X. Tantalum rod implantation and vascularized iliac grafting for osteonecrosis of the femoral head. Orthopedics. 2013;36:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Mao Q, Wang W, Xu T, Zhang S, Xiao L, Chen D, Jin H, Tong P. Combination treatment of biomechanical support and targeted intra-arterial infusion of peripheral blood stem cells mobilized by granulocyte-colony stimulating factor for the osteonecrosis of the femoral head: a randomized controlled clinical trial. J Bone Miner Res. 2015;30:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Liu Y, Su X, Zhou S, Wang L, Wang C, Liu S. A modified porous tantalum implant technique for osteonecrosis of the femoral head: survivorship analysis and prognostic factors for radiographic progression and conversion to total hip arthroplasty. Int J Clin Exp Med. 2015;8:1918-1930. [PubMed] |
| 38. | Tanzer M, Kantor S, Bobyn JD. Enhancement of bone growth into porous intramedullary implants using non-invasive low intensity ultrasound. J Orthop Res. 2001;19:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |