Published online Jul 18, 2014. doi: 10.5312/wjo.v5.i3.328
Revised: March 12, 2014
Accepted: April 25, 2014
Published online: July 18, 2014
Processing time: 206 Days and 23.8 Hours
Rheumatoid arthritis is a chronic autoimmune inflammatory disease associated with increased cardiovascular risk and higher mortality in respect to general population. Beyond joint disease, inflammation is the major determinant of accelerated atherosclerosis observed in rheumatoid arthritis. We review the relationship between inflammation, atherosclerosis and cardiovascular risk in rheumatoid arthritis, focusing on the assessment of subclinical atherosclerosis by functional and morphological methods. These tools include flow mediated dilatation, carotid intima-media thickness, ankle/brachial index, coronary calcium content, pulse wave analysis and serum biomarker of subclinical atherosclerosis.
Core tip: In this paper we briefly review the role of subclinical atherosclerosis in rheumatoid arthritis, its relationship with inflammatory process and the current available method to detect early atherosclerotic changes.
- Citation: Scarno A, Perrotta FM, Cardini F, Carboni A, Annibali G, Lubrano E, Spadaro A. Beyond the joint: Subclinical atherosclerosis in rheumatoid arthritis. World J Orthop 2014; 5(3): 328-335
- URL: https://www.wjgnet.com/2218-5836/full/v5/i3/328.htm
- DOI: https://dx.doi.org/10.5312/wjo.v5.i3.328
Rheumatoid arthritis (RA) is a systemic autoimmune inflammatory disease that affects synovial joints and lead to chronic pain, bone erosions and progressive disability. Approximately 1% of the adult population in the United States has RA, and the overall world prevalence range from 0.5% to 1%, qualifying it as the most common chronic inflammatory condition[1,2]. Beyond joint disease, evidences support the hypothesis that chronic inflammation could increase cardiovascular risk: patients with RA die earlier than the general population[3], in particular, mortality risk in RA patients is 1.5 higher than general population and occurs largely as a result of higher rates of cardiovascular death[4]. A recent meta-analysis shows that standardized mortality ratio (SMR) ranges from 0.99 to 3.82 for myocardial infarction and from 1.08 to 2 for cerebrovascular diseases[5], while risk to develop peripheral arterial diseases is 2.35 in a large cohort of United States patients. Risk to develop non fatal cardiovascular and cerebrovascular diseases, that lead to significant disability care cost is also increased in RA. These diseases are strictly related to an accelerated atherosclerotic process and, although several factors contribute independently to the heightened cardiovascular risk observed in patients with RA, systemic inflammation contributes importantly[6]. Different authors suggested in fact that the higher prevalence of cardiovascular events in RA patients could be explained by other mechanisms than the classic atherosclerotic risk factors[7-10].
We briefly review the role of subclinical atherosclerosis in RA, its relationship with inflammatory process and the non-invasive methods to detect early atherosclerotic changes and to estimate risk of cardiovascular events.
Atherosclerosis and RA share a number of similarities, including T-cell and mast cell activation, production of pro-inflammatory cytokines such as tumor necrosis factor (TNF) alpha and interleukin (IL)-6, and increased expression of leukocyte adhesion molecules[11].
Patients with RA have elevated levels of the acute-phase reactant C reactive protein (CRP), a marker of inflammation associated with increased cardiovascular risk. Moreover, CRP causes endothelial dysfunction by decreasing endothelial nitric oxide synthase, a potent anti-atherogenic factor[12].
Patients with RA with elevated erythrocyte sedimentation rate (ESR) have a higher rate of cardiovascular death than those without elevated ESR. This inflammatory marker also increases linearly with increased carotid artery intima-media thickness in both patients with RA and healthy controls[6].
Immune system plays an important role in the progression and development of atherosclerotic disease and associated complications. Atherosclerosis is in fact now considered as an autoimmune disease[6,13,14]. The presence of inflammatory cells, such as macrophages and activated lymphocytes within atherosclerotic plaque, is a strong indicator of immune system involvement. Furthermore, the inflammatory burden in RA and other rheumatologic diseases increases the process of oxidation of low density lipoproteins (ox-LDL), responsible for the formation and progression of atherosclerotic plaque[15]. ox-LDL amplifies the inflammatory response through the expression of adhesion molecules by endothelial cells and through the production of pro-inflammatory cytokines (TNF alpha, IL-1, IL-6) by macrophages[13,16]. Mature dendritic cells (DC) express CCL17 that favoring T-lymphocytes recruitment; moreover the presence of modified or native LDL, induce up-regulation of co-stimulatory molecules on DCs that lead to T-lymphocyte proliferation. Modified LDL determine the formation of new antigenic epitopes which can be presented by DCs and brought to clonal expansion of LDL-specific T-lymphocytes. Indeed, about 10% of all T-lymphocytes detectable in human atherosclerotic plaques specifically recognize modified or native LDL. Of note, LDL-specific T-lymphocytes are also present in the circulation[17]. The elevated levels of pro-inflammatory cytokines can elicit a systemic inflammatory state that could lead to pro-atherogenic phenotype: cytokines, in addition to their role in regulating immune responses, mediate a number of metabolic effects that, in the short term, mediate appropriate responses to injury or infection, but on a chronic basis prove detrimental: systemic release of IL-1, IL-17, IL-6, and TNF-α, produced in synovial tissue in RA patients, promotes a number of pro-atherogenic functions of the liver, adipose tissue, skeletal muscle, and vascular endothelium, including insulin resistance, dyslipidemia, endothelial activation, and prothrombotic and antifibrinolytic effects[14]. CRP and other factors local released by leukocytes, contributes to early endothelial dysfunction and damage.
Immunological abnormalities such as auto-antibodies production may be involved in endothelial dysfunction and in the process of progression and rupture of the atherosclerotic plaque. Rheumatoid factor could be found in the atheroma as immunocomplex and is associated with impaired endothelial function and increased mortality[18].
Atherosclerotic vascular involvement and cardiac abnormalities including pericardial, myocardial, and endocardial involvement, were higher among anti citrullinated peptide antibodies (ACPA) positive RA patients[19]. Citrullinated proteins, including citrullinated fibrinogen, are present within atherosclerotic plaque, and co-localized with peptidylarginine deiminase type 4 (PAD-4). Moreover, ACPA serum levels correlates with subclinical atherosclerosis indices. These, and other observations, support the hypothesis that citrullinated epitopes within the atherosclerotic plaque may be targeted by RA-associated ACPAs, thus forming immune complexes capable of locally perpetuating plaque inflammation and progression[20].
Several studies demonstrated that endothelial dysfunction plays a central role in the pathogenesis of atherosclerosis, promotes early atherosclerotic changes and is predictive for the development of cardiovascular events[21,22]. Patients with RA have a greater prevalence of arterial atherosclerotic plaques than controls[23,24] and the presence of atherosclerotic plaques correlate with disease duration, radiological damage index and systemic inflammation[25].
Early detection of subclinical atherosclerosis in RA could be useful to prevent cardiovascular events, death and disability. Different non-invasive methods are available to detect atherosclerosis and to estimate risk of cardiovascular events. These tools include functional and morphological assessment of artery physiology.
The normal, healthy endothelium regulates vascular tone and structure and exerts anticoagulant, anti-platelet, and fibrinolitic properties. The maintenance of vascular tone is accomplished by the release of numerous dilator and constrictor substances. The major vasodilator substance released by the endothelium is nitric oxide (NO). Endothelial dysfunction occurs when NO bioavailability is reduced[10,21,22,26,27].
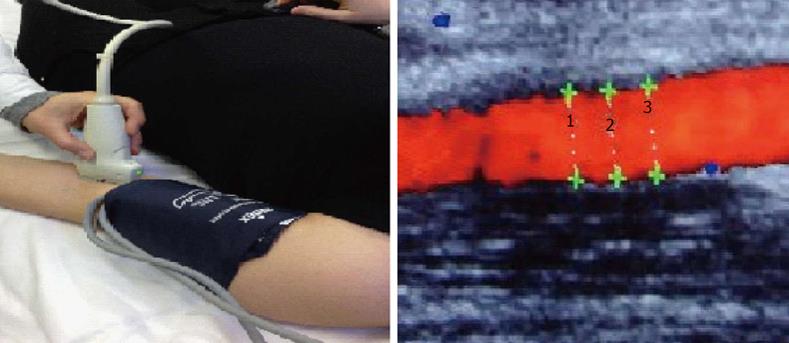
Among different methods, the assessment of flow-mediated dilatation (FMD) is one of the most used to assess the endothelial function in vivo, with a non invasive approach[28]. FMD reflects the ability of brachial artery to dilate after reactive hyperaemia induced by shear stress. It depends on the endothelial production of agents with vasomotor action, specifically NO[29-31](Figure 1).
Traditional cardiovascular risk factors, such as smoking, obesity, abnormal glucose or lipid dismetabolism and hypertension, could alter endothelial function and have been related with impaired FMD. Moreover, impaired FMD predicts the risk of future cardiovascular events and it is a surrogate marker of general atherosclerosis[32-35].
Endothelial function could also be assessed with administration of sublingual nitroglycerin (NTG). NTG induces a vasodilatation that is endothelium-independent to production of local NO[36].
In RA patients, FMD is impaired, compared to controls, independent to the presence of classical atherosclerosis risk factors. RA patients showed similar FMD impairment than those with diabetes, supporting the theory that RA is an independent risk factor for atherosclerosis. Endothelial dysfunction in these patients seems to be related to disease activity (DAS28), disease duration, HLA-DRB1 shared epitope and inflammatory indices[37]. Furthermore, in RA patients disease activity, assessed by DAS28, ESR and CRP, predicts the magnitude of endothelial dysfunction[38]. FMD is impaired even in patients with early disease, suggesting that atherosclerotic process starts early[39]. Few studies demonstrate NTG-mediated vasodilatation impairment in RA patients. Hannawi et al[40] in a longitudinal study on 20 patients with early RA found that both FMD and NTG-mediated vasodilatation were significantly lower in patients in respect to control and negatively correlated with age and CRP.
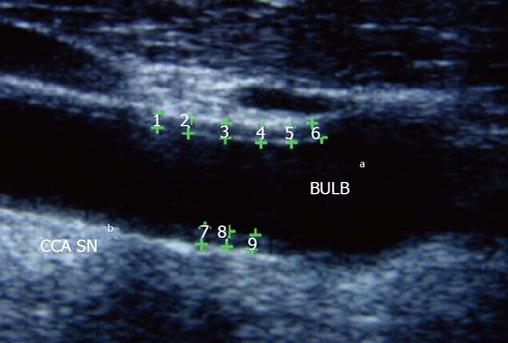
Carotid atherosclerosis may be determined by the assessment of common carotid intima-media thickness (IMT) using high-resolution B-mode ultrasound technique (Figure 2). Increased IMT is associated to the onset of cardiovascular events and is strongly related to the presence of atherosclerosis risk factors, such as hypertension, hypercholesterolemia, smoke, diabetes and obesity[41-43]. Several studies reported increased IMT in patients with rheumatic disease, in particular, patients with systemic lupus erythematosus, psoriatic arthritis and ankylosing spondylitis show increased IMT in respect to control as demonstrated in some study[44,45]. Patients with RA showed increased IMT in respect to age and sex matched controls: a meta-analysis from 22 studies, in 2011, compared carotid IMT data of 1384 RA patients with 1147 control subjects. Seventeen of 22 studies reported a statistical significant higher IMT in RA patients compared with controls. Mean IMT in RA patients was 0.71 mm and in control subjects 0.62 mm even in subject without other cardiovascular risk factors[46,47]. In RA patients IMT correlates with disease activity, severity and disease duration, with CRP, erythrocyte sedimentation rate (ESR) and use of corticosteroids[25]. Furthermore, increased IMT and carotid plaque presence predict the risk of cardiovascular morbidity in RA patients, in particular, carotid artery IMT > 0.9 has a high predictive power for the development of cardiovascular events over a 5-years period follow-up[48,49].
The Ankle/Brachial Index (ABI) or Windsor’s Index represents a simple, useful, reproducible, non-invasive method to detect asymptomatic peripheral artery disease (PAD). It expresses the ratio between the systolic blood pressure in posterior tibial artery measured at the ankle, and the systolic blood pressure measured in the brachial artery. It has been used for many years to assess the severity of peripheral artery disease and to detect the presence of asymptomatic, although hemodynamically relevant, stenosis[50,51].
ABI is an indicator of generalized atherosclerosis and can provide prognostic information about cardiovascular diseases. Moreover, patients with lower values of ABI are at higher risk of myocardial infarction or stroke. In RA patients, disease activity, disease duration and corticosteroid use are risk factors for PAD. Few studies evaluated the prevalence of peripheral artery disease in patients with rheumatic disease. Del Rincòn et al[52] studied 234 patients with RA and demonstrated an increased prevalence of impaired peripheral artery function. In this study there was high percentage of peripheral artery obstruction and incompressibility in RA patients in respect to controls[52].
Coronary atherosclerosis is the major determiner of miocardial infarction, but could be directly detectable only through invasive exams such as angiography. Indirect information about coronary atherosclerosis could be obtained by measurement of calcium content. Coronary Calcium Content (CAC) measurement is considered as surrogate marker of atherosclerosis because of its high correlation with total atherosclerotic plaques demonstrated in angiographic, hystopathologic, and ultrasound studies. CAC is a marker of subclinical atherosclerosis of the coronary district and gives quantitative measurement of the calcified share of coronary atherosclerotic plaque. Moreover, it strongly predicts the risk of cardiovascular disease in general population[53].
Recent evidences suggest that measurement of CAC is predictive of myocardial infarction and cardiovascular disease at 5 years and the use of CAC can provide important informations, independent from the other traditional cardiac risk factors[53,54]. Different cross-sectional studies investigated the role of CAC detection in RA patients. In particular Giles et al[55] showed a higher prevalence and extent of CAC in RA patients compared with controls after adjustments for the main cardiovascular risk factors. Furthermore, patients with longstanding RA have more extensive subclinical atherosclerosis assessed by CAC compared to patients of the same age as a consequence of accelerated atherosclerosis[55]. Finally, in RA patients, higher CAC is significantly associated with serum TNF-alpha and IL-6 levels. This evidence supports the role of inflammation in the promotion of atherosclerosis, and specifically of coronary calcification in RA.
Coronary flow reserve (CFR) gives indirect information on the status of coronary district through the analysis of artery flow signals. Impairment of endothelial function and reduced CFR, which reflects coronary microvascular function, has been shown to be early manifestation of atherosclerosis and coronary artery disease. CFR is measured with non invasive trans-thoracic Doppler transducer used to identify patients with known or suspected cardiovascular diseases[56,57]. CFR is impaired in patients with connective tissue diseases and RA without clinical evidence of heart disease, as a result of impaired microcirculation, as demonstrated by reduced CFR in a cohort of 81 RA patients compared with healthy controls[58]. Moreover, in RA CFR correlated with disease duration and with left ventricular function[58,59].
Arterial stiffness is one of the events that occur in the natural process of aging, but it could also be related to pathological process as arteriosclerosis. Pulse Wave Analysis (PWA) is one of the most used and reproducible method to assess arterial stiffness. PWA consists of two fundamental components: pulse wave velocity (PWV) and augmentation index (AIx). PWV is an excellent indicator of arterial compliance of large vessels. Several studies demonstrated that hypertension, diabetes and smoke, reduce the compliance of artery wall. Furthermore, PWV is associated to coronary atherosclerosis and to an higher mortality. Significantly increase in PWV was observed also in inflammatory rheumatic diseases such as SLE and RA and correlates with impaired FMD and increased IMT[60-62]. Chronic and systemic inflammation could enhance arterial stiffness increasing the presence of fibroblasts cells at endothelial level, interfering with the processes that regulates arterial vasodilation and constriction. In RA patients arterial elasticity is also inversely related with inflammation indices[60]. PWA appears to be a more sensitive test of vascular dysfunction than FMD in RA and may be the preferred marker of vascular dysfunction in RA patients. Significantly increased PWV was observed in RA patients and PWV was correlated with impaired FMD and increased IMT. Instead, arterial elasticity is decreased and is inversely associated with measures of inflammation. In a recent study Provan et al[62] demonstrated the predictive value of CRP to increased arterial stiffness in 15 year follow up RA patients, confirming the role of inflammation on early atherosclerotic changes.
In addition to traditional risk factors in RA other possible biomarkers that could be associated with the development of subclinical atherosclerosis were investigated. In particular, it has been suggested that serum biomarker could be useful to assess the presence of subclinical atherosclerosis or to estimate the risk to develop cardiovascular events. The levels of ox-LDL are associated with increased cardiovascular risk and were significantly more elevated in RA patients compared to controls. Moreover serum levels of NO in patients with RA are significantly lower than in controls and, also, correlated inversely with IMT[63].
Asymmetric-dimethylarginine (ADMA) is a molecule that inhibits endothelial NO synthase (eNOS). Elevated ADMA levels are an independent risk factor for endothelial dysfunction, and they have been associated with hypertension, diabetes, hypercholesterolemia, renal failure, and atherosclerosis in both experimental models and humans. Recent evidences demonstrated that ADMA levels are increased in RA patients in respect to control and its levels decreased after therapy[64].
Apeline is a recently described peptide that is known to be produced by several cell types. It causes endothelium-dependent vasorelaxation by triggering the release of NO. Apeline serum levels are significantly lower in RA patients[64]. Other potential biomarkers of subclinical atherosclerosis are anti-oxidant substances such as beta-carotene, vitamin E, D and C. Further studies are needed to define the role of these molecules in clinical practice.
Vascular function is abnormal in RA and the atherosclerotic process seems to be accelerated: increased arterial stiffness, reduced arterial elasticity, impaired endothelial response, increased IMT and coronary calcium content are related to presence of systemic inflammation and with increased risk of cardiovascular morbidity and mortality. The management of RA patients should be in line with the European League Against Rheumatism recommendations for the management of cardiovascular risk in patients with RA[65]: these recommendations provide adequate control of arthritis and periodic evaluations of the cardiovascular risk; in particular, treatment strategy to control cardiovascular risk factors should be based on the use of statins and ACE-inhibitors or angiotensin II blockers because of their potential anti-inflammatory effects[65]. Further studies are needed to understand the impact of traditional therapies and new biologic drugs on the development of subclinical atherosclerosis and cardiovascular risk prevention. Traditional DMARDs and anti-TNF therapy could potentially decrease cardiovascular risk and improve endothelial function through the impact on inflammation. To support this hypothesis, some studies showed improvement in endothelial function, assessed by FMD and no progression of carotid IMT, in RA patients treated with adalimumab and infliximab[66,67]. Effect of B-cells depletion therapy on subclinical atherosclerosis is still debated: some study reported no effects on arterial stiffness during 6 mo therapy with rituximab, while other evidences demonstrated beneficial effects on FMD and IMT[68,69]. In conclusion, patients and physicians should be aware of the potential cardiovascular risk of rheumatoid arthritis and take specific diagnostic and therapeutic measures to assess and reduce this risk.
P- Reviewer: Bener A S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 589] [Article Influence: 29.5] [Reference Citation Analysis (2)] |
| 2. | Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care. 2012;18:S295-S302. [PubMed] |
| 3. | Gabriel SE, Crowson CS, Kremers HM, Doran MF, Turesson C, O’Fallon WM, Matteson EL. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 335] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 1053] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 5. | Meune C, Touzé E, Trinquart L, Allanore Y. High risk of clinical cardiovascular events in rheumatoid arthritis: Levels of associations of myocardial infarction and stroke through a systematic review and meta-analysis. Arch Cardiovasc Dis. 2010;103:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med. 2008;121:S21-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Del Rincón I, Williams K, Stern MP, Freeman GL, O’Leary DH, Escalante A. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Gonzalez-Gay MA, Gonzalez-Juanatey C, Piñeiro A, Garcia-Porrua C, Testa A, Llorca J. High-grade C-reactive protein elevation correlates with accelerated atherogenesis in patients with rheumatoid arthritis. J Rheumatol. 2005;32:1219-1223. [PubMed] |
| 10. | Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. Rheumatoid arthritis: a disease associated with accelerated atherogenesis. Semin Arthritis Rheum. 2005;35:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Pasceri V, Yeh ET. A tale of two diseases: atherosclerosis and rheumatoid arthritis. Circulation. 1999;100:2124-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 243] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 749] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 13. | Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15621] [Cited by in RCA: 15527] [Article Influence: 597.2] [Reference Citation Analysis (0)] |
| 14. | Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045-2051. [PubMed] |
| 15. | Lusis AJ. Atherosclerosis. Nature. 2000;407:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4039] [Cited by in RCA: 4162] [Article Influence: 166.5] [Reference Citation Analysis (0)] |
| 16. | Reiss AB, Glass AD. Atherosclerosis: immune and inflammatory aspects. J Investig Med. 2006;54:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Murdaca G, Colombo BM, Cagnati P, Gulli R, Spanò F, Puppo F. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis. 2012;224:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Hjeltnes G, Hollan I, Førre Ø, Wiik A, Mikkelsen K, Agewall S. Anti-CCP and RF IgM: predictors of impaired endothelial function in rheumatoid arthritis patients. Scand J Rheumatol. 2011;40:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Arnab B, Biswadip G, Arindam P, Shyamash M, Anirban G, Rajan P. Anti-CCP antibody in patients with established rheumatoid arthritis: Does it predict adverse cardiovascular profile? J Cardiovasc Dis Res. 2013;4:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Sokolove J, Brennan MJ, Sharpe O, Lahey LJ, Kao AH, Krishnan E, Edmundowicz D, Lepus CM, Wasko MC, Robinson WH. Brief report: citrullination within the atherosclerotic plaque: a potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum. 2013;65:1719-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27-III32. [PubMed] |
| 22. | Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 615] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 23. | Roman MJ, Moeller E, Davis A, Paget SA, Crow MK, Lockshin MD, Sammaritano L, Devereux RB, Schwartz JE, Levine DM. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med. 2006;144:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Kobayashi H, Giles JT, Polak JF, Blumenthal RS, Leffell MS, Szklo M, Petri M, Gelber AC, Post W, Bathon JM. Increased prevalence of carotid artery atherosclerosis in rheumatoid arthritis is artery-specific. J Rheumatol. 2010;37:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Tanasescu C, Jurcut C, Jurcut R, Ginghina C. Vascular disease in rheumatoid arthritis: from subclinical lesions to cardiovascular risk. Eur J Intern Med. 2009;20:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Puissant C, Abraham P, Durand S, Humeau-Heurtier A, Faure S, Rousseau P, Mahé G. [Endothelial function: role, assessment and limits]. J Mal Vasc. 2014;39:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Spadaro A, Perrotta FM, Carboni A, Cardini F, Annibali G, Lubrano E, Scarno A. Flow-mediated dilatation and its role in chronic rheumatic diseases. OAMM. 2013;1:2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3348] [Cited by in RCA: 3466] [Article Influence: 150.7] [Reference Citation Analysis (0)] |
| 29. | Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Lüscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 1097] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 30. | Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 381] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 31. | Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 511] [Cited by in RCA: 509] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 32. | Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 33. | Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 354] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 34. | Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 313] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 35. | Gokce N, Keaney JF, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 604] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 36. | Akamatsu D, Sato A, Goto H, Watanabe T, Hashimoto M, Shimizu T, Sugawara H, Sato H, Nakano Y, Miura T. Nitroglycerin-mediated vasodilatation of the brachial artery may predict long-term cardiovascular events irrespective of the presence of atherosclerotic disease. J Atheroscler Thromb. 2010;17:1266-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Martin J, Llorca J. Endothelial dysfunction, carotid intima-media thickness, and accelerated atherosclerosis in rheumatoid arthritis. Semin Arthritis Rheum. 2008;38:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Sarli B, Baktir AO, Cebicci M, Dogan Y, Demirbas M, Kurtul S, Saglam H, Akpek M, Sahin O, Arinc H. Predictors of Endothelial Dysfunction in Patients With Rheumatoid Arthritis. Angiology. 2013;Sep 26; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Foster W, Lip GY, Raza K, Carruthers D, Blann AD. An observational study of endothelial function in early arthritis. Eur J Clin Invest. 2012;42:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Hannawi S, Marwick TH, Thomas R. Inflammation predicts accelerated brachial arterial wall changes in patients with recent-onset rheumatoid arthritis. Arthritis Res Ther. 2009;11:R51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke. 2006;37:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 476] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 42. | Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Dal Palù C, Hansson L, Magnani B, Rahn KH, Reid J. Risk factors associated with alterations in carotid intima-media thickness in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis. J Hypertens. 1998;16:949-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 213] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 43. | Tell GS, Howard G, McKinney WM. Risk factors for site specific extracranial carotid artery plaque distribution as measured by B-mode ultrasound. J Clin Epidemiol. 1989;42:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Perrotta FM, Scarno A, Carboni A, Bernardo V, Montepaone M, Lubrano E, Spadaro A. Assessment of subclinical atherosclerosis in ankylosing spondylitis: correlations with disease activity indices. Reumatismo. 2013;65:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Gonzalez-Juanatey C, Llorca J, Amigo-Diaz E, Dierssen T, Martin J, Gonzalez-Gay MA. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 46. | van Sijl AM, Peters MJ, Knol DK, de Vet HC, Gonzalez-Gay MA, Smulders YM, Dijkmans BA, Nurmohamed MT. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum. 2011;40:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Ristić GG, Lepić T, Glisić B, Stanisavljević D, Vojvodić D, Petronijević M, Stefanović D. Rheumatoid arthritis is an independent risk factor for increased carotid intima-media thickness: impact of anti-inflammatory treatment. Rheumatology (Oxford). 2010;49:1076-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Evans MR, Escalante A, Battafarano DF, Freeman GL, O’Leary DH, del Rincón I. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 49. | Gonzalez-Juanatey C, Llorca J, Martin J, Gonzalez-Gay MA. Carotid intima-media thickness predicts the development of cardiovascular events in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2009;38:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 50. | Orchard TJ, Strandness DE. Assessment of peripheral vascular disease in diabetes. Report and recommendations of an international workshop sponsored by the American Diabetes Association and the American Heart Association September 18-20, 1992 New Orleans, Louisiana. Circulation. 1993;88:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 136] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Zierler RE, Sumner DS. Physiologic assessment of peripheral arterial occlusive disease. Vascular surgery. Philadelphia: Saunders 2000; 140-65. |
| 52. | del Rincón I, Haas RW, Pogosian S, Escalante A. Lower limb arterial incompressibility and obstruction in rheumatoid arthritis. Ann Rheum Dis. 2005;64:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 53. | Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med. 2004;164:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 428] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 54. | Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1020] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 55. | Giles JT, Szklo M, Post W, Petri M, Blumenthal RS, Lam G, Gelber AC, Detrano R, Scott WW, Kronmal RA. Coronary arterial calcification in rheumatoid arthritis: comparison with the Multi-Ethnic Study of Atherosclerosis. Arthritis Res Ther. 2009;11:R36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | De Gennaro Colonna V, Bonomo S, Ferrario P, Bianchi M, Berti M, Guazzi M, Manfredi B, Muller EE, Berti F, Rossoni G. Asymmetric dimethylarginine (ADMA) induces vascular endothelium impairment and aggravates post-ischemic ventricular dysfunction in rats. Eur J Pharmacol. 2007;557:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Galderisi M, Cicala S, Caso P, De Simone L, D’Errico A, Petrocelli A, de Divitiis O. Coronary flow reserve and myocardial diastolic dysfunction in arterial hypertension. Am J Cardiol. 2002;90:860-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Atzeni F, Sarzi-Puttini P, De Blasio G, Delfino L, Tomasoni L, Turiel M. Preclinical impairment of coronary flow reserve in patients with rheumatoid arthritis. Ann N Y Acad Sci. 2007;1108:392-397. [PubMed] |
| 59. | Ciftci O, Yilmaz S, Topcu S, Caliskan M, Gullu H, Erdogan D, Pamuk BO, Yildirir A, Muderrisoglu H. Impaired coronary microvascular function and increased intima-media thickness in rheumatoid arthritis. Atherosclerosis. 2008;198:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Wong M, Toh L, Wilson A, Rowley K, Karschimkus C, Prior D, Romas E, Clemens L, Dragicevic G, Harianto H. Reduced arterial elasticity in rheumatoid arthritis and the relationship to vascular disease risk factors and inflammation. Arthritis Rheum. 2003;48:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 61. | Kocabay G, Hasdemir H, Yildiz M. Evaluation of pulse wave velocity in systemic lupus erythematosus, rheumatoid arthritis and Behçet’s disease. J Cardiol. 2012;59:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Provan SA, Angel K, Semb AG, Mowinckel P, Agewall S, Atar D, Kvien TK. Early prediction of increased arterial stiffness in patients with chronic inflammation: a 15-year followup study of 108 patients with rheumatoid arthritis. J Rheumatol. 2011;38:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Profumo E, Di Franco M, Buttari B, Masella R, Filesi C, Tosti ME, Scrivo R, Scarno A, Spadaro A, Saso L. Biomarkers of subclinical atherosclerosis in patients with autoimmune disorders. Mediators Inflamm. 2012;2012:503942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Di Franco M, Spinelli FR, Metere A, Gerardi MC, Conti V, Boccalini F, Iannuccelli C, Ciciarello F, Agati L, Valesini G. Serum levels of asymmetric dimethylarginine and apelin as potential markers of vascular endothelial dysfunction in early rheumatoid arthritis. Mediators Inflamm. 2012;2012:347268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, McInnes IB, Haentzschel H, Gonzalez-Gay MA, Provan S. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1038] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 66. | Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, Gomez-Acebo I, Testa A, Garcia-Porrua C, Sanchez-Andrade A, Llorca J, González-Gay MA. Anti-TNF-alpha-adalimumab therapy is associated with persistent improvement of endothelial function without progression of carotid intima-media wall thickness in patients with rheumatoid arthritis refractory to conventional therapy. Mediators Inflamm. 2012;2012:674265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 67. | Sidiropoulos PI, Siakka P, Pagonidis K, Raptopoulou A, Kritikos H, Tsetis D, Boumpas DT. Sustained improvement of vascular endothelial function during anti-TNFalpha treatment in rheumatoid arthritis patients. Scand J Rheumatol. 2009;38:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 68. | Kerekes G, Soltész P, Dér H, Veres K, Szabó Z, Végvári A, Szegedi G, Shoenfeld Y, Szekanecz Z. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin Rheumatol. 2009;28:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 69. | Mathieu S, Pereira B, Dubost JJ, Lusson JR, Soubrier M. No significant change in arterial stiffness in RA after 6 months and 1 year of rituximab treatment. Rheumatology (Oxford). 2012;51:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |