Published online Nov 18, 2014. doi: 10.5312/wjo.v5.i5.645
Revised: March 26, 2014
Accepted: May 28, 2014
Published online: November 18, 2014
Processing time: 238 Days and 18.6 Hours
The risk of venous thromboembolism (VTE) in rheumatoid arthritis (RA) and the higher incidence of RA patients undergoing major orthopedic surgery is well recognized. The objective of the present study is to describe the incidence of VTE and discuss the correct prophylaxis in RA patients undergoing knee or hip replacement. A systematic review of studies on thromboprophylaxis in RA patients undergoing major orthopedic surgery was performed. Detailed information was extracted to calculate the rate of VTE in RA orthopedic patients and analyze the thromboprophylaxis performed and bleeding complications. Eight articles were eligible for full review. No difference in the overall rate of VTE was observed between RA patients and controls. No significant differences were found in RA patients in terms of bleeding complications. The data on the optimal prophylaxis to be used in RA patients were insufficient to recommend any of the several options available. In the absence of dedicated guidelines for the care of RA patients undergoing orthopedic surgery, management must be individualized to obtain favorable patient outcome, weighing up all the factors that might put the patient at risk for higher bleeding and thrombotic events.
Core tip: The purpose of this review is to quantify the incidence of venous thromboembolism (VTE) in patients with rheumatoid arthritis (RA) undergoing major orthopedic surgery and to discuss the current management of VTE prophylaxis in RA patients undergoing major joint arthroplasty and establish whether these patients are at higher risk for VTE than the general population.
- Citation: Mameli A, Marongiu F. Thromboembolic disease in patients with rheumatoid arthritis undergoing joint arthroplasty: Update on prophylaxes. World J Orthop 2014; 5(5): 645-652
- URL: https://www.wjgnet.com/2218-5836/full/v5/i5/645.htm
- DOI: https://dx.doi.org/10.5312/wjo.v5.i5.645
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disorder that can affect many tissues and organs. It principally attacks synovial joints, resulting in a painful condition and even in disability if inadequately treated. At present, surgical correction in patients with RA is a highly successful solution for those suffering from advanced joint destruction. However, it should be taken into account that venous thromboembolism may be a possible complication after total hip arthroplasty (THA) and total knee arthroplasty (TKA) since orthopedic surgery carries per se a high risk for this thrombotic condition[1]. The link between chronic systemic inflammatory disease, such as RA and thrombotic disease has been documented: it may be the final effect of a hypercoagulability state concomitantly to a reduced fibrinolysis[2]. It is thought that hypercoagulability is induced by active systemic inflammation and production of cytokines such as TNF-α and interleukin-1, that can lead to endothelial dysfunction, down regulation of Protein C, a natural anticoagulant, and then to an inhibition of fibrinolysis[2,3]. Acute hospitalizations, drugs, surgical procedures, physical inactivity and other co-morbidities may represent further risk factors for VTE in RA patients[4].
On the basis of the nine American College of Chest Physicians (ACCP) guidelines on VTE prevention, prophylaxis may be extended to more than 30 d, particularly after THA. The recommended thrombo-prophylactic regimens include low-molecular-weight heparin, fondaparinux, dabigatran, apixaban, rivaroxaban (total hip arthroplasty or total knee arthroplasty but not hip fracture surgery), low-dose unfractionated heparin, adjusted-dose vitamin K antagonist, aspirin (all Grade 1B), or an intermittent pneumatic compression device (IPCD) (Grade 1C) for a minimum of 10 to 14 d[1].
These guidelines are based on investigations in which predominantly osteoarthritis (OA) patients were studied. Paradoxically, although the thromboembolic risk in RA patients is recognized to be high, contradictory results emerge from the few studies performed on RA patients undergoing orthopedic surgery.
The aim of this review is to understand better the incidence of thromboembolic diseases in RA patients undergoing major orthopedic surgery. Furthermore, the authors’ aim is to discuss the current management of VTE prophylaxis in RA patients undergoing major joint arthroplasty and establish whether RA patients undergoing orthopedic surgery are at higher risk than the general population for VTE.
A systematic PubMed search was conducted to identify all articles between January 1970 and 15 November 2013 that analyzed the thromboprophilaxis in RA patients undergoing major orthopedic surgery. The Key words used in the search were: “Arthroplasty” OR “knee surgery,” OR “Hip surgery” OR “Venous thromboembolism” OR “Prophylaxis of venous thromboembolism”. The search results were combined with “Rheumatoid arthritis” using the Boolean search operator AND.
The authors carried out an initial screening of all titles and abstracts retrieved from the search. Articles were eligible for full text assessment if they reported original data on RA patients that had undergone THA and TKA, and reported information on the thromboprophylaxis performed, the rate of VTE and bleeding complications.
Studies pertaining anticoagulation, DVT, pulmonary embolism (PE) or thromboembolic diseases in RA patients having major orthopedic surgery were included. Articles were excluded if they were not in English. Case reports were also excluded. To ensure that the research was thorough, the reference lists of each article were also reviewed for other potentially eligible studies. Data were extracted from each article using a self-composed form to extract the following: (1) number of RA patients; (2) type of surgical procedure; (3) method of prophylaxis; (4) method of surveillance used; (5) the incidence of DVT; (6) the incidence of PE; and (7) incidence of bleeding complications. A meta-analysis was carried out entering only those studies that provided the event rate both in patients with RA and controls. For this purpose MEDCALC software (version 10.0.1.0) was used computing both fixed and random model. Pooled results are reported as odds ratio (OR) and 95%CI. A probability value of 0.05 or less was considered statistically significant. Statistical heterogeneity was evaluated using the I2 statistic which assess the appropriateness of pooling the individual study results. The I2 value provides the estimate of the amount of variance across studies due to heterogeneity rather than chance.
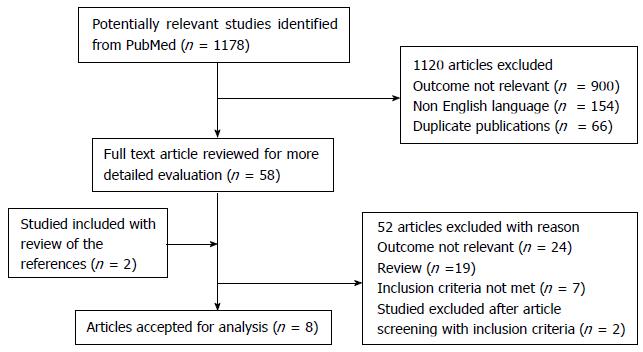
A total of 1178 titles were found through the electronic search on PubMed. After deleting duplicates and screening titles and abstracts, 1120 articles were excluded leaving 58 articles for full-paper review. Review of references revealed 2 further articles. After a detailed full-paper review, another 52 were excluded. A total of 8 articles[5-12] satisfied most of the inclusion criteria for data extraction after full review (Figure 1): Seven observational cohort studies, and 1 randomized clinical trial. Of the 8 studies, 7 were retrospective, 1 was prospective.
Characteristics and extracted data of the studies are summarized in Table 1. A total of 8886 RA patients were included in our review. The agents for prophylaxis were: Nadroparine[5], low doses of unfractionated heparin[6], Aspirin[7], Acenocumarol[8] and mechanical prophylaxes[10].
| Ref. | Study design | Operation | RA patients | Methods of prophylaxis | Duration of prophylaxis | Methods of surveillance | VTE | EP | Bleedingcomplications |
| van Heereveld et al[5] | Retrospective open study of all medical record of patients with RA who underwent a Hip or Knee replacement from Jan 1987 to April 1995 | THA and TKA | 103 patients with RA who underwent 151 surgical procedure 55 (TKRH) 96 (THR) | Subcutaneous SH 5000 UI twice a day, starting two-six ours before surgery and was given twice a day, or nadroparin 7500 IC-U (10.000-20.00 IC U for obese patients) once a day. NSAIDs in 85% daily and continued after hospital discharge | For a minimun of 7 d and discontinued as soon as patient was adequately mobilized | Sonography, phlebography and V/Q scanning only in patients with clinical suspicion of VTE or PE The patients were seen every three months. the total of follow up was one year | 1 | 0 | 20/151 (13%) Fifteen haematoma necessitating blood transfusion in six cases In one instance a surgical decompression was made. In none of bleeding episodes were signs of haemodynamic instability |
| Niki et al[6] | Prospective study of 333 patients who underwent primary TKA between October 2003 and June 2007 with diagnosis of RA and OA | TKA | 199 (238 KNEES) | LOW dose unfractionated heparin (5000 U) for when patients had history of DVT or D-Dimer levels > 10 g/mL) | From second to eight day post-discharge | Sonography (pre-operatively and on POD 7), d-dimer on POD 0, 1 and 7 | 51 | 1 | 1 |
| Sharrock et al[7] | Retrospective review of 571 primary TKA in epidural anesthesia between July 1986 to June 1990 | TKA | 54 RA | Aspirin (650 mg) and elastic streaking | 5 d | Venography at forty and fifty post operative day | 21 (39%) | Not reported | Not reported |
| Swiestra et al[8] | Retrospective randomized study of 101 consecutive patients admitted for primary THA | THA | 14 RA | Acenocumarol started four or one day preoperatively aiming a thrombotest of 25% during the operation (1.5-1.6 INR) | Discontinuation of anticoagulation after negative venogram | Venography with 99mtc labeled macroaggregates of albumin, performed about 10 d after the operation for identifying proximal DVT | 23/101 | 1 patient post-discharge | 2 bleeding complication associated to excessively prolonged protrombine time |
| White et al[9] | Retrospective analysis of in hospitality mortality and morbidity of 721 RA vs 8859 OA patients who underwent a non emergent THR from 1984 to 1985 | THA | 721 RA | Not reported | Not reported | Not reported | 0.3 % of VTE vs 1.2% in OA patients | 4.20% | |
| Nagase et al[10] | Retrospective analysis of 27542 patients who underwent THA or TKA in 723 japan hospital between 2007 and 2008 | THA/TKA | 2153 RA | Mechanical prophylaxis or mechanical prophylaxis and fondaparinux | Not reported | Not reported | 19 (0.89%) | Not reported | |
| Soohoo et al[11] | reviewed discharge data from 138399 patients undergoing primary THA in California from 1995 to 2005 | THA | 5565 RA | Not reported | Not reported | Not reported | OR = 1.46 (95%CI: 0.82-1.61; P = 0.2) | Not reported | |
| Hull et al[12] | A randomized trial was performed in 310 consecutive patients undergoing total hip replacement between 1978 and 1986 | THA | 77 RA | Sequential calf and thigh intermittent compression was begun postoperatively in the recovery room compared with none prophylaxis | Intermittent compression was continued until the patient was discharged from the hospital or for 14 d, at which time most patients were fully ambulant | Leg scanning was performed on the first day after surgery and then daily for 14 d | None prophylaxis: 77/158 Intermittent leg pneumatic compression: 36/152 | Not reported | |
As can be observed in table 1, only 3 studies satisfied all the inclusion criteria[5,6,8]. In the other studies there were several bias: the prophylaxis utilized was not reported in 3 studies, the duration of prophylaxis was not indicated in 3, the method of surveillance for the diagnosis of VTE was not reported in 3, and the incidence of bleeding complications was not reported in 4 studies.
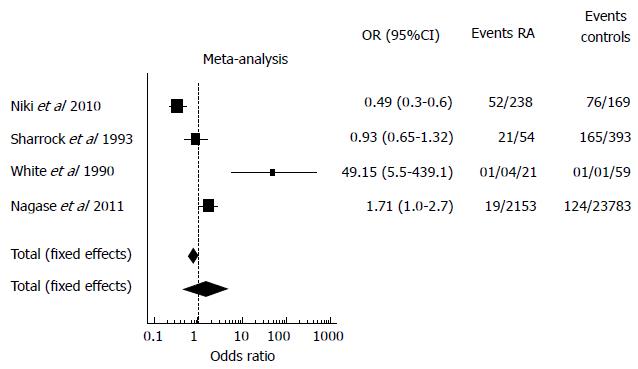
A total of 4 studies which reported the incidence of VTE in RA patients and controls (mainly patients with Osteoarthrosis) undergoing orthopedic surgery were identified[6,7,9,10] and included in the meta-analysis. Three studies were excluded from the meta-analysis since the rate of VTE in RA patients was not reported. Another study was excluded because the incidence of VTE in both the RA group and controls was expressed only as Odds Ratio. The final results of the meta-analysis are shown in Figure 2: the OR calculated with both the fixed and the random effect models shows no difference between AR patients and controls in terms of VTE rates. The random effect analysis shows a wider variance between the studies as expected. This data is confirmed by the great heterogeneity found among the studies (I2 = 92.1). Consequent forest plot is also shown to depict the overall rate of the frequency of VTE in RA and controls undergoing orthopedic surgery.
Since bleeding events that occurred both in the AR group and controls were reported in only two studies it was decided not to perform meta-analysis. Niki et al[6] reported similar figures of bleeding in both groups: 1/238 (0.42 %) in AR patients and 2/169 (1.1 %) (P = 0.57) in the controls while in the study of White et al[9] bleeding events were 30/721 (4.2 %) in RA patients and 345/8859 (3.9 %) (P = 0.68) in controls (OA patients). van Heereveld et al[5] reported a bleeding event in 20/151 (13.2 %) AR patients, Swierstra et al[8] reported 2 bleeding episodes in 14 patients with AR but secondary to anti-vitamin K overdose. In the other studies no data on bleeding were reported.
On the basis of the results obtained from this review we tried to answer some questions with the aim to confer a practical approach to this topic.
Historically the rate of DVT or PE after major orthopedic surgery was estimated to be 40% to 84% in patients undergoing total knee arthroplasty (TKA)[13,14] and in 45% to 57% of those undergoing total hip arthroplasty (THA) in the absence of thromboprophylaxis. Proximal DVT in the absence of thromboprophylaxis has been reported to occur in 9% to 20% of TKA patients and in 23% to 36% of THA patients[12,15,16]. Currently with modern techniques and post-operative care, the estimated risk of developing a symptomatic VTE without prophylaxis is approximately 4.3%[1]. As for RA patients who underwent major orthopedic surgery, Abernethy and Kelly report a rate of DVT and PE r of 70% and 50% respectively in the absence of prophylaxis[17,18]. The meta-analysis we have carried out involved only 4 studies for which the rate of VTE both in RA patients and controls were available. Results show no differences between these group of patients. However, a limitation of these results is the important heterogeneity among the studies documented by the very high value of I2. On the basis of these results it is difficult to conclude that RA patients are different from other groups of patients in terms of thromboembolic risk. We therefore believe that RA patients should be given the same prophylactic approach recommended for orthopedic surgery. Another limitation of the meta-analysis we performed is the extremely different anti-thrombotic prophylaxis used in the 4 considered studies.
If other studies not included in the meta-analysis are considered, an indirect confirmation comes from Soohoo et al[11] who reviewed discharge data from 138399 patients undergoing primary THA in California from 1995 to 2005. Diagnosis of Rheumatoid arthritis was associated to an increase of complications at 90-d after surgery considered as a whole (mortality, infection, dislocation, revision, perioperative fracture, neurologic injury, and thromboembolic disease) compared with patients without RA (OR = 1.53, 95%CI: 1.23-1.91, P < 0.001). In particular, the risk is particularly increased for mortality at 90 d (OR = 1.88, 95%CI: 1.17-3.03, P = 0.01) but not for thromboembolism [OR = 1.46 (0.82-2.61, P = 0.20)[11].
Another study, not included in the meta-analysis because it dealt retrospectively only with RA patients, was that of van Heereveld et al[5] who found only one patient, in 151 surgical procedures, who developed symptoms of post-discharge VTE, despite the short duration of heparin administration (7 d). Interestingly, 85% of the RA patients used NSAIDs daily and thus they may have been protected, at least partially, from venous thromboembolism because of the anti-platelet activity of these drugs. However, this possible favorable effect was offset by a relatively high rate of bleeding complications (13%). In summary, the data show that in the presence of a prophylaxis the incidence of VTE in patients with RA is not only greater than other groups of comparison, but even lower in some studies.
To our knowledge the data about the best prophylaxis to be used in RA patients are insufficient to conclude in favor of any of the several options available. Significantly, different rates of DVT following TKA were observed in different preoperative strategies. Maneuvers to reduce accumulation of blood in the deep vein of the limb during surgery or to dislodge adherent clot may be useful strategies to minimize deep vein thrombosis following TKA. Elevation of the leg after surgery and early ambulation may also contribute to lower deep vein thrombosis rates[5]. In 2012, the ACCP recommends the use of several drugs for antithrombotic prophylaxis in patients who undergo THA or TKA. These drugs range from heparins (unfractioned, LMWH and fondaparinux) to aspirin. VKA and the new oral anticoagulants (dabigatran, rivaroxaban nd apixaban) are also considered. However this recommendation is referred to any anti-thrombotic drug in comparison to no anti-thrombotic prophylaxis. In a further recommendation, the ACCP suggests the use of LMWH, irrespective of the concomitant use of any pneumatic compression device (IPCD), in preference to the other drugs listed above. This choice could be explained by several factors (1) the favorable effect of aspirin is mild and is counterbalanced by the hemorrhagic risk conferred by this drug. Surprisingly we have read the recommendations of ACCP also on the use of aspirin in the thromboembolism prophylaxis after THA and TKA. It worth noting, however, that it was intended that the use of aspirin is to be considered better than nothing so that it is not certainly the drug of choice in that orthopedic setting[1]; (2) the difficulty in the peri-operative management of AVK; and (3) the similar efficacy of the new oral anticoagulants (NOAC) in comparison to LMWH, their longer half-life and the lack of post-marketing studies. In particular, NOAC (dabigatran, rivaroxaban and apixaban) show an increased risk for major bleeding[19] and a poor adherence[20]. Moreover, there is no data about both the safety and the efficacy of these drugs in patients with RA since the inclusion criteria of the clinical trials comparing NOAC with LMWH did not reflect the typical patient with RA who undergone major orthopedic surgery, that is a subject with several co-morbidities and that frequently use NSAID. Another point of concern may be the number of possible drug-interactions due to the metabolism of NOAC by the cytochrome P450 CYP 3A4 (rivaroxaban and apixaban) and the p-glycoprotein system (dabigatran, rivaroxaban and apixaban)[21]. These aspects may be not negligible when considering the Disease Modifying Antirheumatic Drugs (DMARDs) commonly utilized in the management of RA.
The historical data suggest that both pre and post-operative initiation of thromboprophylaxis are similar in terms of safety and efficacy. Meta-analysis or systematic review comparing pre- and post-operative initiation of therapy have found no consistent difference in efficacy and safety (bleeding rates) between the two strategies[22,23,24]. In many European countries LMWH is considered the standard therapy for prophylaxis following THA or TKA and is initiated pre-operatively to maximize its efficacy[24]. Preoperative thrombo-prophylaxis is initiated on the assumption that the surgery per se and the accompanying immobility are the main causes of thrombosis[22,25]. However, as most thrombi develop post-operatively, starting anticoagulant therapy following surgery could also prevent VTE[26].
Since RA is a medical condition with increased risk of venous thrombotic events, the use of prophylaxis with heparin to prevent venous thrombosis should be administered even several days before surgery if the patient is bed ridden. In other words, if a patient with RA is immobilized and has been scheduled for surgery anti-thrombotic, prophylaxis should be started regardless of the waiting time for surgery. Immobilization per se may be related per se to disease activity and inflammation which in turn may induce a hypercoagulable state[27].
Most VTE events occur after hospital discharge. Consequently, extended thromboprophylaxis after discharge should be considered and is particularly important after major surgery. The peak of DVT incidence is observed around the fifth postoperative day[28]. After the first postoperative week a second coagulation process occurs, as demonstrated by an increase of thrombin-antithrombin III complexes and D-dimer, markers of coagulation activation, which may persist for up to six weeks or longer[29]. This might be attributable to a relative immobilization of the patient after discharge.
In summary, RA patients should undergo physical therapy since physical activity is necessary to prevent disabilities and restore functions, decrease pain and joint inflammation and increase ROM and strength. The early mobilization is the primary objective for the physician in order to assess the duration of prophylaxis Anti-thrombotic prophylaxis should be last at least 5 wk as recommended by the ACCP but in RA patients a longer period of anti-thrombotic prophylaxis should be considered depending on recovery of mobility.
A delicate balance exists between VTE prophylaxis and systemic and surgical site of bleeding, which can lead to surgical wound complications including infection, haematoma and gastrointestinal bleeding. Many orthopedic surgeons fear the risk of bleeding associated with the introduction of anticoagulant prophylaxis for VTE prevention. Bleeding may occur earlier than VTE, and seriously compromise the result of surgery, or later as a complication of prophylaxis. A meta-analysis of 9 trials of extended duration (up 42 d) of VTE prophylaxes with LMWH after TKA or THA showed that there was no significant increase in major bleeding episodes despite the marked reductions in symptomatic VTE[30]. Only a small (1.2%) increase in minor bleeding was observed compared with patients receiving post-discharge placebo. In summary, in the single RA patient it is important to balance the bleeding risk against that of thromboembolism whilst keeping in mind that the latter represents a priority to be managed.
RA patients who undergo major orthopedic surgery for joint destruction typically have severe disease. In these patients medical therapy has generally failed. In the absence of dedicated guidelines for the care of patients with RA undergoing orthopedic surgery, management must be individualized to obtain favorable patient outcome, weighing up all the factors that place the patient at the same time at a higher bleeding and thrombotic risk.
Ideally, preoperative evaluation by an orthopedic surgeon should start several weeks before elective surgery for an optimal management of thrombotic and bleeding risk of RA patients.
RA patients may be at increased risk of VTE due to active inflammatory disease, specific joint problems and the surgical procedures themselves. The presence of co-morbidities, as impaired renal function, cardiovascular and liver diseases and some drugs, especially NSAIDs should be carefully examined prior to starting thromboprophylaxis. Finally, RA patients should be treated as the other candidates for orthopedic surgery but special care should be paid to their comorbidities before and after surgery. Dedicated clinical trials should be planned to respond to the several still unanswered questions we have tried to discuss here.
We thank Mr. Barry Mark Wheaton for his editing assistance.
P- Reviewer: Undas A S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Falck-Ytter Y, Francis CV, Johanson N, Curley C, Dahl O, Schulman S, Ortel T, Pauker S, Colwell Jr CW. Prevention of VTE in Orthopedic surgery patients. The Nineth ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2012;141:278S–325S. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1341] [Cited by in RCA: 1569] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 2. | Mameli A, Barcellona D, Marongiu F. Rheumatoid arthritis and thrombosis. Clin Exp Rheumatol. 2009;27:846-855. [PubMed] |
| 3. | Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost. 2005;94:362-365. [PubMed] |
| 4. | Chung WS, Peng CL, Lin CL, Chang YJ, Chen YF, Chiang JY, Sung FC, Kao CH. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis. 2013;Aug 13; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis. 2001;60:974-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Niki Y, Matsumoto H, Hakozaki A, Mochizuki T, Momohara S. Rheumatoid arthritis: a risk factor for deep venous thrombosis after total knee arthroplasty? Comparative study with osteoarthritis. J Orthop Sci. 2010;15:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Sharrock NE, Hargett MJ, Urquhart B, Peterson MG, Ranawat C, Insall J, Windsor R. Factors affecting deep vein thrombosis rate following total knee arthroplasty under epidural anesthesia. J Arthroplasty. 1993;8:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Swierstra BA, Stibbe J, Schouten HJ. Prevention of thrombosis after hip arthroplasty. A prospective study of preoperative oral anticoagulants. Acta Orthop Scand. 1988;59:139-143. [PubMed] |
| 9. | White RH, McCurdy SA, Marder RA. Early morbidity after total hip replacement: rheumatoid arthritis versus osteoarthritis. J Gen Intern Med. 1990;5:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Nagase Y, Yasunaga H, Horiguchi H, Hashimoto H, Shoda N, Kadono Y, Matsuda S, Nakamura K, Tanaka S. Risk factors for pulmonary embolism and the effects of fondaparinux after total hip and knee arthroplasty: a retrospective observational study with use of a national database in Japan. J Bone Joint Surg Am. 2011;93:e146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Soohoo NF, Farng E, Lieberman JR, Chambers L, Zingmond DS. Factors that predict short-term complication rates after total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2363-2371. [PubMed] [DOI] [Full Text] |
| 12. | Hull RD, Raskob GE, Gent M, McLoughlin D, Julian D, Smith FC, Dale NI, Reed-Davis R, Lofthouse RN, Anderson C. Effectiveness of intermittent pneumatic leg compression for preventing deep vein thrombosis after total hip replacement. JAMA. 1990;263:2313-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 112] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66:194-201. [PubMed] |
| 14. | Stringer MD, Steadman CA, Hedges AR, Thomas EM, Morley TR, Kakkar VV. Deep vein thrombosis after elective knee surgery. An incidence study in 312 patients. J Bone Joint Surg Br. 1989;71:492-497. [PubMed] |
| 15. | Lassen MR, Borris LC, Christiansen HM, Boll KL, Eiskjaer SP, Nielsen BW, Schøtt P, Olsen AD, Rodenberg JC, Lucht U. Prevention of thromboembolism in 190 hip arthroplasties. Comparison of LMW heparin and placebo. Acta Orthop Scand. 1991;62:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Hoek JA, Nurmohamed MT, Hamelynck KJ, Marti RK, Knipscheer HC, ten Cate H, Büller HR, Magnani HN, ten Cate JW. Prevention of deep vein thrombosis following total hip replacement by low molecular weight heparinoid. Thromb Haemost. 1992;67:28-32. [PubMed] |
| 17. | Abernethy PJ. Surgery of the rheumatoid knee. Ann Rheum Dis. 1990;49 Suppl 2:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Kelly IG. Surgical treatment of the rheumatoid hip. Ann Rheum Dis. 1990;49 Suppl 2:858-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Adam SS, McDuffie JR, Lachiewicz PF, Ortel TL, Williams JW. Comparative effectiveness of new oral anticoagulants and standard thromboprophylaxis in patients having total hip or knee replacement: a systematic review. Ann Intern Med. 2013;159:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Pengo V, Crippa L, Falanga A, Finazzi G, Marongiu F, Palareti G, Poli D, Testa S, Tiraferri E, Tosetto A. Questions and answers on the use of dabigatran and perspectives on the use of other new oral anticoagulants in patients with atrial fibrillation. A consensus document of the Italian Federation of Thrombosis Centers (FCSA). Thromb Haemost. 2011;106:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013;52:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Strebel N, Prins M, Agnelli G, Büller HR. Preoperative or postoperative start of prophylaxis for venous thromboembolism with low-molecular-weight heparin in elective hip surgery? Arch Intern Med. 2002;162:1451-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Hull RD, Pineo GF, MacIsaac S. Low-molecular-weight heparin prophylaxis: preoperative versus postoperative initiation in patients undergoing elective hip surgery. Thromb Res. 2001;101:V155-V162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Hull RD, Raskob GE. Venous thromboembolic disease. Curr Opin Cardiol. 1991;6:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Perka C. Preoperative versus postoperative initiation of thromboprophylaxis following major orthopedic surgery: safety and efficacy of postoperative administration supported by recent trials of new oral anticoagulants. Thromb J. 2011;9:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Pottier P, Fouassier M, Hardouin JB, Volteau C, Planchon B. D-dimers, thrombin-antithrombin complexes, and risk factors for thromboembolism in hospitalized patient. Clin Appl Thromb Hemost. 2009;15:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Kakkar VV. Low molecular weight heparins: prophylaxis of venous thromboembolism in surgical patients. Semin Hematol. 1997;34:9-19. [PubMed] |
| 29. | Dahl OE, Aspelin T, Arnesen H, Seljeflot I, Kierulf P, Ruyter R, Lyberg T. Increased activation of coagulation and formation of late deep venous thrombosis following discontinuation of thromboprophylaxis after hip replacement surgery. Thromb Res. 1995;80:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Eikelboom JW, Quinlan DJ, Douketis JD. Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of the randomised trials. Lancet. 2001;358:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 334] [Article Influence: 13.9] [Reference Citation Analysis (0)] |