Published online Sep 18, 2014. doi: 10.5312/wjo.v5.i4.550
Revised: June 14, 2014
Accepted: June 20, 2014
Published online: September 18, 2014
Processing time: 176 Days and 23.7 Hours
AIM: To investigate donor site’s area histological and immunohistochemical knee cartilage appearances after resurfacing iatrogenic defects with biosynthetic plugs orautografts.
METHODS: Thirty New Zealand White rabbits were used in this study. A full-thickness cylindrical defect of 4.5 mm (diameter) × 7 mm (depth) was created with a hand drill in the femoral groove of every animal. In Group A (n = 10) the defect of the donor site was repaired with a biosynthetic osteochondral plug, in Group B (n = 10) with an osteochondral autograft, while in Group C (control group of 10) rabbits were left untreated.
RESULTS: Twenty-four weeks postoperatively, smooth articular cartilage was found macroscopically in some trocleas’ surfaces; in all others, an articular surface with discontinuities was observed. Twenty-eight out of 30 animals were found with predominantly viable chondrocytes leaving the remaining two -which were found only in the control group- with partially viable chondrocytes. However, histology revealed many statistical differences between the groups as far as the International Cartilage Repair Society (ICRS) categories are concerned. Immunofluoresence also revealed the presence of collagen II in all specimens of Group B, whereas in Group A collagen II was found in less specimens. In Group C collagen IIwas not found.
CONCLUSION: The matrix, cell distribution, subchondral bone and cartilage mineralization ICRS categories showed statistically differences between the three groups. Group A was second, while group B received the best scores; the control group got the worst ICRS scores in these categories. So, the donor site area, when repairing osteochondral lesions with autografting systems, is better amended with osteochondral autograft rather than bone graft substitute implant.
Core tip: The donor site during the autografting process or during synthetic plugging when dealing with osteochondral defects is usually not well evaluated or addressed. This is an innovative original article, in which the donor site is repaired with autografts or synthetic plugs and after 24 wk it is histologically and immunohistochemically evaluated and compared.
- Citation: Intzoglou KS, Mastrokalos DS, Korres DS, Papaparaskeva K, Koulalis D, Babis GC. Donor’s site evaluation after restoration with autografts or synthetic plugs in rabbits. World J Orthop 2014; 5(4): 550-556
- URL: https://www.wjgnet.com/2218-5836/full/v5/i4/550.htm
- DOI: https://dx.doi.org/10.5312/wjo.v5.i4.550
Many different methods have been documented in literature, trying to restore articular cartilage. In the 1950’s, the first attempts were made to restore articular cartilage using skin. Even though since then, much progress has been made[1-6], there is still lacking evidence in how to best manage these lesions. The avascular and denervated environment, the immobility of chondrocytes and the limited ability of mature chondrocytes to proliferate, are considered as the main reasons that obstruct intrinsic articular regeneration[7-9].
An abundance of surgical techniques, each with their advantages and disadvantages, have been developed in order to repair articular cartilage defects. The choice of treatment usually depends on the surgeon’s preference and the size of the lesion. The current treatments include: (1) palliative (debridement), marrow stimulating techniques (microfractures, drilling, abrasion) and transfer of osteochondral unit (autograft or allograft) for lesions up to 2.5 cm2; and (2) for lesions bigger than 2.5 cm2 , use of chondrogenic potential cells (autologous chondrocyte implantation) and osteochondral allografts[10].
Increasing interest appears in one-time surgery techniques as they provide shorter recovery time, with the transfer of an entire osteochondral autograft being the most appealing[2-6,9,11]. Autografting represents a reasonable solution for osteochondral defects. In the most widespread system of mosaicplasty, an osteochondral cylindrical graft is received from a healthy region (donor site) and after appropriate preparation of the osteochondral defect region with special instrumentation, it is inserted in the recipient site (osteochondral defect). The donor site, depending on the surgeon’s preference remains uncovered, is covered with biosynthetic implant or is covered with the autograft been taken from the defect region.
To date, the histological faith of the donor site remains unanswered. No consensus exists regarding the best approach to achieve optimal results[4-6,9,12]. The donor site, could be a major source of pain. Nonetheless, it is underestimated because all surgeons and all reports focus on the damaged area. We performed a controlled laboratory animal study in order to find an answer about the faith of the donor site region. In order to identify the method which achieves the best outcomes for the International Cartilage Repair Society (ICRS) scoring system, and which ends up expressing more collagen of type II(marking the presence of hyaline cartilage) we used a rabbit model[13] where the same standardized cartilage defect was treated with three different options; bone graft substitute (BGS) plug, osteochondral autograft transplantation and conservative approach. Our null hypothesis was that there would be no significant difference between the control, the OA and the BGS group. P value < 0.05 was deemed to indicate statistical significance.
The following controlled laboratory study was approved by the local veterinarian department and the scientific committee of our University’s laboratory. Thirty male, New Zealand White rabbits, which were 3 mo old and had a mean weight of 3.8 kg (range, 3.5 to 4 kg) were used in this study. Under moderate sedation we administrated IV ketamine to achieve general anesthesia according to a previously published protocol[14]. The rabbits were placed in the supine position and have had their right leg shaved and sterilized. A 4-cm medial parapatellar arthrotomy was made and the patella was dislocated laterally. Thus, the femoral condyles and groove were exposed. The region of the femoral groove, which contacted with the patella when the knee was flexed at 90o, was selected as the site for the osteochondral defect[15]. A full thickness osteochondral defect, using the smallest instrument of the provider (Smith and Nephew, Memphis, Tennessee) was made using a hand drill, leading to a defect of 4.5 mm in diameter and 7 mm depth. The defect was then carefully debrided of any cartilaginous remnants and was dilated with a 5 mm dilator, at the same time the bottom of the defect was flattened under the slight load of the dilator. During the drilling process, the depth was checked continuously for an accurate depth of 7 mm. All implants were inserted press-fit without any additional fixation or glue. The rabbits were divided into 3 groups (10 per group) depending on the treatment method used for cartilage repair, In group A, the defect was reconstructed using the Smith and Nephew’s TruFit (Memphis, Tennessee) BGS (Bone Graft Substitute) plug of 5 mm in diameter and of 7 mm height, which is a composite material of polylactide-co-glycolide, calcium sulfate and polyglycolide fibers. Special attention was paid in order to apply the graft equally to the host cartilage surface, following the specific guidelines of the manufacturer. In group B, an osteochondral autograft (OA), 5 mm in diameter and of 7 mm height, harvested from an area above the recipient site of the same femoral groove was used to restore the cartilage lesion[5,9], following the “mosaicplasty” surgical technique initially described by Hangody et al[16]. The donor area was selected in a manner that a minimum critical space of 3 mm was left between the host and the donor area. The region of the donor site of this group had had its cartilage disrupted and destroyed with a curette before harvesting. This was done in order to mimic the unhealthy cartilage seen when osteochondral autograft transfer system (OATS) is performed. In the control group (group C) the defect was left untreated.
The capsule was closed with simple interrupted sutures (3-0 absorbable), followed by skin closure with a running subcuticular suture (4-0 absorbable). All rabbits underwent a perioperative course of enterofloxin, with post-operative pain control using aspirin. All surgical procedures were made by the same surgeon.
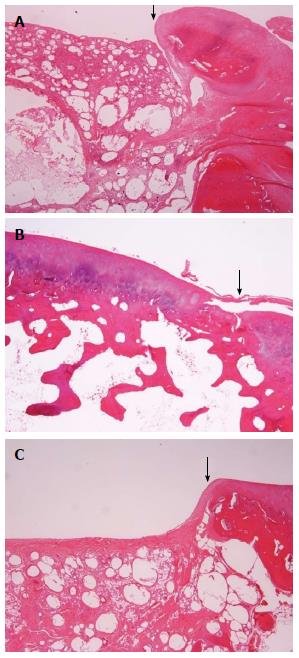
All evaluations were made by the same assessor, who was blind to the procedure used in each specimen. After 24 wk the animals were sacrificed using a lethal dose of phenobarbital IV injection under general anesthesia, which was achieved using the protocol of the surgery[4,14]. The type and degree of integration of reparative tissue were evaluated. Joint surfaces were grossly examined (Figure 1). Repair tissue was assessed macroscopically according to the ICRS recommendations[7]. ICRS Visual Histologic Assessment Scale is used to histologically evaluate the repaired lesions[18,19]. For macroscopic assessment 3 main characteristics are evaluated: (1) the defect depth compared with the surrounding cartilage (control group) and the survival of the initially grafted surface (BGS plug and the autograft); (2) the integration of the repaired tissue to the border zone (size of the gap); and (3) the macroscopic appearance of the repair tissue surface (smooth, fissured, degenerated, etc.).
After macroscopic evaluation distal thirds of all femurs were removed and placed for 2 d in 10% neutral buffered formalin. Specimens were decalcified and followed the routine procedure of dehydration of tissues and were embedded in paraffin. 1.5 μm thickness sections were stained for hematoxylin and eosin stain (H and E). The histochemical stains (Toluidine Blue and Wan-Giesson) as well as immunohistostaining with antibodies against collagen type II (Dako A/S, Denmark) were performed, as long as the extend of collagen type II in cartilage is considered a marker of degree of differentiation towards hyaline cartilage[12,18-20]. Each H and E sample took a score according to the ICRS scoring system[7,18] (Table 1). A total of 30 knees underwent histological evaluation.
| BGS group | OA group | Control group | |
| Surface (0,3) | 1.2 ± 1.55 | 1.5 ± 1.58 | 0.9 ± 1.45 |
| Matrix (0-3) | 1.6 ± 1.35 | 3 | 0.7 ± 0.67 |
| Cell distribution (0-3) | 0.7 ± 0.82 | 2 | 0.1 ± 0.32 |
| Cell viability (0,1,3) | 3 | 3 | 2.6 ± 0.84 |
| Subchondral bone (0-3) | 1.4 ± 0.84 | 2 | 0.9 ± 1.19 |
| Cartilage mineralization (0,3) | 1.2 ± 1.55 | 3 | 0.6 ± 1.26 |
Data were analyzed using SPSS ver.16. Histological grading scores were analyzed statistically using the Kruskal-Wallis and the Mann-Whitney test. Categorical variables (surface and cartilage mineralization) were analyzed using the χ2 test.
Twenty-four weeks after surgery the rabbits were sacrificed and the repaired site was examined macroscopically, histologically and immunohistochemically for an overall assessment, of whether bone graft substitute is better graft than the autograft. All rabbits were left free to move in their cages postoperatively. No evidence of postoperative infection at the wound site was observed, and all wounds healed uneventfully. No rabbit died before the scheduled sacrifice time.
In group A, the grafted areas were well recognized with distinct margins and a reddish appearance. The surface was opaque, almost smooth and seemed well incorporated with the surrounding, healthy cartilage. The BGS plug was even with the host articular surface (Figure 1A). In group B, femurs the margins between the host and the repaired tissue were not easily discerned. The grafted area had a light yellow, smooth, continuous surface (Figure 1B). In group C, the defects sites seemed to be filled with white to reddish, soft, irregular tissue. This repair tissue almost filled the defects, which were grossly distinguishable from the surrounding tissue and had irregular surfaces (Figure 1C). No apparent synovitis and no degenerative changes on the opposing articular surfaces in either the tibiofemoral or patellofemoral joints were observed in any of the three groups.
The mean scores of each group for each ICRS category are summarized in Table 1. Immunohistostain evaluation with collagen type II antibodies can be seen in Table 2. Significant differences were found between the groups for the ICRS categories of matrix, cell distribution and subchondral bone (Table 3). Furthermore, there was significant difference in the mineralization category (Table 3). No significant difference was found between the three groups for the surface and viability ICRS categories.
| Presence of collagen II | Presence of collagen II | Presence of collagen II | |||
| Α1 | Yes | Β1 | Yes | C1 | No |
| Α2 | No | Β2 | Yes | C2 | No |
| Α3 | Yes | Β3 | Yes | C3 | No |
| Α4 | No | Β4 | Yes | C4 | No |
| Α5 | No | Β5 | Yes | C5 | Yes |
| Α6 | Yes | Β6 | Yes | C6 | No |
| Α7 | Yes | Β7 | Yes | C7 | No |
| Α8 | Yes | Β8 | Yes | C8 | No |
| A9 | No | Β9 | Yes | C9 | No |
| A10 | Yes | Β10 | Yes | C10 | No |
Significant difference was found in the ICRS categories matrix, cell distribution, and subchondral bone between the OA and BGS group, with the OA group showing much improved healing. Comparing the OA and the control group significant differences were also found in the same ICRS categories; the OA got better outcome measures than the control group. In the comparison between the BGS and the control group significant difference was observed only in the cell distribution category, with the BGS group receiving a better score.
Utilizing the χ2 test in order to make the same three comparisons (group A vs group B, the A vs the control group and the B vs the control group) no significant difference was noted as far as the surface category is concerned. No significant difference was observed when comparing all three groups per two for the cell viability category, as well (Table 3).
A lot of basic research in articular cartilage repair has been conducted[16,21,22]. The purpose of this study was to evaluate and compare three options when restoring osteochondral defects with OATS (mosaicplasty) in a rabbit model. We used an animal model, which underwent repair of an osteochondral lesion, created by a hand drill, with specified diameter and depth. It is known that cartilage defects smaller than 4 mm tend to heal spontaneously into cartilage in the rabbit model[23]. In order to prevent spontaneous healing, we designed a protocol which included a larger defect. With an exact-fit plug of prespecified measurements, but different composition (OA or BGS) we repaired the site, or left it without intervening (control group). The patella, tibial plateau and menisci did not show any increased degenerative changes as a result of articulating against the donor or recipient sites of the osteochondral grafts a finding which comes in agreement with the Lane et al[5] findings. Our transplanted grafts had a similar gross macroscopic appearance at the sacrifice time as the one 24 wk before; an observation stated by Lane et al[5], though for a shorter period of time (12 wk). We managed to successfully transfer an osteochondral plug with maintenance of cellular viability which was also recorded by Lane et al[5] in the goat model 12 wk post-operatevely.
The underlying subchondral bone appeared to undergo a routine fracture healing, while the superficial cartilage layer appeared to interdigitate with the host cartilage. Lane et al[5] stated the presence of a cleft between the host and the transplanted cartilage in the goat model 12 wk post-operatevely, while Nam et al[9] in the rabbit model found that the interdigitation of the cartilage is not in all sides of the chondral part 12 wk post-operatevely. Nakaji et al[24] report a fracture healing process after the implantation of the graft with “improvement of the continuity of the articular cartilage surface after the 12th week, which is then almost as normal”. Makino et al[6] reported full embedding of the autograft in the rabbit model 24 wk post-operatively; an observation which comes in agreement with our findings.
In group B, we noticed subchondral bone healing with excellent trabecular interdigitation with the host bone, something that has been published before by Lane et al[5] and Nam et al[9]. We did not notice any difference on the thickness of the chondral part of the graft, compared to the host cartilage. It is well known that as the cartilage matures it becomes thinner and the number of cells decreases[25,26]. Makino et al[15] reported an increase of the thickness of the chondral part of the graft 24 wk post-operatevely, when - due to their technique- the implanted graft was slightly undersized of the host lesion. When they used slightly oversized graft they did not notice any thickness differences. It can be assumed that the initial stability of the graft was obtained because the size of the graft was slightly larger than the created defect. It should be pointed out that our donor site has less thickness cartilage. Thus, a stable graft changes its biomechanical properties in order to meet the loading needs of the area that it is transplanted to. Consequently, the graft is adapted to the biomechanical properties of the host cartilage.
The quality of the healing response of the control group was not good. The defect was clearly different from the surrounding cartilage, had a white to red-brownish appearance, was softer in palpation and had irregular surface. This observation comes in agreement with previously published data[9,15]. The host surrounding cartilage maintained its normal structure 24 wk post-operatively, something stated by Nam et al[9] (12 wk post-op), Lane et al[5] (12 wk post-op) and Makino et al[6,15] (12 and 24 wk post-op) also.
The articular surface 24 wk post-operatively presented with no significant difference between the three groups, as far the smoothness is concerned. Thus, it has been clearly shown that the congruity of the articular surface can be preserved excellently if the plug is perfectly grafted to the defect. However, the histological examination of the repaired site revealed differences between the three groups. The ICRS categories of matrix, cell distribution, subchondral bone and mineralization had significant differences, with the group B receiving the best scores, group A being second and the control group getting the worst ICRS scores in these categories. The absence of significant difference when comparing the three groups, as far as the surface category and the cell viability are concerned, states that the good macroscopic appearance of the repaired site and the viability of the graft do not imply an equally histologically and immunochemically healthy graft.
This study has provided some new data and insights but also has some limitations. One limitation concerns the rabbit model. It is not an entirely suitable animal model to study articular cartilage repair procedures in preclinical studies. Hunziker noted that “the matrix domain sustained and remodeled by an individual cellular unit is, in the human, approximately 8 to 10 times larger than that in the rabbit”[27]. It likely would lead to substantial enhancement in the rabbit to maintain surrounding cartilage compared to the human. Nevertheless, the rabbit is probably the most often used model for economic reasons and the literature contains interpretations based on rabbit data. Although we believe our rabbit model represents the clinical situation, cartilage repair procedures using this model should still be interpreted with caution before proceeding to clinical studies and conclusions.
Another limitation is that the defects were located on the trochlear of the rabbits. The trochlear were selected in order for us to have the critical space to create a 5 mm cylindrical defect. The patellofemoral joints of rabbits have some degree of compressive force because the rabbit knees are always in the flexed position. However, no direct weight bearing appears in the patellofemoral joint. To find animal knees that resemble the human knee in terms of biomechanics, we would have to use bigger experimental animals. Also, when autografting our animal model suggests an osteochondral repair with an autograft that has been iatrogenically destroyed, whereas in clinical practice the defect from the donor site (which actually is the recipient site of the healthy osteochondral graft) is degenerative most of the times.
Previous studies report no degeneration of the grafted autologous osteochondral graft in 12 wk[9]. We saw no degeneration of the graft in 24 wk either, an observation that agrees with previous reports for other experimental animals in the literature[4,6]. Further investigation concerning the histological, mechanical and immunohistochemical properties of grafted cartilage needs to be done to verify the longer effects of OA and BGS transplantation, bearing always in mind that in the clinical situation a transplanted knee with osteochondral problems continues to improve even after 6 mo post-operatively.
In a conclusion, we repaired full-thickness defects with three different ways. We compared the results per two, showing that outcomes of the OA graft were significantly better than those reported with the BGS, which in turn was significantly better than the control group, shooting this way down our initial hypothesis. Therefore, the donor site in mosaicplasty technique is better amended with osteochondral autograft rather than with BGS implants. Choice of procedure lies upon donor size morbidity, lesion’s size, quality and viability of the present cartilage, knee’s overall evaluation (possible meniscal lesions, rupture of ACL, etc.) and surgeon’s preference. Other strategies as well are under investigation, that deserve our attention and more thorough experimental studies[28,29].
The current experimental work took place in the Laboratory for Research of the Musculoskeletal System, University of Athens, KAT Hospital, Maroussi, 14561, Athens.
Autografting is a well known option as far as resurfacing osteochondral defects is concerned. Usually the donor site when not left empty, is covered with an autograft or a biosynthetic plug. The donor site area, though a possible source of postsurgical pain, is not well investigated.
There are controversial publications in the area. Everybody agrees about the fate of the autografts (inferior than hyaline cartilage) but the fate of the synthetic plugs is not universally agreed. Depending on the post-surgery time and on the publication, synthetic plugs appear in the literature with hyaline-like cartilage to fibrous tissue.
This is the first study to the knowledge that deals with the donor site area of the autografting procedure.
This study proves that the donor site area is better amended with autografts than synthetic plugs. So, when available, an autograft-though its surface injured- could be used for the donor site area.
ICRS score: International Cartilage Repair Society scoring system, which evaluates and scores cartilage appearences in the microscope. BGS: Biosynthetic Graft Substitute.
This is an excellent study evaluating 2 different methods of repairing the donor site following knee cartilage resurfacing. The authors concluded that the donor site in mosaicplasty technique is better amended with osteochondral autograft rather than with BGS implants. The manuscript is well written and easy to follow.
P- Reviewer: Lykissas MG S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Hunter W. On the structure and diseases of articulating cartilages. Trans R Soc Lond. 1743;42B:514-521. |
| 2. | Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 284] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33:443-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Bardos T, Farkas B, Mezes B, Vancsodi J, Kvell K, Czompoly T, Nemeth P, Bellyei A, Illes T. Osteochondral integration of multiply incised pure cartilage allograft: repair method of focal chondral defects in a porcine model. Am J Sports Med. 2009;37 Suppl 1:50S-57S. [PubMed] |
| 5. | Lane JG, Tontz Jr WL, Ball ST, Massie JB, Chen AC, Bae WC, Amiel ME, Sah RL, Amiel D. A morphologic, biochemical, and biomechanical assessment of short-term effects of osteochondral autograft plug transfer in an animal model. Arthroscopy. 2001;8:856-863. [RCA] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Makino T, Fujioka H, Kurosaka M, Matsui N, Yoshihara H, Tsunoda M, Mizuno K. Histologic analysis of the implanted cartilage in an exact-fit osteochondral transplantation model. Arthroscopy. 2001;17:747-751. [PubMed] |
| 7. | Brittberg M, Aglietti T, Gambardella R. ICRS cartilage injury evaluation package. 2012; Available from: http://www.cartilage.org/_files/contentmanagement/ICRS_evaluation.pdf. Accessed November 28th. |
| 8. | Mandelbaum BR, Browne JE, Fu F, Micheli L, Mosely JB, Erggelet C, Minas T, Peterson L. Articular cartilage lesions of the knee. Am J Sports Med. 1998;26:853-861. [PubMed] |
| 9. | Nam EK, Makhsous M, Koh J, Bowen M, Nuber G, Zhang LQ. Biomechanical and histological evaluation of osteochondral transplantation in a rabbit model. Am J Sports Med. 2004;32:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Panseri S, Russo A, Cunha C, Bondi A, Di Martino A, Patella S, Kon E. Osteochondral tissue engineering approaches for articular cartilage and subchondral bone regeneration. Knee Surg Sports Traumatol Arthrosc. 2012;20:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Bekkers JE, Inklaar M, Saris DB. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37 Suppl 1:148S-155S. [PubMed] |
| 12. | Roberts S, Menage J, Sandell LJ, Evans EH, Richardson JB. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee. 2009;16:398-404. [PubMed] |
| 13. | Hurtig M, Buschmann M, Fortier L. Preclinical Studies for Cartilage Repair. Recommendations from the International Cartilage Repair Society. Cartilage. 2011;2:p137-152. |
| 14. | Borkowski R, Karas AZ. Sedation and anesthesia of pet rabbits. Clin Tech Small Anim Pract. 1999;14:44-49. [PubMed] |
| 15. | Makino T, Fujioka H, Terukina M, Yoshiya S, Matsui N, Kurosaka M. The effect of graft sizing on osteochondral transplantation. Arthroscopy. 2004;20:837-840. [PubMed] |
| 16. | Hangody L, Kish G, Kárpáti Z, Szerb I, Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 303] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Koulalis D, Schultz W, Heyden M, König F. Autologous osteochondral grafts in the treatment of cartilage defects of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2004;12:329-334. [PubMed] |
| 18. | Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85-A Suppl 2:45-57. [PubMed] |
| 19. | Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38:880-890. [PubMed] |
| 20. | Moriya T, Wada Y, Watanabe A, Sasho T, Nakagawa K, Mainil-Varlet P, Moriya H. Evaluation of reparative cartilage after autologous chondrocyte implantation for osteochondritis dissecans: histology, biochemistry, and MR imaging. J Orthop Sci. 2007;12:265-273. [PubMed] |
| 21. | Outerbridge HK, Outerbridge AR, Outerbridge RE. The use of a lateral patellar autologous graft for the repair of a large osteochondral defect in the knee. J Bone Joint Surg Am. 1995;77:65-72. [PubMed] |
| 22. | Pearce SG, Hurtig MB, Clarnette R, Kalra M, Cowan B, Miniaci A. An investigation of 2 techniques for optimizing joint surface congruency using multiple cylindrical osteochondral autografts. Arthroscopy. 2001;17:50-55. [PubMed] |
| 23. | Lietman SA, Miyamoto S, Brown PR, Inoue N, Reddi AH. The temporal sequence of spontaneous repair of osteochondral defects in the knees of rabbits is dependent on the geometry of the defect. J Bone Joint Surg Br. 2002;84:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Nakaji N, Fujioka H, Nagura I, Kokubu T, Makino T, Sakai H, Kuroda R, Doita M, Kurosaka M. The structural properties of an osteochondral cylinder graft-recipient construct on autologous osteochondral transplantation. Arthroscopy. 2006;22:422-427. [PubMed] |
| 25. | Mankin HJ, Mow VC, Buckwalter JA. Form and function of articular cartilage. In Simon SR, ed. Orthopaedic basic science. Chicago: American Academy of Orthopaedic Surgeons 1994; 1-44. |
| 26. | Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185-192. [PubMed] |
| 27. | Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilag. 2007;15:403-413. |
| 28. | Chen H, Yang X, Liao Y, Zeng X, Liang P, Kang N, Tan J, Liang Z. MRI and histologic analysis of collagen type II sponge on repairing the cartilage defects of rabbit knee joints. J Biomed Mater Res B Appl Biomater. 2011;96:267-275. [PubMed] |
| 29. | Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007;23:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |