Published online Nov 18, 2014. doi: 10.5312/wjo.v5.i5.574
Revised: April 7, 2014
Accepted: May 29, 2014
Published online: November 18, 2014
Processing time: 235 Days and 12 Hours
Ankle involvement is frequent in patients with inflammatory rheumatic diseases, but accurate evaluation by physical examination is often difficult because of the complex anatomical structures of the ankle. Over the last decade, ultrasound (US) has become a practical imaging tool for the assessment of articular and periarticular pathologies, including joint synovitis, tenosynovitis, and enthesitis in rheumatic diseases. Progress in power Doppler (PD) technology has enabled evaluation of the strength of ongoing inflammation. PDUS is very useful for identifying the location and kind of pathologies in rheumatic ankles as well as for distinguishing between inflammatory processes and degenerative changes or between active inflammation and residual damage. The aim of this paper is to illustrate the US assessment of ankle lesions in patients with inflammatory rheumatic diseases, including rheumatoid arthritis, spondyloarthritis, and systemic lupus erythematosus, focusing on the utility of PDUS.
Core tip: Over the last decade, ultrasound (US) has become a practical imaging tool for the assessment of articular and periarticular pathologies in rheumatic diseases. Progress in power Doppler (PD) technology has enabled evaluation of the strength of ongoing inflammation. PDUS is useful not only for identifying the pathologies in rheumatic ankles, but also for distinguishing between inflammatory processes and degenerative changes or between active inflammation and residual damage. The aim of this paper is to illustrate the US assessment of ankle lesions in patients with inflammatory rheumatic diseases, including rheumatoid arthritis and spondyloarthritis, focusing on the utility of PDUS.
- Citation: Suzuki T. Power Doppler ultrasonographic assessment of the ankle in patients with inflammatory rheumatic diseases. World J Orthop 2014; 5(5): 574-584
- URL: https://www.wjgnet.com/2218-5836/full/v5/i5/574.htm
- DOI: https://dx.doi.org/10.5312/wjo.v5.i5.574
Many kinds of systemic arthritic diseases affect the ankle. Among them, there are also diseases for which ankle involvement is a hallmark. As the ankle is one of the major weight-bearing joints, it is prone to being affected by injury and/or by degenerative disorders. In the ankles of patients with inflammatory rheumatic diseases, this extrinsic damage commonly coexists, and it is not always easy to distinguish between them.
Anatomical structures in the ankle lie close together and are sometimes overlying, making differentiation between adjacent structures problematic[1]. Inflamed synovia are relatively small in size and located relatively deep beneath the skin. Obesity, peripheral edema, and predisposing degenerative conditions such as osteophytes, especially in elderly patients, also impede the detection of synovitis.
In this context, a diagnostic imaging technique is important for the evaluation of ankle lesions. However, plain radiography has limited value for the detection of soft tissue changes, and it lacks tissue specificity. Although magnetic resonance (MR) imaging is a high resolution imaging method that can disclose both bone and soft tissue abnormalities, it involves problems such as MR-incompatible metallic implants, patient claustrophobia, accessibility issues, and high cost, all of which prevent it from being widely used for the examination of ankles in rheumatic diseases.
In contrast, ultrasound (US) is a versatile, accurate, safe, and relatively cheap modality that is increasingly used in daily rheumatologic practice. It offers multiplanar evaluation and allows parallel dynamic assessment of multiple target structures, including joints, tendons, ligaments, and bony cortex[2].
In addition to the advantages of grey-scale ultrasound (GSUS), power Doppler (PD) technology has allowed the visualization of the hyperemia of soft tissues in inflammatory articular diseases[3]. A comparison of PDUS findings with histopathologic findings of synovial membrane vascularity in rheumatoid arthritis (RA) patients who have undergone knee arthroplasty has shown that PD signals represent abnormal hypervascularity in RA synovial tissue[4]. Many reports indicate that the intensity of PD signals in the synovium correlates with the intensity of articular and tenosynovial inflammation in RA patients[5,6]. Moreover, PD techniques seem to help differentiate inflammatory enthesis diseases[7].
The aim of this paper is to illustrate the US assessment of ankle lesions in patients with inflammatory rheumatic diseases, focusing on the utility of PDUS.
The important pathologies addressed here are synovial inflammations, including articular synovitis and tenosynovitis, and enthesitis of tendon insertion, including subtendinous bursitis. Structural damage such as bone erosion and tendon degeneration are addressed collaterally and ligament lesions are not mentioned here.
The Outcome Measures in Rheumatology Clinical Trials (OMERACT) US definitions for the most common pathological findings occurring in inflammatory rheumatic diseases are presented in Table 1[8]. For detailed information regarding the US machine settings and fundamental scanning techniques, please refer to the relevant published papers[9].
| RA bone erosion | An intra-articular discontinuity of the bone surface that is visible in two perpendicular planes |
| Synovial fluid | Abnormal hypoechoic or anechoic (relative to subdermal fat, but sometimes may be isoechoic or hyperechoic) intra-articular material that is displaceable and compressible, but does not exhibit a Doppler signal |
| Synovial hypertrophy | Abnormal hypoechoic (relative to subdermal fat, but sometimes may be isoechoic or hyperechoic) intra-articular tissue that is nondisplaceable and poorly compressible and which may exhibit a Doppler signal |
| Tenosynovitis | Hypoechoic or anechoic thickened tissue with or with- out fluid within the tendon sheath, which is seen in two perpendicular planes and which may exhibit a Doppler signal |
| Enthesopathy | Abnormally hypoechoic (loss of normal fibrillar architecture) and/or thickened tendon or ligament at its bony attachment (may occasionally contain hyperechoic foci consistent with calcification), seen in two perpendicular planes that may exhibit a Doppler signal and/or bony changes, including enthesophytes, erosions, or irregularity |
Data regarding the positioning of the patient and anatomical structures to be scanned in the ankle region are presented in Table 2[10]. An encompassing scanning technique makes it possible for the examiners not to overlook pathologies, regardless of whether they were symptomatic or asymptomatic[11,12]. Utilizing these techniques, we have shown the frequencies of various pathologies in symptomatic ankles with either early or established RA in a previous study (Table 3)[13]. This study revealed that ankle tenosynovitis is observed more frequently than ankle synovitis among early RA patients.
| Supine, with flexed knee, foot on the examination bed |
| Tibiotalar joint: anterior recess |
| Talonavicular joint |
| Subtalar joint: lateral and medial recess |
| Tendon compartments |
| Anterior: Tibialis anterior tendon |
| Extensor hallucis longus tendon |
| Extensor digitorum longus tendon |
| Lateral: Peroneus brevis tendon |
| Peroneus longus tendon |
| Medial (frog position): |
| Tibialis posterior tendon |
| Flexor digitorum longus tendon |
| Flexor hallucis longus tendon |
| Prone, with the foot hanging over the examination bed |
| Achilles tendon |
| Superficial and retrocalcaneal bursae |
| Subtalar joint: posterior recess |
| Early RA | Established RA | Overall | |
| Number of ankles | 62 | 38 | 100 |
| Joint synovitis | |||
| Talocrural joint synovitis | 32.2% | 39.5% | 35.0% |
| Subtalar joint synovitis | 30.7% | 36.8% | 33.0% |
| Talonavicular joint synovitis | 27.4% | 26.3% | 27.0% |
| Overall | 48.4% | 68.4% | 56.0% |
| Tenosynovitis | |||
| Ankle flexors (TP, FDL, FHL) | 54.8% | 31.6% | 46.0% |
| Peroneal tendons (PB, PL) | 33.9% | 21.1% | 29.0% |
| Ankle extensors (TA, EHL, EDL) | 12.9% | 5.3% | 10.0% |
| Overall | 69.4% | 47.4% | 61.0% |
| Achilles tendon involvement | |||
| Retrocalcaneal bursitis | 35.5% | 13.2% | 27.0% |
| AT enthesitis | 19.4% | 26.3% | 22.0% |
| AT tendonitis | 12.9% | 13.2% | 13.0% |
| AT paratenonitis | 8.1% | 2.6% | 6.0% |
| Overall | 38.7% | 39.5% | 39.0% |
The major inflammatory rheumatic diseases addressed in this paper are RA, psoriatic arthritis (PsA), reactive arthritis (ReA), undifferentiated spondyloarthritis (uSpA), and systemic lupus erythematosus (SLE). US assessment of crystal-associated diseases and degenerative disorders related to aging or trauma will be addressed elsewhere. Multiplanar scanning using both GSUS and PDUS should be carried out. Synovial fluid detected by GSUS and/or hyperemia detected in or adjacent to the synovial membrane by PDUS represents synovitis[14]. Although the degree of hyperemia is indicative of the extent of inflammation, hyperemia itself is not specific to a certain disease[15].
Synovial hypertrophy detected by GSUS represents cellular infiltration into synovial tissues with or without synovial cell proliferation. Fine and slow blood flow detected in thickened synovium by PDUS represents proliferative synovitis associated with vascularization, which is a hallmark of the active synovitis observed in RA, PsA, and sometimes SLE[16].
The respective features of the pathologies in inflammatory rheumatic disease depicted in each anatomical structure are addressed below.
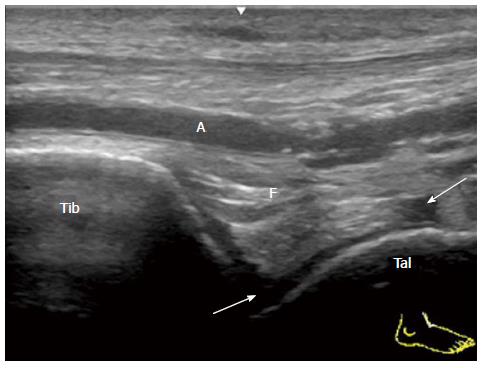
To visualize the anterior recess of the tibiotalar joint, place the transducer in the sagittal plane distal to the tibia. In the normal joint, a hyperechoic anterior fat pad can be seen to fill the space between the tibia and the talus anteriorly with a layer of anechoic hyaline cartilage on the surface of the talar dome[17].
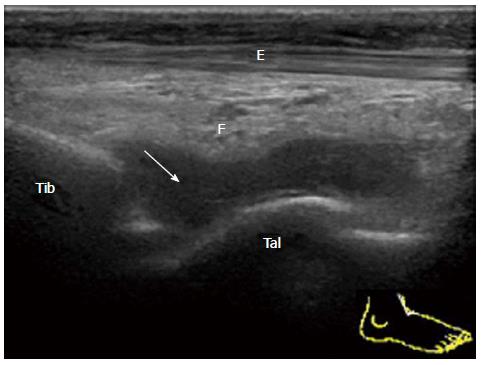
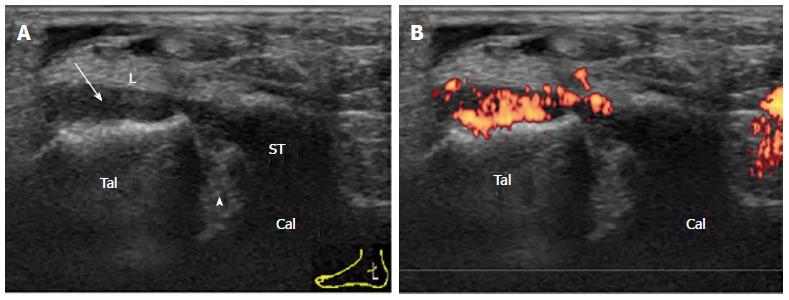
A small simple effusion in mild acute synovitis of the tibiotalar joint in a patient with reactive arthritis is depicted as an anechoic area (Figure 1). Severe proliferative synovitis of the tibiotalar joint in rheumatoid arthritis is depicted as a hypoechoic area over the talar dome (Figure 2). Large effusion and/or proliferative synovium lead to the displacement of the fat pad covering the talus neck and creating capsule distension.
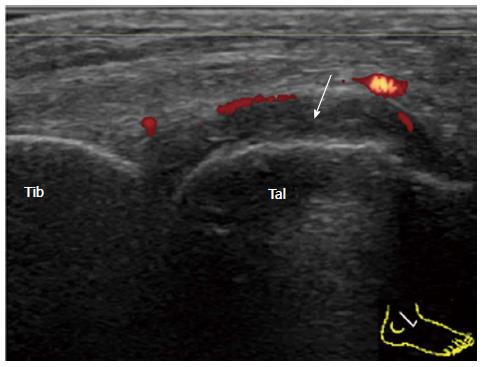
Complex joint fluid can be seen as a hypoechoic area. To distinguish synovium from complex joint fluid, blood flow detected by PD sonography is helpful[18]. However, PD sensitivity is relatively low at the median part of the anterior recess because the synovium is located deep beneath the tendons and the fat body. Strong blood flow in the dorsalis pedis artery also hinders the identification of the fine synovial vessels. In order to increase the sensitivity of the PD detection, scanning both lateral and medial sides of the anterior recess must be performed (Figure 3).
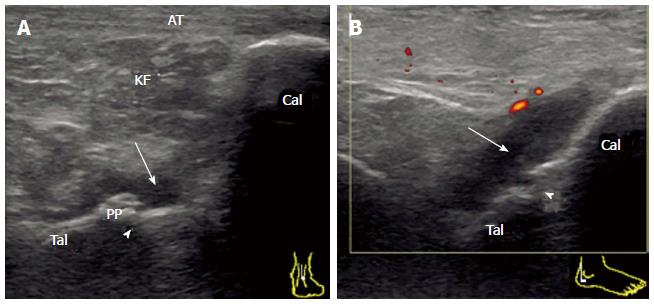
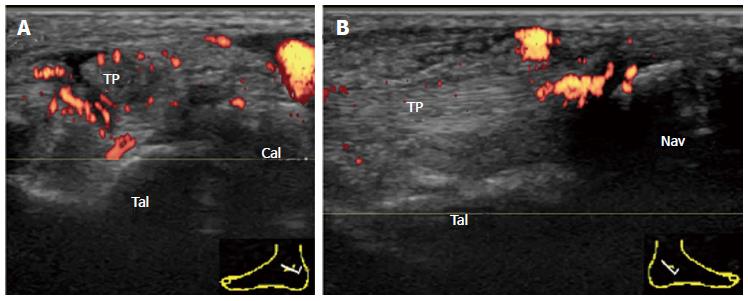
The subtalar or talocalcaneal joint is composed of three facets: a broad posterior facet representing the primary articulating surface, a medially located middle facet in which the sustentaculum tali articulates with the medial process of the talus, and an anterior facet. There are limited sonographic windows for imaging the subtalar joint (STJ), and the posterior subtalar joint (PSTJ) is usually the target. Synovitis of the PSTJ is best evaluated by scanning its posterior recess. The joint space of the PSTJ is easy to determine in the sagittal view crossing the posterior talar process. In order to fully visualize the posterior recess, which tends to distend laterally, the transducer should be placed just lateral to the Achilles tendon in an anatomic parasagittal plane by medially angling the transducer face (Figure 4). Because synovium is depicted as relatively shallow through this posterolateral window, better detection of the PD signal and a safer route for aspiration are provided[19].
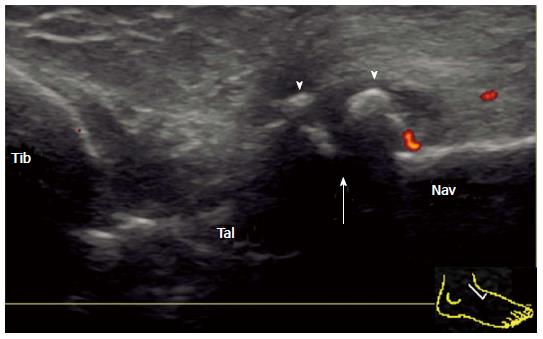
The medial facet of the PSTJ can be partly observed in the coronal view crossing the medial malleolus. In the normal joint, the joint space between the talus and the sustentaculum tali can be depicted beneath the tibiocalcaneal ligament. In the case of synovitis, effusion and/or proliferated synovium stretch cranially along the tibiocalcaneal ligament (Figure 5).
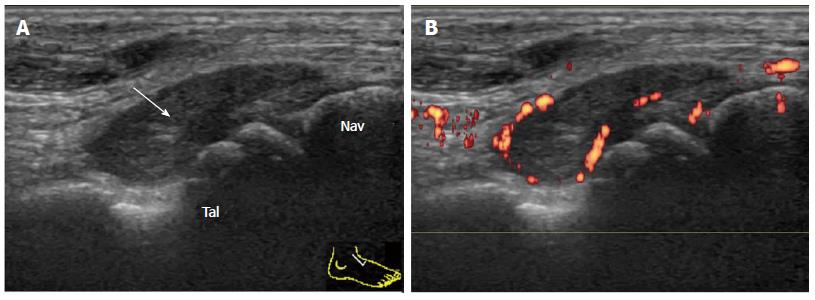
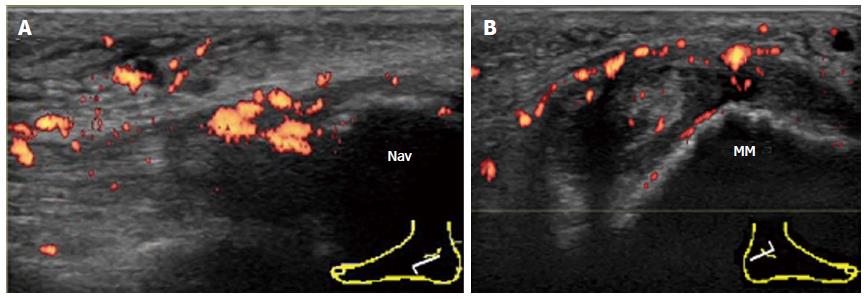
The talonavicular joint (TNJ) is best evaluated by the longitudinal dorsal view of the joint. In the case of TNJ synovitis in RA patients, proliferated synovium bulges out of the joint space dorsally and the PD signal can be easily detected (Figure 6).
It has been reported that TNJ synovitis is frequently detected by US in both symptomatic and asymptomatic ankles in RA patients[13]. Similarly, proliferative synovitis of the TNJ can arise in PsA patients (Figure 7).
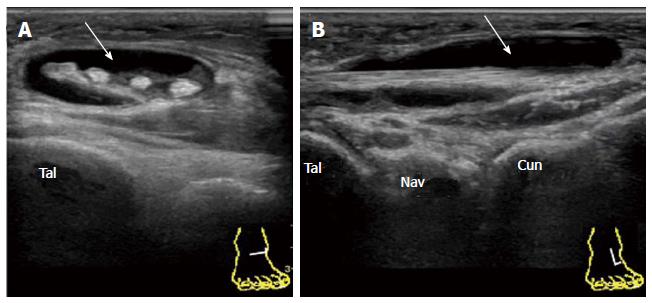
It has also been reported that TNJ synovitis is often detected by US in the ankles of healthy control subjects[20]. The ankle is one of the sites of predilection for osteoarthritis. In long-standing RA patients, degenerative changes in the TNJ are frequently encountered (Figure 8). PDUS can add to the information regarding the activity of ongoing inflammation in the joint.
The three tendons in the anterior aspect of the ankle from medial to lateral are the tibialis anterior (TA), extensor hallucis longus (EHL), and extensor digitorum longus (EDL)[17]. These extensor tendons pass beneath retinacula and have sheaths to protect them. The main pathology that occurs in inflammatory rheumatic diseases in this area is tenosynovitis. Tenosynovitis of the EDL is most frequently observed, followed by that of the TA. Tenosynovitis of the EHL seems to be less frequent while the myotendinous junction should not be confounded with dilated tenosynovium. These extensor tendons tend to present with serous tenosynovitis rather than proliferative tenosynovitis, unlike the medial flexors and lateral peroneal tendons, which frequently present with proliferative tenosynovitis (Figure 9)[21].
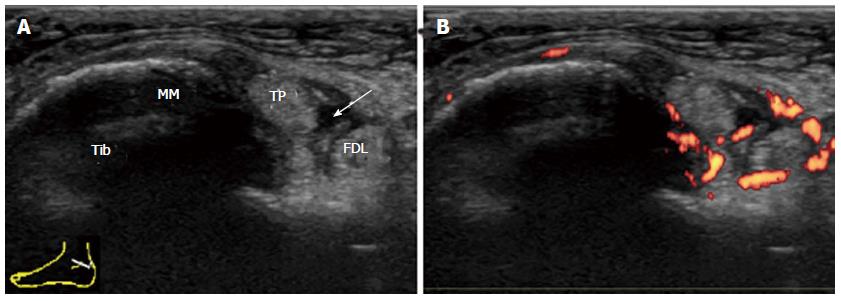
By transverse scanning of the medial ankle tendons, the tibialis posterior (TP) tendon, flexor digitorum longus (FDL) tendon, and flexor hallucis longus (FHL) tendon can be visualized from the anterior to the posterior side[22].
As the TP and FDL hook around the medial malleolus and the FHL runs in a groove along the calcaneus, all tendons have individual sheaths. Tenosynovitis frequently occurs in this area in inflammatory rheumatic diseases. Tenosynovitis of TP is most frequently observed, and is sometimes accompanied by FDL tenosynovitis (Figure 10). Pathology usually occurs at or distal to the medial malleolus. Fluid collection within the synovial sheath is predominantly observed near the distal end of the sheath, while small effusion often exists at the same site in the ankles of healthy subjects. Proliferative tenosynovitis of TP is frequently observed in RA patients[21]. Thickening and hyperemia of the sheath with or without fluid in it are the typical features. It has been reported that tenosynovitis is observed more frequently than joint synovitis in symptomatic ankles with early RA[13].
The second site of pathology on the TP is where it inserts into the navicular bone. A longitudinal scan is more informative in proximity to its insertion. An important active inflammatory pathology in rheumatic diseases at tendon insertion is enthesitis. Enthesitis frequently occurs in patients with SpA (Figure 11A). The distal end of a normal TP tendon splays at the insertion and may appear hypoechoic due to anisotropy. Therefore, the presence of intratendinous hyperemia permits a more confident diagnosis of enthesitis.
Although enthesitis is the hallmark of PsA, ankle tenosynovitis is also often observed in PsA patients. In some cases, the implication of enthesitis in a functional enthesis formed by fibrocartilage on the surface of bone and tendon is suggested (Figure 11B)[23].
Periarticular involvement is an important characteristic of musculoskeletal manifestation in SLE patients[24]. In particular, tendinopathies including tendonitis, tenosynovitis and tendon rupture are thought to be common. Although there have only been a few reports about the sonographic feature of tenosynovitis only in hands or wrists with SLE, ankle tendinopathies do not seem to be rare (Figure 12). The appearance of tenosynovitis or enthesitis, however, seems to be unspecific.
The lateral ankle tendons are the peroneus longus and brevis. They hook behind the retromalleolar sulcus of the fibula and are stabilized by the superior peroneal retinaculum. At the level of the lateral malleolus, the peroneus longus and brevis share a common sheath. Distal to the lateral malleolus, the peroneals diverge[22].
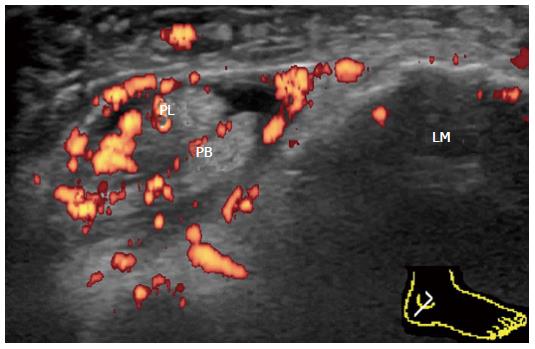
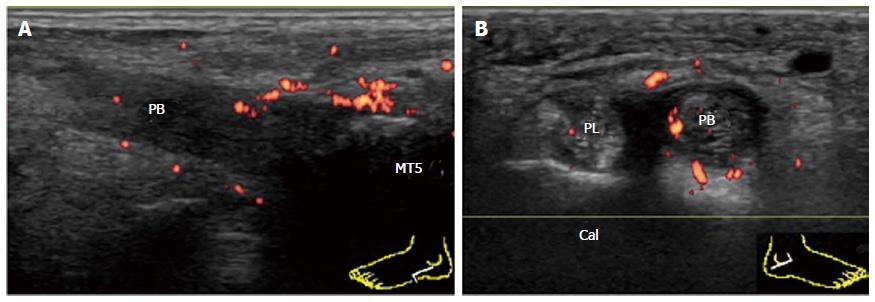
Pathology usually occurs at or distal to the lateral malleolus (Figure 13). Tenosynovitis of peroneus longus and brevis can occur either independently or concurrently. Proliferative tenosynovitis of peroneal tendons and TP is frequently observed in RA patients[21]. Thickening and hyperemia of the sheath with or without fluid in it are sonographic findings suggestive of active inflammation.
In addition to TP, enthesitis of PB at its insertion into the base of the fifth metatarsal is frequently observed in PsA patients (Figure 14A). Tenosynovitis of peroneal tendons is also commonly observed in PsA patients (Figure 14B).
The Achilles tendon is a typical tendon with a linear fibrillar pattern. It inserts into a relatively small footprint on the posterosuperior aspect of the calcaneus. Between the calcaneus and the Achilles tendon there is a retrocalcaneal bursa. A normal bursa is thin walled and contains only minimal fluid[25].
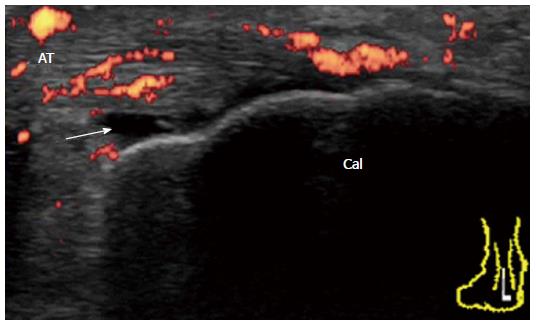
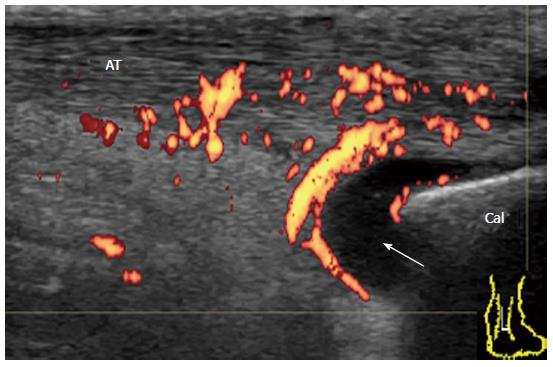
Achilles tendon enthesopathy is a common finding in SpA, and can be present in both symptomatic and asymptomatic patients[7]. In the OMERACT definition (Table 1), acute and chronic inflammatory aspects in gray-scale US (i.e., loss of normal echo structure, increased thickness, or focal calcific deposits) and Doppler US are combined with findings of structural damage (i.e., enthesophytes and bony erosions). This combination may be helpful for diagnostic purposes (i.e., presence or absence of enthesis involvement) but probably not for responsiveness or for the differential diagnosis of inflammatory diseases (i.e., SpA or RA versus mechanical or metabolic entheseal involvement). Enthesitis rather than enthesopathy is important to evaluate in inflammatory rheumatic diseases. For defining enthesitis, a Doppler signal should be detected at the cortical enthesis insertion and should be differentiated from a reflecting surface artifact or nutrition vessel signal[7]. A Doppler signal may be detected even in the absence of either bone changes (cortical irregularities, erosions, or enthesophytes) or tendon degeneration (tendinosis, tendon erosions and tears). For example, ReA patients with subacute enthesitis together with enthesial and intratendinous hyperemia and a small amount of fluid in retrocalcaneal bursa with minimal structural damage (Figure 15).
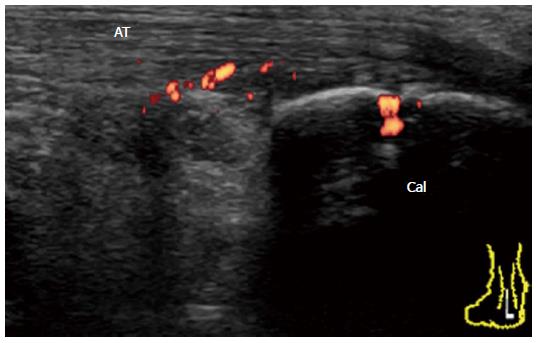
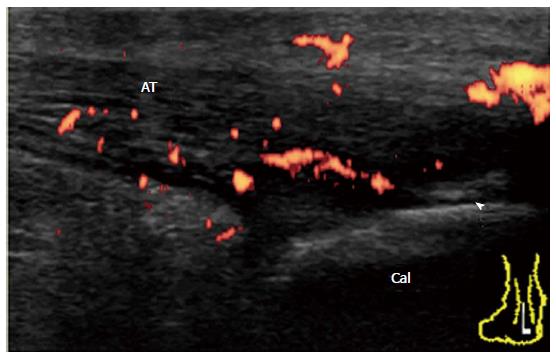
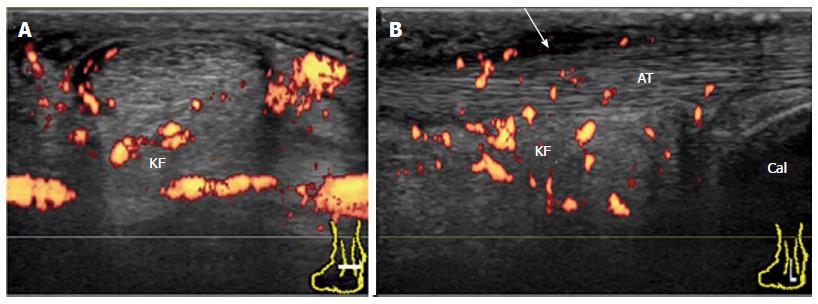
It has been suggested that inflammation at the bursal part of the fibrocartilage is important for the pathogenesis of enthesitis in SpA patients[26]. Bone erosion that is cranially adjacent to the insertion is characteristic of enthesitis in SpA. In early undifferentiated SpA patients, a Doppler signal at the enthesis insertion that is associated with cortical erosion can be observed with minimal tendinosis (Figure 16). In chronic active enthesitis, increased thickness, hypoechogenicity, and loss of fibrillar pattern of the Achilles tendon are associated with abnormal vascularization at the enthesis insertion with bone changes (Figure 17). Enthesitis is also often observed among RA patients. It has been suggested that retrocalcaneal bursa is primarily involved in the rheumatoid enthesitis symptom (Figure 18)[7].
The Achilles tendon does not have a tendon sheath. Instead, it has a U-shaped paratenon wrapping around the tendon from its dorsal aspect, which is normally barely visible. Achilles paratenonitis is inflammation in the paratenon and can occur in isolation or in association with tendinopathy. The paratenon becomes thickened, hypoechoic and hyperemic in the case of paratenonitis (Figure 19). Achilles paratenonitis is sometimes observed among RA patients, especially in the early stage of disease[13].
US is useful for identifying the location and kind of pathologies in rheumatic ankles. Furthermore, PDUS is useful for distinguishing between inflammatory processes and degenerative changes or between active inflammation and residual damage. PDUS is of vital use in the assessment of ankles in patients with inflammatory rheumatic diseases in daily practice.
P- Reviewer: Azzoni R S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Wakefield RJ, Freeston JE, O’Connor P, Reay N, Budgen A, Hensor EM, Helliwell PS, Emery P, Woodburn J. The optimal assessment of the rheumatoid arthritis hindfoot: a comparative study of clinical examination, ultrasound and high field MRI. Ann Rheum Dis. 2008;67:1678-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Szkudlarek M, Narvestad E, Klarlund M, Court-Payen M, Thomsen HS, Østergaard M. Ultrasonography of the metatarsophalangeal joints in rheumatoid arthritis: comparison with magnetic resonance imaging, conventional radiography, and clinical examination. Arthritis Rheum. 2004;50:2103-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 262] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 3. | Schmidt WA, Völker L, Zacher J, Schläfke M, Ruhnke M, Gromnica-Ihle E. Colour Doppler ultrasonography to detect pannus in knee joint synovitis. Clin Exp Rheumatol. 2000;18:439-444. [PubMed] |
| 4. | Walther M, Harms H, Krenn V, Radke S, Faehndrich TP, Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Wakefield RJ, Brown AK, O’Connor PJ, Emery P. Power Doppler sonography: improving disease activity assessment in inflammatory musculoskeletal disease. Arthritis Rheum. 2003;48:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Hammer HB, Kvien TK. Ultrasonography shows significant improvement in wrist and ankle tenosynovitis in rheumatoid arthritis patients treated with adalimumab. Scand J Rheumatol. 2011;40:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 357] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D’Agostino MA, Sanchez EN, Iagnocco A, Schmidt WA, Bruyn GA. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485-2487. [PubMed] |
| 9. | Torp-Pedersen ST, Terslev L. Settings and artefacts relevant in colour/power Doppler ultrasound in rheumatology. Ann Rheum Dis. 2008;67:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Micu MC, Nestorova R, Petranova T, Porta F, Radunovic G, Vlad V, Iagnocco A. Ultrasound of the ankle and foot in rheumatology. Med Ultrason. 2012;14:34-41. [PubMed] |
| 11. | Naredo E, Valor L, De la Torre I, Martínez-Barrio J, Hinojosa M, Aramburu F, Ovalles-Bonilla JG, Hernández D, Montoro M, González CM. Ultrasound joint inflammation in rheumatoid arthritis in clinical remission: how many and which joints should be assessed? Arthritis Care Res (Hoboken). 2013;65:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Freeston JE, Coates LC, Nam JL, Moverley AR, Hensor EM, Wakefield RJ, Emery P, Helliwell PS, Conaghan PG. Is there subclinical synovitis in early psoriatic arthritis? A clinical comparison with gray-scale and power Doppler ultrasound. Arthritis Care Res (Hoboken). 2014;66:432-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Suzuki T, Okamoto A. Ultrasound examination of symptomatic ankles in shorter-duration rheumatoid arthritis patients often reveals tenosynovitis. Clin Exp Rheumatol. 2013;31:281-284. [PubMed] |
| 14. | Brown AK, Wakefield RJ, Conaghan PG, Karim Z, O’Connor PJ, Emery P. New approaches to imaging early inflammatory arthritis. Clin Exp Rheumatol. 2004;22:S18-S25. [PubMed] |
| 15. | Hau M, Kneitz C, Tony HP, Keberle M, Jahns R, Jenett M. High resolution ultrasound detects a decrease in pannus vascularisation of small finger joints in patients with rheumatoid arthritis receiving treatment with soluble tumour necrosis factor alpha receptor (etanercept). Ann Rheum Dis. 2002;61:55-58. [PubMed] |
| 16. | Hau M, Schultz H, Tony HP, Keberle M, Jahns R, Haerten R, Jenett M. Evaluation of pannus and vascularization of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis by high-resolution ultrasound (multidimensional linear array). Arthritis Rheum. 1999;42:2303-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Fessell DP, Jamadar DA, Jacobson JA, Caoili EM, Dong Q, Pai SS, van H, Marnix T. Sonography of Dorsal Ankle and Foot Abnormalities. Am J Roentgenol. 2003;181:1573-1581. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Newman JS, Adler RS, Bude RO, Rubin JM. Detection of soft-tissue hyperemia: value of power Doppler sonography. AJR Am J Roentgenol. 1994;163:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 187] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Smith J, Finnoff JT, Henning PT, Turner NS. Accuracy of sonographically guided posterior subtalar joint injections: comparison of 3 techniques. J Ultrasound Med. 2009;28:1549-1557. [PubMed] |
| 20. | Sant’Ana Petterle G, Natour J, Rodrigues da Luz K, Soares Machado F, dos Santos MF, da Rocha Correa Fernandes A, Vilar Furtado RN. Usefulness of US to show subclinical joint abnormalities in asymptomatic feet of RA patients compared to healthy controls. Clin Exp Rheumatol. 2013;31:904-912. [PubMed] |
| 21. | Suzuki T, Tohda E, Ishihara K. Power Doppler ultrasonography of symptomatic rheumatoid arthritis ankles revealed a positive association between tenosynovitis and rheumatoid factor. Mod Rheumatol. 2009;19:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | De Maeseneer M, Marcelis S, Jager T, Shahabpour M, Van Roy P, Weaver J, Jacobson JA. Sonography of the normal ankle: a target approach using skeletal reference points. AJR Am J Roentgenol. 2009;192:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503-526. [PubMed] |
| 24. | Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol. 2009;23:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Riente L, Delle Sedie A, Iagnocco A, Filippucci E, Meenagh G, Valesini G, Grassi W, Bombardieri S. Ultrasound imaging for the rheumatologist V. Ultrasonography of the ankle and foot. Clin Exp Rheumatol. 2006;24:493-498. [PubMed] |
| 26. | Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61:905-910. [PubMed] |