INTRODUCTION
The around the knee skin and soft tissue defects represents a challenge to the plastic surgeon. A thin, pliable coverage of the knee joint is a prerequisite to promote wound healing and to any concomitant orthopedic procedure. As a rule, the upper one third of the tibia can be covered effectively with rotational muscle flaps[1]. Nevertheless, complex lower limb wounds are best managed with a distant flap, because avoids additional skin incisions to the already damaged with impaired perfusion limb. Moreover, introduces a well-perfused tissue from an uninjured area, thus facilitating wound healing[2]. On the other hand, the free tissue transfer is more challenging option for around the knee, due to the difficulties in recipient vessel selection positioned deeply around the knee[3].
The purpose of this article is to analyze the reconstructive challenges and reconstructive options for around the knee defects and to propose a reconstructive algorithm.
REVIEW OF THE LITERATURE
The literature search revealed 138 articles related to the reconstruction of soft tissue defects around the knee, whereas 124 articles consider the open and/or infected knee joint salvage with flaps. Thirteen articles are related to the use of local flaps and 51 articles are related to the use of pedicled flaps in peri-knee soft tissue reconstruction. Forty-one articles discuss the use of free flaps whilst 11 articles discuss the recipients’ vessels that can be used.
Five review articles have been identified in English literature: 3 related to wound complications in total knee arthroplasty and flap reconstruction[4-6], 1 related to the recipient vessel selection[3], and 1 related to lateral genicular artery flap[7].
One article proposes an algorithm of treatment of the exposed total knee prosthesis[8], but no article has been identified proposing a reconstructive algorithm of the around the knee defects.
THE CAUSES AND RISK FACTORS OF THE SOFT TISSUE DEFECT AROUND THE KNEE
Apart from posttraumatic defects[9,10] and oncological resections[11-14] knee soft tissue defects may arise from chronic infection, post surgical radiation[15], or surgical release of postburn flexion contractures[16]. Moreover, can be caused by multiple previous operations[6,17,18]. Wound complications following total knee arthroplasty can occur up to 20% of patients, and are related to skin/soft tissue necrosis and possible exposure of the implant. Gross infection may lead to loss of the prosthesis or even of the limb[6,17,19].
Risk factors for knee wound complications could be related to the patient’s general status and to local wound factors[20]. Diabetes is associated with dehiscence and infection[19], smoking is related to bleeding complications and infection[2], obesity may induce dehiscence and deep-venous thrombosis[2].
Local factors predisposing to complications are previous scars, major vessel trauma, hematoma, local infection, tension at the skin closure, and previous irradiated skin[20].
RECONSTRUCTIVE CHALLENGES
The knee is a hinge type synovial joint, which permits flexion and extension about a transverse axis, and a small medial and lateral rotation about the axis of the lower leg in the flexed position[21]. The total range of motion is dependent on several parameters such as active insufficiency, hamstring tightness and soft-tissue restraints. The overlying skin is thin and pliable with remarkable distensibility. The size of the skin defect should be estimated with the knee at maximum flexion, and the “like tissue replacement” principle should be ideally applied. In other words the defect should be replaced with plenty pliable skin from the adjacent area. In extensive or complex defects local flaps may not be available, therefore it requires either a distant muscle flap[2] or microvascular tissue transfers to aid in soft tissue reconstruction[22,23]. Distant muscle flaps are associated with variable morbidity and further trauma in the already traumatized limb, but is a fast procedure that requires less reconstructive expertise. Free tissue transfers offers less donor and recipient site morbidity, have the advantage of single stage, but requires expertise and infrastructure[22]. However, the main challenge is the proper selection of recipient vessels[3]. Some surgeons prefer to choose the suitable recipient vessels first allowing this choice to direct the proper flap choice[24,25]. Others advocate the selection of the proper flap first (according the size and depth of the defect), influencing the recipient vessels choice[26]. The fact is that in the severely damaged limb the recipient vessel selection is the main challenge and will determine the success of the reconstruction[3].
OPTIONS OF TREATMENT
The treatment of choice depends on the wound dimensions and geometry, presence of gross contamination and/or infection, and most importantly if there is bone, tendon or implant exposure[8]. The options are conservative wound management or debridement with reconstruction in the presence of necrotic tissues[23,25]. The cornerstone of the treatment is the thorough debridement and removal of any devitalized and infected tissue and/or infected foreign material.
In cases of small split-thickness skin loss, early skin grafting is preferable to secondary healing, in order to avoid hypertrophic or contracted scars. In deep wounds (without exposure of the patella, bone or implant) associating the vacuum assisted closure (VAC) therapy reduces the wound exudate, controls the microbial load and the speeds the granulation tissue formation, thus facilitating secondary skin grafting[26].
Nevertheless, if the resulting defect is deeper with exposed bone or hardware, or infection is documented, a flap is needed[23]. If the defect is associated with intra-articular infection, drainage through an arthrotomy and continuous irrigation could be indicated[27].
LOCAL FASCIOCUTANEOUS AND PERFORATOR FLAPS
Local skin are used in small skin defects (less than 4 cm). The prerequisite is the absence of infection and no bone or prosthesis exposure[23]. More recently, local perforator flaps have been used, with significant advantages due to their low donor morbidity and better esthetic outcome[2]. Panni et al[4], in their review paper, emphasized on the use of a flap from the inferomedial thigh based on perforators arising from the descending genicular artery. The preoperative investigation of the perforator vessels with color doppler is essential[28]. Occasionally the flap may suffer from venous congestion, and an additional venous microanastomosis with a local vein could be valuable. The flap presents wide arc of rotation and may cover the whole knee[29].
PEDICLED MUSCLE AND MUSCULOCUTANEOUS FLAPS
Pedicled muscle and musculocutaneous flaps still represent the workhorse for coverage of knee defects, because of the straightforwardness, and are specifically indicated more complex soft defects with joint and/or prosthesis exposure. Muscle flaps obliterate the three-dimensional defect and provide rich blood supply to the wound that facilitates the antibiotics delivery.
Although, gastrocnemius flap was introduced since 1978[30] is still the most commonly used flap for knee coverage, due to its reliable axial blood supply and ease of dissection. The gastrocnemius flap is a type I (single vascular pedicle) according to the Mathe et al[31] classification with dominant vessel in most patients the media sural artery. The medial gastrocnemius is greater than the lateral one, with longer vascular pedicle and greater arc of rotation, reaching the knee easily and thus is more frequently used (Figure 1). The lateral gastrocnemius carries the risk of damaging the peroneal nerve while it turns around the neck of the fibula, and is used for lateral knee defects. The medial half musculotendinous unit has been used to reconstruct in one stage the extensor apparatus and to provide soft tissue cover in open knee joint[10]. The combined “gastrocnemius with soleus bi-muscle flap” has been introduced for large patella/infrapatella defects, and is based on the perforators of the distal half of the gastrocnemius muscle to the soleus muscle[32]. The soleus muscle (Type II: dominant pedicles branches from popliteal artery, proximal two branches of posterior tibial, proximal two branches of peroneal artery and minor pedicle 3 or 4 segmental branches of the posterior tibial[31]), based proximally, can be reliably carried to a point approximately 5 cm above its tendinous insertion, thus to cover the lower portion of the knee[32] The soleus is a “slow” muscle that aids in posture stabilization and slow gait. Transfer even of the entire soleus muscle creates little if any functional deficit. Gastrocnemius flap has been the workhorse in exposed total knee prosthesis with variable but generally good results[33-35].
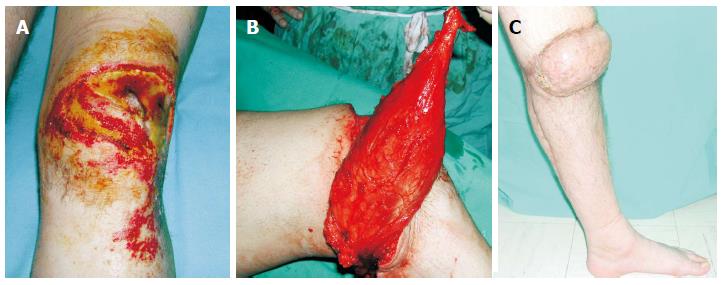
Figure 1 A 28-year-old man suffered a postburn unhealed wound around the right knee that was managed with pedicled gastrocnemius flap.
A: The exposed patella was associated with flexion contracture; B: A medial gastrocnemius muscle flap was elevated as an island flap based on the medial sural artery. The muscle was freed from its origin and its motor nerve, as well, to allow greater arc of rotation; C: Four weeks postoperatively, gait without external supports was achieved. From the cosmetic point of view, although the muscle was denervated, a bulky pivot point was present that resulted in a cosmetic deformity.
Recently, gracilis muscle that is also associated with negligible donor site morbidity has been described for knee resurfacing. Gracilis muscle is type II (dominant pedicle medial circumflex femoral artery and minor pedicle 1-2 branches from superficial femoral artery) according to the Mathes and Nahai classification[31]. The reversed gracilis pedicle flap has been suggested as a substitute to, or combination with a gastrocnemius for the treatment of large patella or suprapatella defects[36].
The Sartorius muscle flap was first used in 1978 to close an exposed knee joint. The blood supply to this muscle is by numerous segmental vessels from the femoral artery (type IV according to the Mathes and Nahai classification[31], and the main feeding vessel is located about 8 cm below the inguinal ligament[37]. The distally based Sartorius is best prefabricated by denervation and vascular delay and then transposed based on the distal pedicle to cover the defect[38]. In 2012 Shen et al[39] presented 12 cases of covering proximal tibia by the sartorius flap based on the rich anatomotic network of the descending genicular artery, together with the perforators of the posterior tibial artery and the medial inferior genicular artery.
Arnold et al[40] in 1981 were the first to publish an article for Vastus medialis flap (type II: dominant pedicle branch from superficial femoral and minor pedicle branches of descending genicular artery[31]) to cover an exposed knee joint, and Swartz et al[41] in 1987 evaluated the blood supply to the vastus lateralis by dye injection techniques in fresh cadaver type II: dominant pedicle descending branch of lateral circumflex femoral artery and minor pedicle branches of lateral superior genicular artery[31]). Thereafter, the authors have used successfully the distally based vastus lateralis muscle flap to cover defects above the knee. These two papers were the precursors of distally based antromedial and anteromedial thigh perforator flap, respectively[40,41].
PEDICLED PERFORATOR FLAPS
Zhang was the first to introduce the reversed anterolateral thigh island flap, and reported that the flap is reliable based on the anastomoses of the lateral superior genicular artery with the descending branch of the lateral circumflex femoris artery[42]. Nevertheless, Yildirim et al[16] reported that the reliability of the flap is questionable. Gravvanis et al[43] comparing the option of distally based ALT vs gastrocnemius flap concluded that the technical complexity of the former flap is justified by the greater flexibility of size and shape, and by the better color and texture match. ALT is less bulk as compared to gastrocnemius, has longer arc of rotation to reach above and below the knee defects, and provides enough skin to resurface the whole knee[44] (Figure 2). However, the authors emphasized that the reconstructive surgeon should always be alert for vascular compromise of the distally based flap, and thus for flap recharging[43]. Later, Gravvanis et al[45] presented the successful use of a distally based ALT flap with a venous supercharge, to reconstruct the tibial tuberosity. They recommended the venous supercharging of this flap as a routine procedure because could eliminate any vascular problems[45].
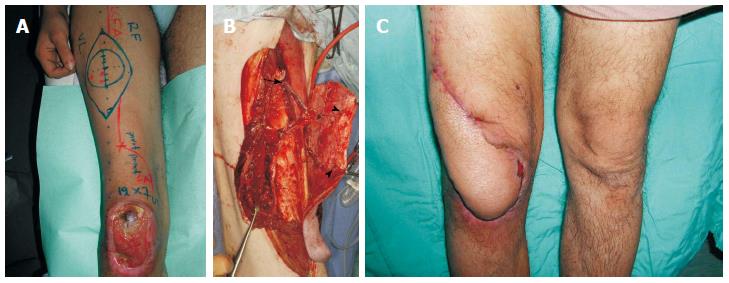
Figure 2 A 24-year-old man suffered from skin and soft tissue defect over the right knee with exposure of the patella that was managed with a distally based anterolateral thigh flap.
A: An anterolateral thigh flap was designed; B: The distally based flap was elevated, based on 2 perforators (arrowheads). Note the 20 cm length of the pedicle (perforator and descending branch of LCFA), as well as, the preservation of the proximal end of the LCFA (arrow) that was added to the flap; C: Satisfactory healing and contour achieved.
The Anteromedial thigh perforator flap is based on perforator of the descending branch of the lateral circumflex femoris artery that is perforating the rectus femoris muscle (instead of the vastus lateralis in the case of the ALT)[46] As a knee defect coverage was presented in 2010 by Hupkens et al[47] in a study on cadavers. Lu et al[48] in 2011 applied this flap on eleven cases covering the exposed knee joint.
Peroneal Artery perforator flap based on perforators from the peroneal artery running in the posterior intermuscular septum can be designed and transferred in a propeller fashion to cover inferior knee defects. Yoshimura et al[49] in 1985, first described this flap for knee defects and Ruan in 2009 presented the extended peroneal artery perforator (EPAP) flap[50,51]. The limitation of the peroneal flap in knee reconstruction is the variable number and location of the perforators. A dominant perforator should be located proximal enough to allow an adequate arc of rotation to cover the knee. Thus, a preoperative study with duplex ultrasound or CT-angiography is mandatory.
Li et al[52] in 1990 was the first to describe the coverage of the knee with the lateral sural flap, based on sural cutaneous artery, preserving all the big vessels of the lower leg. Umemoto et al[53] followed in 2005, and Shim et al[54] in 2006 presented the medial sural artery flap on six patients, covering knee defects.
Nguyen et al[55] described on cadaveric studies the lateral supragenicular pedicled perforator flap, and subsequently they used successfully this perforator flap in two patients with exposed knee joint.
Likewise, Rad et al[56] described the anterior tibial artery perforator flap on cadavers, and then reported the management of four patients with good results. Adhikari et al[57] has used the same flap to reconstruct post-burn flexion contractures in seven patients with excellent outcomes.
The introduction of free-style local perforator flaps[58] and propeler flaps[59] presented another reliable and predictable technique to cover skin defects over the knee with a low morbidity rate. The method is based on the use of Echo-Doppler Tracing of good perforator vessels around the knee defect and the administration of Freestyle supero-medial or supero-lateral flaps[59].
FREE FLAPS
Free flaps are used for more extensive soft tissue defects. In the case of a complex, three-dimensional defect a free flap is a better option, because it avoids further scaring and trauma to an already injured limb. This well-perfused tissue from outside the zone of injury brings stability to the wound and promotes healing. When considering free tissue transfer for around the knee defects, the recipient vessel selection is the main difficulty and challenge[3].
RECIPIENT VESSELS
The use of various vessels has been reported, however each has drawbacks. The popliteal artery, the continuation of the femoral, is the main vessel in the knee region, and divides into anterior tibial, posterior tibial and peroneal. Popliteal artery gives of branches such as sural, superior genicular, middle genicular and the inferior genicular artery. The size, position and depth of the soft tissue loss will change the normal anatomy of the region and will drive the surgeon to different options according to the quality of the available vessels.
The popliteal artery is a reliable choice of recipient vessels[60], but is risky for free tissue transfer to the anterior surface of the knee-joint[3] due to potential compression of the vascular pedicle and/or microanastomosis. End-to-side anastomosis is necessary to preserve the distal flow of the major artery, and is associated with limb ischemia during microanastomosis. Tibial arteries can be used as recipient vessels but the anterior tibial artery is frequently involved in trauma as compared with the posterior artery[61]. Nevertheless, the smaller branches can reliably provide inflow, thus one does not have to isolate the popliteal or tibial system in the majority of cases.
The sural arteries are two branches that arise from the popliteal artery across the knee joint, and can be used as recipients when popliteal vessels are absent or severly damaged[62]. In trauma cases the gastrocnemius muscle heads may protect the sural vessels from injury. Therefore are preserved as reliable recipient vessels to simplify the required microanastomoses in an end-to-end fashion, instead of end-to-side to the popliteal system. Hallock proposed a medial approach to the sural vessels that permits the patient to remain in a supine or lateral position for simultaneous dissection of the donor and recipient site[63]. Beumer et al[24] has proved that the interruption of medial sural artery has no subsequent functional effect to the gastrocnemius head, and indicated that should be used with confidence as recipient vessel for free flap to the knee.
In either side of the politeal artery, two superior genicular arteries arise and wind around the femur above the condyles in front of the knee joint. Park et al[25] used them successfully in four cases of soft tissue defect in the posterior region of the knee. Rees-Lee et al[64] further popularized their use for anterior and medial knee defects. The middle genicular artery arises from the anterolaterar surface of the popliteal artery, and generally presents short, intraarticular course. The descending genicular artery arise from the femoral artery and descents between the vastus medialis and the adductor magnus, and is used as a recipient vessel for the anterior knee defects[25,65]. Remarkably, Chien et al[66] has used this vessel as recipient for an ALT free flap in reverse flow pattern.
The descending branch of the lateral circumflex femoral vessels can be dissected out and serve as a recipient vessel after being placed adjacent to the defect for free tissue transfer to the difficult areas of the lower extremity[67-69] (Figure 3). However, the descending branch has variable anatomy and size, especially at its distal end, thus a meticulous preoperative imaging with duplex ultrasound and/or CT-angiography is mandatory.
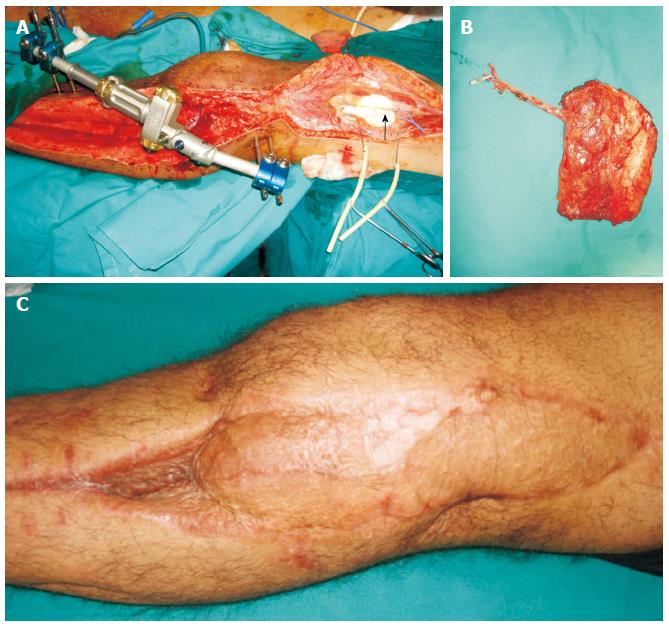
Figure 3 Patient with anterior compartment syndrome and vascular (femoral-popliteal) bypass, presented with skin and soft tissue defect over the lateral aspect of the knee.
A: The descending branch of the lateral circumflex femoral vessels was dissected as recipients (arrow); B: The contralateral vastus lateralis muscle was dissected as a free flap and was anastomosed in end-to-end fashion to the distal end of the descending branch of the lateral circumflex femoral vessels; C: Satisfactory healing and contour achieved.
The distal superficial femoral arterial branch was used as recipient vessel for a free flap reconstruction involving knee and proximal tibia[70].
Suprafascial perforators of the knee area were also introduced as recipients, but this approach demands advanced microsurgical expertise. The random perforators were traced with Doppler sonography, computer tomography angiography or magnetic resonance angiography in 25 different cases[71].
In high-energy injuries all superficial sited vessels may be are involved, consequently their use is unreliable. Such cases require the use of vein grafts or long arteriovenous fistulas that are associated with higher failure rate[72]. Alternatively, the Superficial Femoral can be used as an ultimate recipient vessel. Gravvanis et al[73] described a reproducible technique to use femoral vessels before the adductor canal as recipients (Figure 4). The femoral present substantial advantages: constant anatomy, ease of dissection and learning curve, and most-importantly well-protected anastomoses that are not positional depended (e.g., cannot be compressed due to the limb’s position). The few drawbacks are: deep recipient vessel position that requires long pedicle or long flap to reach the defect and the temporal occlusion of limb’s perfusion during anastomoses[73].
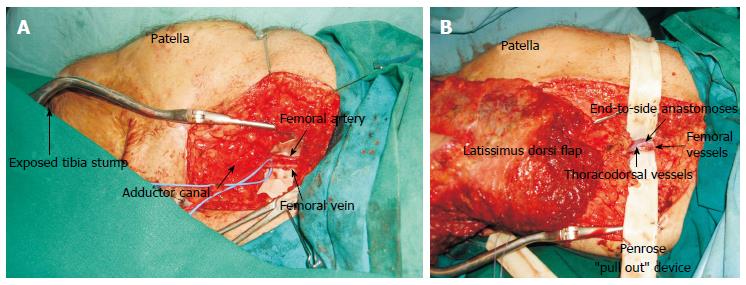
Figure 4 The use of superficial femoral vessels as recipient in free tissue transfer for around the knee defects.
A: Femoral vessels skeletonised and dissected free from surrounding fat of the area; B: Penrose ”pull out” device in place facilitated the end to side anastomoses.
FREE MUSCLE FLAPS
Free muscle flaps traditionally have been used to reconstruct lower extremity defects and are generally covered with skin grafts. They confront better the three-dimensional defect; they control infection and bring stability to the wound. On the other hand they are associated with variable donor site morbidity, and shorter vascular pedicle (as compared to perforator flaps). The latter may restrict the free tissue transfer, given that in the mobile knee area the selection of recipient vessels is critical.
The latissimus dorsi muscle flap (type V: dominant pedicle thoracodorsal artery and secondary segmental branches of posterior intercostal and lumbar artery[31]) is probably the most popular free flap in knee coverage, in the literature[26,74-79], followed by rectus abdominis[76,79] (type III two dominant pedicles: superior and deep inferior epigastric artery) and fascia lata[80] (type I single vascular pedicle: ascending branch of lateral circumflex femoral artery). Rectus femoris muscle as a functional graft for restoration of knee extension and defect coverage after trauma was published by Wechselberger et al[81] in 2006. Tibial composite bone and soft tissue defects of the knee were reconstructed with free composite serratus anterior and rib flaps by Lin et al[82] in 1997.
Taking into account the considerable morbidity of the aforementioned flaps, muscles with documented less morbidity can be used in the majority of cases. Vastus lateralis muscle flap[83] can be used in extended defects (Figure 3) instead of latissimus dorsi and gracilis muscle[84] can be used in smaller defects instead of rectus abdominis. Both muscles can be used as functional muscles for knee extension.
FREE PERFORATOR FLAPS
Free perforator flaps are cutaneous flaps without muscle that gave another rebirth to reconstructive surgery. The most commonly used in knee coverage are ALT (anterolateral thigh, Figure 5), TDAP (thoracodorsal artery perforator), and superficial iliac circumflex perforator flap[85]. Cavadas et al[86] reported the use of the medial sural artery perforator free flap and Herrera et al[70] the superficial artery epigastric flap. Among their advantages are reliability and the long pedicle that enables to easily reach the recipient vessels facilitating microanastomosis. Most importantly, perforator flaps are associated with low donor site morbidity[87], given that no muscle is sacrificed and typically the donor site is closed directly resulting in a linear scar. They present great versatility and adaptability. They can be safely thinned in the plane immediately under the superficial fascia, in order to provide thin pliable coverage. On the other hand, if there is a three-dimensional defect or a grossly contaminated wound, part of the underlying muscle (e.g., vastus lateralis, latissimus dorsi) may be included in a chimeric fashion[88]. Most importantly, complex knee trauma with knee joint exposure and patellar tendon deficiency can be reconstructed in a single stage with ALT myocutaneous flap combined with vascularized fascia lata[88].
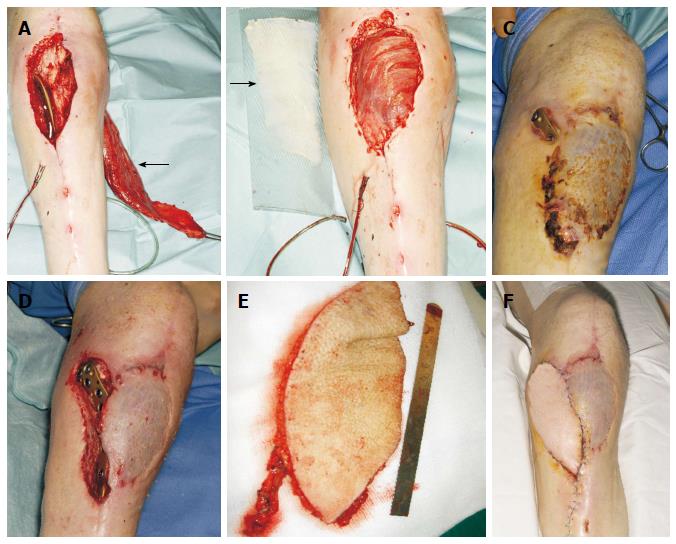
Figure 5 Infrapatellar exposure of a poorly placed plate in a 60-year-old man.
A: Due to the questionable viability of the lateral gastrocnemius head, the medial head was dissected (arrow); B: The flap was medially rotated and covered the defect. The muscle was resurfaced with a split-thickness skin graft (arrow); C: Two months later, the plate was exposed in a more proximal position; D: Defect following thorough debridement; E: A free Anterolateral thigh flap based on a single perforator was raised; F: The free flap was anastomosed on end-to-end fashion to the anterior tibial vessels, and a satisfactory healing was achieved.
RECONSTRUCTIVE ALGORITHM
Successful salvage of the lower extremity following flap reconstruction ranges from 75% to 100%, while salvage of the knee prosthesis is achieved in 75%-85% of patients[23]. Although effective treatment options of the exposed knee joint are available today, there is still no universally accepted management. This is mainly due to the lack of soft tissues over the knee joint that precludes the use of local flaps frequently, and due to the challenging selection of recipient sites for microanastomoses. The optimal management of knee defects is still debated, depending on the size of the wound, location of the wound, presence of deep infection, and the level of microsurgical expertise. The decision either for pedicled or free flap should be based on the existing vascular anatomy, thus a meticulous preoperative vascular study either with Duplex ultrasound[28] or CT angiography[89,90] should be done.
Taking into account all the existed literature, a reconstructive algorithm is proposed (Figure 6).
Figure 6 Knee reconstructive algorithm.
VAC: Vacuum assisted closure; ALT: Alanine aminotransferase.
For small (< 4 cm) defects without exposure of the patella, split thickness skin grafts with or without Vacuum Assisted Closure (VAC) therapy is the treatment of choice.
For deeper wounds with exposure of the patella, bone or the artificial joint, flap coverage is mandatory. For small defects (< 4 cm) without infection, skin flaps present the advantages of less morbidity, pliability and better cosmetic outcomes. Perforator flaps such as free style propeller perforator flaps and medial sural artery perforator flap are indicated for suprapatellar and patellar defects, whilst peroneal artery perforator flap is indicated for infrapatellar defects. Nevertheless, perforator flaps are more technically demanding and needs a significant amount of microsurgical expertise.
Therefore, pedicled muscle flaps have been the workhorses for decades. Moreover, muscle flaps are better indicated for deep infection because they obliterate the dead space arising from the debridement and bring stability to the wound. Gastrocnemious flap has been proved to be reliable, safe and easy surgical option. Medial gastro head is more suitable for patellar, infrapatellar defects and tibial tuberosity. Lateral gastro head is more suitable for lateral defects. Distally based thigh muscle flaps, such as sartorius and gracilis, are suggested in the case of suprapatellar infected wounds or absent/traumatised sural arteries (not reliable gastrocnemius flap).
For moderate defects (4-6 cm) not excessively deep, medial sural artery perforator flap and distally based ALT flap is indicated. If the defect is extended to the infrapatellar area, ALT flap supercharging of the flap is considered. If the defect is deep and there is a space to fill gastrocnemius flap and distally based vastus lateralis musle flap are suggested.
For large defects (> 6 cm) or severely damaged limb, a free flap is better solution. Anterolateral thigh as a perforator flap for superficial wounds or as a chimeric flap (with part of vastus lateralis muscle) for deeper wounds will be the flap of choice in the majority of cases. If the thickness of ALT is not suitable, vastus lateralis (associated with minimal morbidity) or latissimus dorsi muscle (associated with considerable morbidity) flap will reconstruct large, deep and infected wounds. The recipient vessel of choice will be identified by the preoperative vascular imaging[28,89,90]. If the study suggests a smaller branch (such as sural, genicular arteries) close to the defect with good inflow and outflow, this choice will enable straightforward end-to-end microanastomoses. Occasionally, smaller branches are absent or traumatized, then Superficial Femoral Vessels can be used in an end-to-side fashion. The choice of the recipient vessels may direct the choice of the most suitable flap, in order to ensure the highest success rate.
Above-the-knee amputation is considered only for life-threatening sepsis or massive bone and soft tissue loss in elderly patients.
CONCLUSION
With the meticulous preoperative planning, by identifying the reconstructive needs, and by understanding the reconstructive algorithm, the surgeon should be able to manage knee defects with high success rate.