Published online Sep 18, 2014. doi: 10.5312/wjo.v5.i4.504
Revised: March 26, 2014
Accepted: May 31, 2014
Published online: September 18, 2014
Processing time: 234 Days and 10.2 Hours
Tofacitinib is the first in a new class of nonbiologic disease-modifying antirheumatic drugs (DMARDs), a targeted, synthetic DMARD, approved for the treatment of rheumatoid arthritis (RA) as monotherapy or in combination with methotrexate or other non-biologic DMARD. Tofacitinib, an orally administered Janus kinase (JAK) inhibitor, decreases T-cell activation, pro-inflammatory cytokine production, and cytokine signaling by inhibiting binding of type I cytokine receptors family and γ-chain cytokines to paired JAK1/JAK3 receptors. The net effect of tofacitinb’s mechanism of action is decreased synovial inflammation and structural joint damage in RA patients. To date, six phase 3 trials have been conducted to evaluate the safety and efficacy of tofacitinib under the oral rheumatoid arthritis triaLs (ORAL) series. This review describes the pharmacology of the novel agent, tofacitinib, and details the safety and efficacy data of the ORAL trials.
Core tip: Tofacitinib, a Janus kinase inhibitor, is a targeted, synthetic, disease-modifying antirheumatic drug (DMARD) approved for the treatment of moderately to severely active rheumatoid arthritis in patients who have had an inadequate response to methotrexate. In numerous phase 2 and 3 trials, tofacitinib has proven to be safe and effective as monotherapy or in combination with methotrexate or other non-biologic DMARDs.
- Citation: Lundquist LM, Cole SW, Sikes ML. Efficacy and safety of tofacitinib for treatment of rheumatoid arthritis. World J Orthop 2014; 5(4): 504-511
- URL: https://www.wjgnet.com/2218-5836/full/v5/i4/504.htm
- DOI: https://dx.doi.org/10.5312/wjo.v5.i4.504
The 2012 American College of Rheumatology (ACR) guidelines on management of rheumatoid arthritis (RA) recommends the use of disease-modifying anti-rheumatic drugs (DMARDs) in early RA of less than six months duration as monotherapy for patients with low disease activity and combination therapy for moderate or high disease activity[1]. They also recommend the use of anti-tumor necrosis factor (TNF) alpha biologic DMARDs with or without methotrexate for early RA with high disease activity and poor prognostic factors. Approved biologic DMARDs include cytokine inhibitors of TNF alpha (adalimumab, etanercept, infliximab, certolizumab pegol, golimumab), interleukin-6 (IL-6) receptor (tocilizumab), and interleukin-1 receptor (anakinra); cell depleting agent targeting of CD20 of B cells (rituximab); and costimulation blocker of cytotoxic T lymphocyte antigen 4 (abatacept). Limitations of biologic DMARDs, which require parenteral administration (intravenous or subcutaneous), has necessitated the development of orally effective treatment options for RA. Although, the European Medicines Agency has twice refused the marketing authorization for tofacitinib based on major concerns of the overall safety profile, tofacitinib, a Janus kinase (JAK) inhibitor, is the first oral non-biologic DMARD approved by the United States Food and Drug Administration in more than a decade[2].
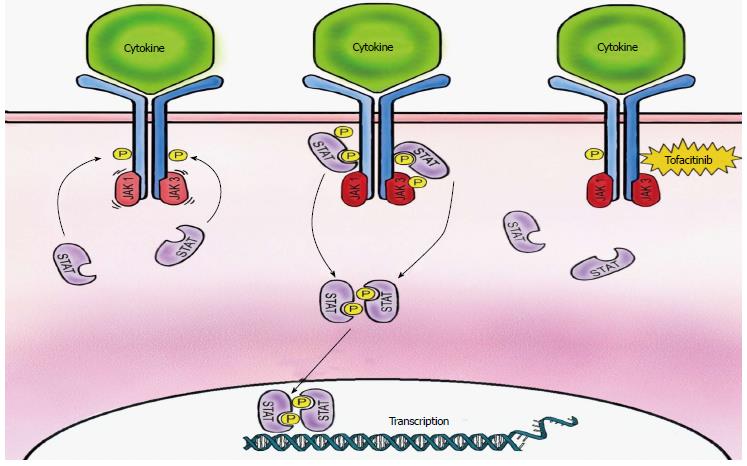
Cytokine signaling, pro-inflammatory cytokine production and immune cell activation are key functions of activated JAK in the perpetuation of autoimmune inflammatory disease[3]. The JAK family, JAK1, JAK 2, JAK 3 and Tyk2, are nonreceptor tyrosine kinases with a variety of intercellular domains, a pseudokinase domain, and SH2- and FERM domains[4]. Binding of cytokines to paired JAK receptors (JAK1/JAK3, JAK1/JAK2, JAK1/Tyk2, JAK2/JAK2) induces autophosphorylation, phosphorylation of tyrosine residues on the cytokine receptor, and phosphorylation with subsequent activation of various signal transducer and activator of transcription (STAT) molecules. This leads to increased JAK activity, further recruitment of cytokines, and changes in gene expression through JAK-STAT pathway. The synovium of RA patients has increased expression of the JAK-STAT pathway[3]. JAK1 and JAK2 play a role in growth, neurodevelopment, hematopoiesis, and host defense while JAK3 and Tyk2 are engaged in immune responses. Tofacitinib is a pan JAK inhibitor with potent inhibition of JAK3 and JAK1 and to a minor degree JAK2. JAK3 binds to the common IL-2Rγ chain of the type I cytokine receptor family (IL-2, IL-4, IL-7, IL-9, IL-15, IL-21), which is crucial for T-cell activation. JAK1 binds with γ-chain cytokines (IL-6, IL-10, IL-13, IL-22, granulocyte colony-stimulating face, interferons). Inhibition of JAKs is responsible for decreased pro-inflammatory cytokines signaling via IL-2 and IL-4 inhibition, decreased IL-6 production by synovial fibroblasts, decreased receptor activator of nuclear factor-κB ligand production, decreased IL-8 production by CD14+ monocytes, and decreased production of TNF-stimulated fibroblast-like synoviocytes. The net effect of tofacitinib is decreased synovial inflammation and structural joint damage in RA patients by limiting T cell and other leukocyte recruitment[3]. Other immune cells involved in RA pathogenesis express JAKs and may also be affected by tofacitinib inhibition. Figure 1 illustrates tofacitinib’s mechanism of action.
Tofacitinib is well absorbed from the gastrointestinal tract following oral administration[2]. Peak plasma concentration (Tmax) occurs within 0.5-1 h with an absolute oral bioavailability of 74%. Administration of tofacitinib with a high-fat meal resulted in a decrease in maximum plasma concentration (Cmax) by 32% with no changes to the area under the plasma concentration time curve (AUC); therefore, tofacitinib was given without regard to meals during clinical trials. Steady state concentrations are achieved in 24-48 h with twice daily administration with minimal accumulation.
Tofacitinib is distributed between plasma and red blood cells equally with a half-life of approximately 3 h and is 40% bound to plasma proteins, mainly albumin[2]. Hepatic metabolism, via CYP3A4 (major) CYP2C19 (minor) accounts for 70% of tofacitinib clearance with the remaining 30% excreted in the urine. The activity of tofacitinib is related to the parent compound, with 8 metabolites retaining less than 10% of potency. No dosage adjustments are necessary for patients with mild hepatic impairment; however, tofacitinib should be reduced to 5 mg once daily in patients with moderate hepatic impairment or moderate to severe renal impairment. Safety and efficacy for patients with severe hepatic impairment, or positive Hepatitis B or Hepatitis C serology has not been established.
Tofacitinib is predominately metabolized via CYP3A4 and drug-drug interactions are of concern[2]. Results from a recent, small in vitro study utilizing midazolam, a highly sensitive CYP3A4 substrate used to evaluate CYP isoenzyme drug interactions, and in vitro data has established a relative lack of effect of tofacitinib on the CYP enzyme system[5]. However, the manufacturer recommends the dose of tofacitinib be reduced by 50% (i.e., 5 mg once daily) when administered with potent CYP3A4 inhibitors (e.g., ketoconazole) or drugs exhibiting both moderate CYP3A4 inhibition and potent CYP2C19 inhibition (e.g., fluconazole)[2]. Concomitant administration of tofacitinib with potent CYP3A4 inducers (e.g., rifampin) can significantly reduce AUC and clinical efficacy necessitating dosage adjustment, though specific recommendations are not provided by the manufacturer. Caution should be exercised during concomitant administration of tofacitinib with cyclosporine and tacrolimus, given the risk of severe infection due to added immunosuppression when co-administered.
Tofacitinib has demonstrated significant ACR20 response in phase 2 trials as monotherapy and with background therapy with methotrexate[6-10]. Six phase 3 trials have been conducted to evaluate the efficacy of tofacitinib under the oral rheumatoid arthritis triaLs (ORAL) series. To date, five trials were available as full publications[11-15] and one as a conference abstract[16]. Three primary efficacy outcome measures were central to the five fully published trials: (1) percentage of patients achieving an ACR20 response, which is defined as 20% reduction from baseline in tender and swollen joints and at least 20% improvement in three of the five ACR core set measures; (2) change from baseline in the Health Assessment questionnaire disability index (HAQ-DI), in which scores range from 0-3 and higher scores indicate greater disability; and (3) percentage of patients with a Disease Activity Score for 28 joint counts based on erythrocyte sedimentation rate (DAS28-4[ESR]) of less than 2.6 with score ranging from 0-9.4. A summary of the phase 3 trial details and results can be found in Tables 1 and 2, respectively.
| Study | Duration | Participants | Demographics | Intervention | Primary outcome |
| ORAL solo | 6 mo | Active RA patients with inadequate response to at least one DMARD (biologic or nonbiologic) receiving stable doses of antimalarial | n = 611 Age: 49.7-52.4 yr Female: 85.2%-88.2% Duration of RA: 7.7-8.6 Baseline HAQ-DI: 1.50-1.53 Baseline DAS-28: 6.65-6.71 | Tofacitinib 5 mg bid; Tofacitinib 10 mg bid; placebo for 3 mo then Tofacitinib 5 mg bid; placebo for 3 mo then Tofacitinib 10 mg bid | ACR20 response at month 3; DAS 28-4 ESR < 2.6 at month 3; HAQ-DI at month 3 (change from baseline) |
| ORAL step | 6 mo | Moderate to severe RA patients with inadequate response to TNF alpha inhibitors | n = 399 Age: 54.4-55.4 yr Female: 80.3%-86.36% Duration of RA: 11.3-13.0 yr Baseline HAQ-DI: 1.5-1.6 Baseline DAS-28: 6.4-6.5 | Tofacitinib 5 mg bid; Tofacitinib 10 mg bid; placebo for 3 mo then Tofacitinib 5 mg bid; placebo for 3 mo then Tofacitinib 10 mg bid | ACR20 response at month 3; DAS 28-4 ESR < 2.6 at month 3; HAQ-DI at month 3 (change from baseline) |
| ORAL standard | 12 mo | Active RA patients receiving stable doses of methotrexate | n = 717 Age: 51.9-55.5 yr Female: 75.0%-85.3% Duration of RA: 6.9-9.0 yr Baseline HAQ-DI: 1.4-1.5 Baseline DAS-28: 6.3-6.6 | Tofacitinib 5 mg bid; Tofacitinib 10 mg bid; adalimumab 40 mg SC every 2 wk; placebo for 6 mo then Tofacitinib 5 mg bid; placebo for 6 mo then Tofacitinib 10 mg bid | ACR20 response at month 6; DAS 28-4 ESR < 2.6 at month 6; HAQ-DI at month 3 (change from baseline) |
| ORAL sync | 12 mo | Active RA patients with inadequate response to one or more DMARD | n = 792 Age: 50.8-53.3 yr Female: 75.0%-83.8% Duration of RA: 8.1-10.2 yr Baseline HAQ-DI: 1.24-1.45 Baseline DAS-28: 6.14-6.44 | Tofacitinib 5 mg bid; Tofacitinib 10 mg bid; Placebo | ACR20 response at month 6; DAS 28-4 ESR < 2.6 at month 6; HAQ-DI at month 3 (change from baseline) |
| ORAL scan | 24 mo | Active RA patients receiving background methotrexate | n = 797 Age: 52.0-53.7 yr Female: 80.2%-91.1% Duration of RA: 8.8-9.5 yr Baseline HAQ-DI: 1.23-1.41 Baseline DAS-28: 6.25-6.34 | Tofacitinib 5 mg bid; Tofacitinib 10 mg bid; placebo for 3 mo then Tofacitinib 5 mg bid; placebo for 3 mo then Tofacitinib 10 mg bid | ACR20 response at month 6; DAS 28-4 ESR < 2.6 at month 6; HAQ-DI at month 3 (change from baseline); SHS at month 6 (change from baseline) |
| ORAL start | 24 mo | Methotrexate naïve patients with active RA | n = 952 Baseline TSS: 16.51-20.30 | Tofacitinib 5 mg bid; Tofacitinib 10 mg bid; methotrexate 10 mg per week with 5 mg increments every 4 wk to 20 mg per week | Modified Total Sharp Score at month 6; ACR70 response at month 6 |
| Primary outcomes | ORAL solo | ORAL step | ORAL standard | ORAL sync | ORAL scan | ||||||||||
| Placebo(n = 122) | Tofacitinib 5 mg bid(n = 243) | Tofacitinib 10 mg bid(n = 245) | Placebo(n = 132) | Tofacitinib 5 mg bid(n = 133) | Tofacitinib 10 mg bid(n = 134) | Placebo(n = 108) | Tofacitinib 5 mg bid(n = 204) | Tofacitinib 10 mg bid(n = 201) | Placebo(n = 159) | Tofacitinib5 mgbid(n = 315) | Tofacitinib 10 mgbid(n = 318) | Placebo(n = 160) | Tofacitinib5 mgbid(n = 321) | Tofacitinib 10 mgbid(n = 316) | |
| ACR20 response (%) | 26.7 | 59.8d | 65.7d | 24.4 | 41.7b | 48.1d | 28.3 | 51.5d | 52.6d | 30.8 | 52.1d | 56.6d | 25.3 | 51.5d | 61.8d |
| Change from baselineHAQ-DI (LSM change) | -0.19 | -0.50d | -0.57d | -0.18 | -0.43d | -0.46d | -0.24 | -0.55a | -0.61a | -0.16 | -0.44d | -0.53d | -0.15 | 0.402 | -0.54d |
| DAS-28-4(ESR) less than2.6 (%) | 4.4 | 5.61 | 8.71 | 1.7 | 6.7a | 8.8a | 1.1 | 6.2a | 12.5a | 2.6 | 8.5b | 12.5d | 1.6 | 7.22 | 16.0d |
ORAL Solo was a 6-mo, multicenter, multinational, randomized, double-blind, placebo-controlled trial[11]. Primary endpoints of this trial were percentage of patients with an ACR20 response, the change from baseline in physical function measured by HAQ-DI, and the percentage of patients with a DAS28-4(ESR) less than 2.6 at month 3. Secondary objectives included percentage of patients with ACR20, ACR50, and ACR70 response rates at all visits, the change in baseline at all visits in the HAQ-DI and DAS28-4(ESR), and the score at month 3 on the functional assessment of chronic illness therapy (FACIT) fatigue instrument. The use of nonsteroidal anti-inflammatory drugs and glucocorticoids (≤ 10 mg of a prednisone equivalent) were permitted. A total of 555 patients completed the trial. All patients who received tofacitinib had statistically significant improvement in ACR20, ACR50, and ACR70 response criteria and HAQ-DI scores at month 3 (P < 0.001 for all comparisons). There were not significant benefits of tofacitinib seen in DAS28-4(ESR). The changes in the FACIT-fatigue score from baseline at month 3 were statistically significant compared with placebo (P < 0.001).
ORAL Step was a 6-mo, multicenter, multinational, randomized, double-blind, placebo-controlled trial[12]. Primary endpoints of this trial were percentage of patients with an ACR20 response, the change from baseline in physical function measured by HAQ-DI, and the percentage of patients achieving DAS28-4(ESR) less than 2.6 at month 3. Secondary objectives were the percentage of patients with ACR20, ACR50, and ACR70 response over time, changes from baseline in the HAQ-DI and DAS28-4(ESR) over time, pain (rated from 0-100), and fatigue measured by the FACIT. Stable doses of methotrexate 7.5 mg to 20 mg weekly for 6 wk prior to the start of the trial were required. The use of nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 inhibitors, or glucocorticoids (≤ 10 mg of a prednisone equivalent) were permitted. A total of 399 patients completed the trial. At month 3, ACR 20, ACR50, ACR70 response rates were significant (P < 0.01 for all comparisons) and changes from baseline in HAQ-DI were significant (P < 0.0001) for tofacitinib compared to placebo. The proportion of patients with DAS28-4(ESR) less than 2.6 at month 3 were significant in tofacitinib 10 mg twice daily group compared to placebo. Improvements in arthritis pain and FACIT assessments were statistically significant for tofacitinib groups compared to placebo.
ORAL Standard was a 12-mo, multicenter, multinational, randomized, double-blind, placebo-controlled trial[13]. Primary endpoints of this trial were percentage of patients with an ACR20 response at month 6, the change from baseline in physical function measured by HAQ-DI at month 3, and the percentage of patients achieving DAS28-4(ESR) less than 2.6 at month 6. Secondary objectives were the percentage of patients with ACR20, ACR50, and ACR70 response over time and changes from baseline in the HAQ-DI and DAS28-4(ESR) over time. A total of 717 patients were included in the full analysis. Patients receiving active treatment achieved a significantly greater percentage of ACR20 response compared to placebo at month 6 (P < 0.001 for all comparisons). Percentage of patients with DAS-28-4(ESR) less than 2.6 at month 6 and mean change from baseline in HAQ-DI score at month 6 were also statistically significant when compared to placebo. For secondary endpoints, greater ACR50 and ACR70 response and significant changes from baseline in DAS28-4(ESR) and HAQ-DI were seen over time (P < 0.05 for all comparisons).
ORAL Sync was a 12-mo, multicenter, multinational, randomized, double-blind, placebo-controlled trial[14]. Primary endpoints of this trial were percentage of patients with an ACR20 response, the change from baseline in physical function measured by HAQ-DI, and the percentage of patients with a DAS28-4(ESR)-defined remission at month 6. Secondary objectives were ACR20, ACR50, and ACR70 response rates, change from baseline HAQ-DI, DAS28-4(ESR) assessments, and FACIT-fatigue score over time. The use of oral corticosteroid therapy (≤ 10 mg of a prednisone equivalent) was permitted. DMARDs disallowed were biologics, cyclosporine, and azathioprine. A total of 792 patients were included in the primary analysis data set with methotrexate being the most frequently prescribe background DMARD (79%). For both tofacitinib groups compared to placebo at month 6, statistically significant differences were seen in ACR20 response rates, improvements from baseline in HAQ-DI and DAS-28 (P < 0.005 for all comparisons). For secondary endpoints, changes from baseline in HAQ-DI, DAS28-4(ESR) less than 2.6, and FACIT-fatigue for both tofacitinib groups compared with placebo were statistically significant. For tofacitinib 10 mg twice daily, ACR20, ACR50, and ACR70, significant response rates were observed by week 2. For tofacitinib 5 mg twice daily, significant response rates were observed by week 2 for ACR20 and ACR50, and by week 4 for ACR70.
ORAL Scan is a 24-mo, multicenter, multinational, randomized, double-blind, placebo-controlled trial[15]. Primary endpoints of this trial were percentage of patients with an ACR20 response at month 6, the change from baseline in physical function measured by HAQ-DI at month 3, percentage of patients achieving DAS-28-4 (ESR) less than 2.6 at month 6, and change from baseline in total modified Sharp/van der Heijde Score (SHS) at month 6. Stable doses of methotrexate were required. The use of nonsteroidal anti-inflammatory drugs and glucocorticoids (≤ 10 mg of a prednisone equivalent) were permitted. A total of 797 patients were randomized and treated. ACR20 response rates for both tofacitinib doses were significant compared to placebo (P < 0.0001 for both comparisons). Significant changes from baseline SHS scores (P < 0.05), HAQ-DI (P < 0.0001), and DAS28-4(ESR) (P < 0.0001) were seen with tofacitinib 10 mg twice daily; non-significant results with tofacitinib 5 mg twice daily.
ORAL Start is a 24-mo, multicenter, multinational, randomized, double-blind, placebo-controlled trial[16]. Primary endpoints of this trial were mean change from baseline in van der Heijde modified Total Sharp Score (mTSS) and percentage of patients with an ACR70 response at month 6. To date, complete methodology and results are unavailable as ORAL Start is published as a conference abstract. A total of 952 patients were randomized and treated. At month 6, mean changes from baseline in mTSS and percentage of patients achieving ACR70 were statistically significant.
Safety of tofacitinib was evaluated in six phase 3 clinical trials[11-16], four phase 2 trials[6-9], two phase 1 trials[17-18], and a study evaluating the impact on latent tuberculosis infection (LTBI) in a mouse model due to concerns with a risk of reactivation with treatments (i.e., tumor necrosis factor alpha inhibitors) for chronic inflammatory disorders, including rheumatoid arthritis[19]. Several of the phase 2 and 3 trials have been reported in two meta-analyses to evaluate efficacy and safety of tofacitinib for treatment of rheumatoid arthritis[20-21]. In phase 1 studies, including a study with patients randomized to receive supratherapeutic doses of tofacitinib (i.e., 100 mg), there were no serious adverse events reported[17-18]. Additionally, there were no discontinuations or dose reductions of study medication due to adverse events reported. All reported events were mild to moderate and resolved quickly, including two reports of anemia. Additional adverse events reported included headache, nausea, vomiting, dizziness, and disorientation. There were no clinically meaningful changes in laboratory values or ECG parameters.
Adverse events attributed to treatment with tofacitinib were similar in phase 2 trials with the most common events being headache, diarrhea, nausea, upper respiratory tract infections, and nasopharyngitis[6-9]. Patients receiving doses greater than the FDA-approved dose of 5 mg twice daily experienced the highest number of adverse events (i.e., 10 mg twice daily, 15 mg twice daily, and 30 mg twice daily) in phase 2 trials with few treatment discontinuations reported[6,8,9]. In the trials reviewed, two deaths were reported in patients receiving tofacitinib[8,9]. One attributed to cerebrovascular accident in a patient receiving 15 mg twice daily and the other experiencing pneumonia that led to respiratory and cardiac failure with 3 mg twice daily dosing. Although infections were reported in patients receiving tofacitinib, the reports were mainly mild to moderate in severity[6-9]. Serious infections included nasopharyngitis, gastroenteritis, pharyngitis, pneumonia, and pneumococcal sepsis [8,9]. No opportunistic infections were reported in the phase 2 trials[6-9]. In one phase 2 trial, non-infectious serious adverse events were reported including foot deformity, osteoarthritis of the hip, femur fracture, cardiac failure, and acute dyspnea[7]. Each of these events resolved following discontinuation of the study drug with the exception of cardiac failure. Patients receiving tofacitinib also experienced decreased neutrophil counts, thrombocytopenia, decreased hemoglobin, and anemia[6-9]. Most adverse effects were reported to be mild to moderate not requiring discontinuation of the study drug; however, several cases of severe anemia were reported leading to the temporary discontinuation of tofacitinib in one patient secondary to gastrointestinal bleeding. In addition to hematologic effects, increases in serum creatinine and lipid parameters [i.e., total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL)] were observed. Most were not of clinical relevance; although, several reports of discontinuation were noted with increases in serum creatinine. Changes in blood pressure were minimal and not considered to be clinically relevant. Increased in transaminase concentrations, including aspartate aminotransferase and alanine aminotransferase were reported with treatment discontinuation in few patients. Most cases resolved spontaneously during treatment and did not require discontinuation of the study medication.
In six phase 3 clinical trials, the adverse events profile for tofacitinib was similar to that observed in phase 2 trials. Safety analyses were conducted at 3 and 6 mo for ORAL Solo and ORAL Step and at 3, 6, and 12 mo for ORAL Standard, ORAL Sync, and ORAL scan[11-15]. Safety information from ORAL Start is currently limited to abstract data and reported for the entire 12-mo period[16]. Table 3 summarizes the number of patients experiencing an adverse event, a serious adverse event, or discontinuation of the study medication due to an adverse event during the analysis period. Table 4 provides a summary of adverse events experienced by patients receiving study medication in the last analysis period for the respective trial. Deaths, serious infection events, reports of tuberculosis, other opportunistic infections, and malignancies are also provided. With concern for reactivation of LTBI in patients receiving immunologic agents, it is important to note that reports of tuberculosis infection in patients receiving tofacitinib were rare in phase 3 trials, with two cases reported in two trials, ORAL Standard and ORAL Sync. Additionally, malignancies reported with tofacitinib were rare and reported only in patients receiving tofacitinib in ORAL Scan.
| ORAL solo | ORAL step | ORAL standard | ORAL sync | ORAL scan | ||||||||||||
| Placebo(n = 122) | Tofacitinib5 mg bid(n = 243) | Tofacitinib10 mg bid(n = 245) | Placebo(n = 132) | Tofacitinib5 mg bid(n = 133) | Tofacitinib10 mg bid(n = 134) | Placebo(n = 108) | Tofacitinib5 mg bid(n = 204) | Tofacitinib10 mg bid(n = 201) | Adalimumab 40 mg once Q2W(n = 204) | Placebo(n = 159) | Tofacitinib5 mg bid(n = 315) | Tofacitinib10 mg bid(n = 318) | Placebo(n = 160) | Tofacitinib5 mg bid(n = 321) | Tofacitinib10 mg bid(n = 316) | |
| Patients with AE, | 67 (54.9) | 124 (51) | 139 (56.7) | 75 (56.8) | 71 (53.4) | 76 (56.7) | 51 (47.2) | 106 (52) | 94 (46.8) | 105 (51.5) | 97 (61) | 166 (52.7) | 173 (54.4) | 73 (45.6) | 157 (48.9) | 171 (54.1) |
| Patients with SAE | 6 (4.9) | 1 (0.4) | 5 (2) | 6 (4.5) | 2 (1.5) | 2 (1.5) | 2 (1.9) | 12 (5.9) | 10 (5) | 5 (2.5) | 6 (3.8) | 9 (2.9) | 8 (2.5) | 5 (3.1) | 12 (3.7) | 10 (3.2) |
| Discontinuation due to AE | 5 (4.1) | 2 (0.8) | 6 (2.4) | 7 (5.3) | 8 (6) | 6 (4.5) | 3 (2.8) | 14 (6.9) | 10 (5) | 10 (4.9) | 2 (1.3) | 13 (4.1) | 13 (4.1) | 5 (3.1) | 15 (4.7) | 14 (4.4) |
| 1ORAL solo | 1ORAL step | 2ORAL standard | 2ORAL sync | 2ORAL scan | 4ORAL start | |||||||||||||||||||
| Tofacit-inib 5 mg bid (after Placebo)(n = 61) | Tofacit-inib 10 mg bid (after Placebo)(n =61) | Tofacit-inib 5 mg bid(n = 243) | Tofaci-tinib 10 mg bid(n = 245) | Tofacit-inib 5 mg bid (after Placebo)(n = 66) | Tofacit-inib 10 mg bid (after Placebo)(n = 66) | Tofacit-inib 5 mg bid(n = 133) | Tofacit-inib 10 mg bid(n = 134) | Tofacit-inib 5 mg bid (after Placebo )(n = 56) | Tofacitinib 10 mg bid (after Placebo)(n = 52) | Tofacit-inib 5 mg bid(n = 204) | Tofacit-inib 10 mg bid(n = 201) | Adalim-umab 40 mg once Q2W(n = 204) | Tofacit-inib 5 mg bid (after Placebo)(n = 79) | Tofacit-inib 10mg bid (after Placebo)(n = 80) | Tofacit-inib 5 mg bid(n = 315) | Tofacit-inib 10 mg bid(n = 318) | Tofacit-inib 5 mg bid (after Placebo)(n = 81) | Tofacit-inib 10 mg bid (after Placebo)(n = 79) | Tofacit-inib 5 mg bid(n = 321) | Tofacit-inib 10 mg bid(n = 316) | Tofacit-inib 5 mg bid(n = 371) | Tofacit-inib 10 mg bid(n =395) | MTX(n = 186) | |
| Patients with AE | 22 (36.1) | 24 (39.3) | 97 (39.9) | 101 (41.2) | 24 (36.4) | 28 (42.4) | 57 (42.9) | 58 (43.3) | 18 (32.1) | 21 (40.4) | 89 (43.6) | 84 (41.8) | 83 (40.7) | 34 (43) | 29 (36.3) | 104 (33) | 135 (42.5) | 34 (42) | 35 (44.3) | 166 (51.7) | 174 (55.1) | (70.1)5 | (74.4)5 | (69.9)5 |
| Patients with SAE | 1 (1.6) | 0 | 5 (2.1) | 6 (2.4) | 3 (4.5) | 2 (3) | 5 (3.8) | 6 (4.5) | 1 (1.8) | 4 (7.7) | 10 (4.9) | 6 (3) | 7 (3.4) | 2 (2.5) | 0 | 7 (2.2) | 9 (2.8) | 1 (1.2) | 4 (5.1) | 13 (4) | 9 (2.8) | (6.5)5 | (6.1)5 | (7.0)5 |
| Discon- tinuation due to AE | 0 | 0 | 1 (0.4) | 3 (1.2) | 1 (1.5) | 2 (3) | 4 (3) | 7 (5.2) | 0 | 2 (3.8) | 6 (2.9) | 3 (1.5) | 4 (2) | 0 | 1 (1.3) | 1 (0.3) | 9 (2.8) | 2 (2.5) | 2 (2.5) | 9 (2.8) | 7 (2.2) | 13 (3.5) | 17 (4.3) | 11 (5.9) |
| Deaths | 0 | 0 | 0 | 1 (0.4) | 0 | 1 (1.5) | 0 | 0 | 0 | 0 | 1 (0.5) | 0 | 1 (0.5) | 0 | 0 | 2 (0.6) | 2 (0.6) | 1 (1.2) | 0 | 2 (0.6) | 1 (0.3) | See note | See note | 0 |
| Patients with serious infection events | 1 (1.6) | 0 | 1 (0.4) | 3 (1.2) | 1 (1.5) | 0 | 2 (1.5) | 2 (1.5) | 2 (3.6) | 0 | 7 (3.4) | 8 (4) | 3 (1.5) | 0 | 0 | 2 (0.6) | 7 (2.2) | 1 (1.2) | 2 (2.5) | 11 (3.4) | 5 (1.6) | (31.8)6 | (38.7)6 | (27.4)6 |
| Pulm- onary Tube- rculosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (1) | 0 | 0 | 0 | 0 | 2 (0.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3Oppor- tunistic infections | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.3) | 1 (0.3) | 0 | 0 | 3 (0.9) | 4 (1.3) | 8 (2.2) | 11 (2.8) | 3 (1.6) |
| Malign- ancies | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 0 | 0 | 5 (1.6) | 4 (1.3) | NR | NR | NR |
Tofacitinib was associated with changes in laboratory tests, specifically lymphocytes, neutrophils, liver enzymes, lipid parameters, and serum creatinine[11-16]. Patients in the tofacitinib groups had decreases in lymphocyte and neutrophil counts. While patients with decreases in lymphocyte counts were more likely to experience an increased incidence of infections, there was no identifiable association between the decrease in neutrophil count and occurrence of serious infection in clinical trials. Similar to results from phase 2 trials, patients receiving tofacitinib experienced increases in liver enzymes greater than 3 times the upper limit of normal; however, normalization of liver enzymes was achieved with modification of study treatment (e.g., dose reduction, interruption, discontinuation). Lipid parameters, including total cholesterol, LDL, and HDL, were also associated with dose-related elevations following initiation of tofacitinib therapy and remained stable throughout the study periods. Dose-related elevations were also observed with serum creatinine; the clinical significance remains unclear given the propensity for elevations to remain within the normal range. However, several trial discontinuations were attributed to elevations in serum creatinine. In addition to more serious events and laboratory changes, other adverse events reported during phase 3 trials included diarrhea, nasopharyngitis, upper respiratory infection, headache, and hypertension. Headache and diarrhea appear to be more common with tofacitinib treatment versus placebo.
Given the risk of reactivation of tuberculosis in patients with LTBI receiving other immunomodulating agents, such as tumor necrosis factor alpha inhibitors, Maiga and colleagues studied the impact of tofacitinib on LTBI in a mouse model[19]. Results indicated a reactivation of latent infection in the presence of tofacitinib due to an increase in bacterial replication and reduction in containment of the bacteria. The investigators concluded that tofacitinib should be prescribed with caution in patients with chronic inflammation and screening for LTBI is warranted prior to use. These results are consistent with reports of tuberculosis cases identified in the phase 3 trial by Kremer and colleagues[14].
ACR 2012 guidelines for treatment of rheumatoid arthritis with use of DMARDs and biologic agents do not specifically address the place in therapy for tofacitinib. However, European League Against Rheumatism (EULAR) recommendations suggest tofacitinib should be considered a targeted, synthetic DMARD for use after treatment failure of at least one biologic DMARD[22]. Safety and efficacy of tofacitinib have been demonstrated in six phase 3 trials [11-16]. Tofacitinib, a Janus kinase inhibitor, offers a novel mechanism of action in the treatment of rheumatoid arthritis and is administered orally, which may be a benefit for patients.
The authors wish to acknowledge Brian D Cole for his medical illustrating.
P- Reviewer: Essex MN, Fioravanti A, Jiang GL, Singh A, Wechalekar MD S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, Moreland LW, O’Dell J, Winthrop KL, Beukelman T. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:625-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 507] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 2. | New York: Pfizer, 2012. . |
| 3. | Bannwarth B, Kostine M, Poursac N. A pharmacokinetic and clinical assessment of tofacitinib for the treatment of rheumatoid arthritis. Expert Opin Drug Metab Toxicol. 2013;9:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Gomez-Puerta JA, Mócsai A. Tyrosine kinase inhibitors for the treatment of rheumatoid arthritis. Curr Top Med Chem. 2013;13:760-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Gupta P, Alvey C, Wang R, Dowty ME, Fahmi OA, Walsky RL, Riese RJ, Krishnaswami S. Lack of effect of tofacitinib (CP-690,550) on the pharmacokinetics of the CYP3A4 substrate midazolam in healthy volunteers: confirmation of in vitro data. Br J Clin Pharmacol. 2012;74:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, Krishnaswami S, Burgos-Vargas R, Wilkinson B, Zerbini CA. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 440] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 7. | Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken). 2011;63:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 8. | Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, Connell CA, Gruben D, Krishnaswami S, Wallenstein G. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 315] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 9. | Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, Gruben D, Kanik KS, Krishnaswami S, Pascual-Ramos V. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 10. | Coombs JH, Bloom BJ, Breedveld FC, Fletcher MP, Gruben D, Kremer JM, Burgos-Vargas R, Wilkinson B, Zerbini CA, Zwillich SH. Improved pain, physical functioning and health status in patients with rheumatoid arthritis treated with CP-690,550, an orally active Janus kinase (JAK) inhibitor: results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010;69:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, Gruben D, Wallenstein GV, Zwillich SH, Kanik KS. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 760] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 12. | Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 553] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 13. | van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, Forejtova S, Zwillich SH, Gruben D, Koncz T. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 733] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 14. | Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, Isaacs JD, Gruben D, Wallenstein G, Krishnaswami S. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 364] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 15. | van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, Cardiel MH, Cohen S, Nash P, Song YW. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 454] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 16. | Lee EB, Fleishmann RM, Hall S, van Vollenhoven RF, Bradley J, Gruben D, Koncz T, Krishnaswami S, Wallenstein G, Zwillich SH. Radiographic, clinical and functional comparison of tofacitinib monotherapy versus methotrexate in methotrexate-naïve patients with rheumatoid arthritis [ACR abstract 2486]. Arthritis Rheum. 2012;64:S1049. |
| 17. | Krishnaswami S, Kudlacz E, Wang R, Chan G. A supratherapeutic dose of the Janus kinase inhibitor tasocitinib (CP-690,550) does not prolong QTc interval in healthy participants. J Clin Pharmacol. 2011;51:1256-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 18. | Cohen S, Zwillich SH, Chow V, Labadie RR, Wilkinson B. Co-administration of the JAK inhibitor CP-690,550 and methotrexate is well tolerated in patients with rheumatoid arthritis without need for dose adjustment. Br J Clin Pharmacol. 2010;69:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Maiga M, Lun S, Guo H, Winglee K, Ammerman NC, Bishai WR. Risk of tuberculosis reactivation with tofacitinib (CP-690550). J Infect Dis. 2012;205:1705-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Kawalec P, Mikrut A, Wiśniewska N, Pilc A. The effectiveness of tofacitinib, a novel Janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. 2013;32:1415-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | He Y, Wong AY, Chan EW, Lau WC, Man KK, Chui CS, Worsley AJ, Wong IC. Efficacy and safety of tofacitinib in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2013;14:298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, Emery P, Gaujoux-Viala C, Gossec L, Nam J. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1540] [Cited by in RCA: 1457] [Article Influence: 132.5] [Reference Citation Analysis (0)] |