BIOMECHANICS OF REVERSE SHOULDER REPLACEMENT
Anatomical total shoulder replacement can restore the full articular surface of the humeral head and the glenoid contour in cases of osteoarthritis. Deficiency of the rotator cuff and capsule can be repaired to provide soft tissue stability for the anatomical shoulder replacement. Massive irreparable rotator cuff tear and a deficient coracoacromial arch result in deficit of concavity compression to allow anterosuperior escape and pseudoparalysis. In such cases, anatomical total shoulder replacement cannot restore shoulder stability[7].
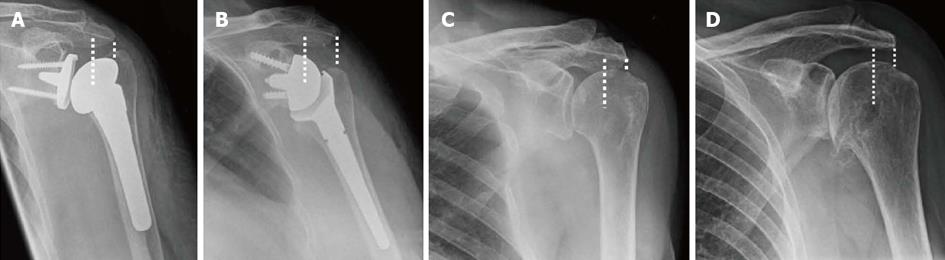
The design features of the reverse shoulder replacement provide stability not inherent in a total anatomical shoulder replacement. The reverse shoulder replacement is partially constrained by a spherical convex glenoid component and a deep and large diameter humeral cup (Figure 1B) in contrast to the shallow concave glenoid and spherical humeral head in a normal glenohumeral joint (Figure 1C). The Grammont et al[6] glenoid component in reverse shoulder replacement does not have a polyethylene component and is fixed with screws onto a base plate which in return is fixed onto the glenoid with divergent screws forming a triangular device. This geometry changes the center of rotation of the reverse shoulder replacement medial and distal onto a more stable point on the osseous surface of the glenoid thus avoiding shear stress of the humeral head on the glenoid component[6,8]. The full surface contact throughout the movement of articulation prevents glenohumeral translation thus removing the rocking horse mechanism by eliminating rim loading[7,9]. The more distal and medial center of rotation restores the deltoid tension, increases its lever arm and indirectly improves its power (Figures 1). The reversal of deltoid action to a centripetal action in reverse shoulder replacement in addition to the structural stability of the design provides a stable semi-constrained joint[6,7].
The current available reverse shoulder replacement systems in the market are based on the successful concept and principles introduced by Professor Grammont et al[6], a prosthesis that is stable, mobilises solely by deltoid and without risk of glenoid support loosening. Common features are the glenoid component (half a sphere in the Grammont design), the humeral cup (a third of a sphere) and the humeral neck with less vertical inclination (155° in the Grammont design). The large ball allows a greater arc of motion and more stability. The short neck of glenoid component medialises the center of rotation to reduce shear force which can loosen the component. The inclination of the humeral cup lowers and medialises the humerus to allow tension of deltoid by increasing the lever arm and recruiting more deltoid bulk[7,9].
INDICATIONS
Following successful reports of reverse shoulder replacement for CTA[6,9,10], its role has expanded with time. It is particularly useful in cases with deficient rotator cuff, such as painful irreparable massive cuff tear without osteoarthritis[11,12], inflammatory arthritis with cuff deficiency[13,14], acute complex proximal humerus fractures[15-17] and proximal humerus bone tumour surgery[18]. Rotator cuff deficiency is a common feature in all and is the best indication for a reverse shoulder replacement. Caution remains in the young population as there is still lack of long term knowledge about the longevity of reverse shoulder replacement but this is anticipated to be clearer in the next few years[8,17]. Non-functioning deltoid muscle is a contraindication for reverse shoulder replacement.
Irreparable massive rotator cuff tear (with and without osteoarthritis)
A large comparative study between age, sex and ASA-score matched patients undergoing hemiarthroplasty and reverse shoulder replacement for CTA using the New Zealand joint registry showed better Oxford Shoulder Score (OSS) in the reverse shoulder group at 6 mo after surgery[19]. Although 102 patients were identified in each group, only 64 hemiarthroplasty and 74 reverse shoulder replacement were available for final analysis. In a small subgroup of patients with 5 year follow up (18 hemiarthroplasty and 14 reverse shoulder), the improvement in OSS was still observed to be greater in the reverse shoulder group[19].
Naveed et al[8] presented a prospective series of the use of a single type of reverse shoulder replacement in 50 shoulders (43 patients) with CTA performed by a single surgeon. Functional outcome scores improved significantly at 8 to 81 mo postoperative follow up. The mean OSS improved by more than a third of the overall score and was a successful pain relieving pain procedure in 84% of the patients.
Wall et al[11] reviewed 191 mixed cases including 59 CTA and 34 massive rotator cuff tear without arthritis up to almost twelve years. These two groups of cases reported the best outcomes in function and subjective scorings following reverse shoulder replacement compared to patients with primary osteoarthritis, posttraumatic arthritis and revision arthroplasty. Movements were also improved but less in external rotation compared to elevation.
Similar observation was reported by Ek et al[20]. In a case series of 40 reverse shoulder replacement in patients younger than 65 years old performed for heterogenous cases, the functional outcome after a mean of 93 mo were similar between patients who did not have glenohumeral arthritis preoperatively and those who did.
Acute complex proximal humerus fracture
Reverse shoulder replacement in acute complex proximal humerus fracture not amenable to surgical fixation can be a good pain relief and functional restoration operation[16,17]. Although hemiarthroplasty is widely performed for complex proximal humeral fracture, there is concern regarding tuberosity union[15,17], integrity of rotator cuff especially in the elderly[21] and potential glenoid wear[22,23].
A prospective series of non-randomised comparison between patients over the age of seventy undergoing hemiarthroplasty and reverse shoulder replacement for complex proximal humerus fracture reported favourable clinical outcomes in the reverse replacement group[17]. Cuff et al[17] reported significantly better shoulder specific outcome scores in the reverse replacement group although the criticisms of the study were the small sample (26 hemiarthroplasty vs 27 reverse shoulder replacement) and the follow-up for reverse shoulder was shorter compared to the group of patients undergoing hemiarthroplasties due to the non-randomised design of the study. Functional results were dependent on the healing of tuberosities as worse outcome was seen in patient undergoing hemiarthroplasty with tuberosities resoption compared to hemiarthroplasty with healed tuberosities. Regardless of the healing of the tuberosities, patients with reverse replacement reported superior functional outcome compared to hemiarthroplasty but healed tuberosities conferred better range of external rotation[17].
A systematic review of 14 studies with 2-4 years of follow up using statistical pooling of outcomes and standard deviation reported 4 times greater odds of developing postoperative complications after reverse shoulder replacement compared to hemiarthroplasty using fracture-specific stem following proximal humerus fracture[16]. Most of the complications in the reverse replacement group were attributed to neurologic complications, reflex sympathetic dystrophy and dislocation[15,24]. The reoperation rates were, however, not different although the exact reason for this is debatable. The authors argued that there could be higher revision surgery in the reverse shoulder group if there was a good alternative salvage procedure. In the hemiarthroplasty group, the follow up period may have been too short to adequately report number of revision surgery. In addition, patients in the reverse shoulder replacement group were significantly older and suffered more fracture dislocations and were followed-up longer compared to patients in reverse shoulder replacement[16].
Chalmers et al[25] retrospectively compared 9 reverse shoulder vs 9 hemiarthroplasty vs 9 open reduction internal fixation for severe proximal humerus fracture and reported better active forward elevation, external rotation, cheaper and faster rehabilitation and total costs in the reverse group but similar outcome scores in all three. This study has its own limitation being a short follow up (minimum 1 year) especially in the reverse shoulder replacement group. In contrast, Gallinet et al[26] reported better abduction, forward flexion and Constant score in the reverse shoulder replacement but worse rotation compared to hemiarthroplasty.
Reverse shoulder replacement can provide good pain relief and allow satisfactory range of motion following complex proximal humerus fracture. In relation to internal fixation or hemiarthroplasty, the shorter and less restrictive postoperative rehabilitation after a reverse shoulder replacement may be an attractive factor for patient and in cost[25]. Similar to other areas of orthopaedic surgery, strong evidence for the use of reverse shoulder replacement in acute complex proximal humerus fracture is still not reported. Studies with a robust methodology and appropriate assessments will better inform the indication of reverse shoulder replacement in acute complex fractures.
Reverse shoulder replacement in the younger population
There is an acceptance that reverse shoulder replacement should be performed cautiously in the younger population[8,9,19]. This is due to the relatively new concept of the modern reverse prosthesis therefore there is at present limited amount of long term outcome on this technology. There is also a lack of salvage options for failed reverse shoulder replacement. Acceptable medium term result from using a stemless reverse shoulder prosthesis in a single surgeon case series represents a step towards preservation of bone stock but still does not solve glenoid complications[27].
Reverse shoulder replacement in the younger population was reported to be a good operation to improve range of motion, function and pain compared to the older population[28,29]. However, subjective reporting was weaker as 20% of the patients were not satisfied (either very dissatisfied, dissatisfied or not satisfied) after an average 36.5 mo following the surgery[29]. In these studies, the patients were younger than 60 years old with mixed cohort of pathologies including rotator cuff deficiency with or without osteoarthritis, revision arthroplasty, rheumatoid arthritis and posttraumatic arthritis which may negatively skewed the results.
Performing a reverse shoulder replacement in the younger population (65 years or younger) with rotator cuff arthropathy did not produce a better functional outcome compared to hemiarthroplasty at 6 mo review[19]. While longer data is required to inform practice surgeons should be reminded that delaying reverse shoulder replacement by performing other procedures could be detrimental to the final outcome[29] as demonstrated that patients with multiple operations before the reverse replacement surgery reported less improvement in functional scores.
Complication rates after reverse shoulder replacement were also high. Muh et al[29] reported 5 revisions and 2 resection arthroplasties in 67 reverse replacements, with survival rate of 89.5% within 6 years in patients age 60 years or younger. Sershon et al[28] reported 14% complication rate including 3 revisions within 4 years after reverse shoulder replacement in 36 shoulders, with total survival rate of 91% in patients with mean age of 54 years. Ek et al[20] reported 37.5% complications in 40 reverse shoulder replacements performed in patients younger than 65 years old. 6 (15%) required removal of prosthesis or conversion to hemiarthroplasty. Their survival rates were reported to be 76% (if any reoperation was taken as endpoint) and 88% (implant survival as endpoint) at 10 years postop. A positive finding from this study showed that the functional outcomes and range of active forward flexion were similar throughout the 10 years of follow up[20].
PITFALLS IN REVERSE SHOULDER REPLACEMENT
Approach
Adequate exposure is required for proper implantation of the reverse shoulder prosthesis especially of the glenoid. The deltopectoral (with or without extension) and deltoid split are the two most commonly used approaches. Naveed et al[8] experienced difficulty exposing the inferior glenoid adequately using deltoid split therefore changed their practice to extended deltopectoral which also allowed them to identify and protect the axillary nerve better. Deltopectoral approach disturbs the integrity of the subscapularis. A disadvantage of deltopectoral approach is a reported higher risk of dislocation in patients with irreparable subscapularis tendon in a prospective series by one surgeon[30]. In contrast, a retrospective study incorporating practices of three surgeons showed similar dislocation rates in patients undergoing reverse shoulder replacement using the deltopectoral approach with or without subscapularis repair[31].
Dislocations
Dislocation is usually due to insufficient soft tissue tension, especially the deltoid or due to worn polyethylene bearing. This could be managed by closed reduction alone or lengthening of the humeral liner[8,9,18,32]. Martinez et al[33] reported two patients with dislocation after reverse shoulder replacement for proximal humerus non-union treated with exchanging to a larger diameter glenosphere. Other causes of dislocation included CAM effect of the tubercle remnants, anteversion of humeral stem and obesity[24,25].
Notching
Notching prevalence increases with the longevity of prosthesis, reported from 40% at year 1 to 87% at 10 year follow up[9,24,29,32]. It is suggested as a cause of glenoid loosening and therefore a clinical concern and negatively affected functional outcomes[20,24] but some reported no effect on Constant score or reoperation rate[9,34]. Notching is not strongly proven to be associated with glenoid component loosening.
A reverse shoulder replacement with notching which extended beyond the inferior fixation screws when examined at post mortem did not show evidence of loosening of the glenoid base plate[35]. Although the true effect of scapular notching is still being investigated and debated, it is best to avoid loss of osseous tissue around prosthesis[9,20,24,34].
Scapular notching is likely related to mechanical impingement by the medial-inferior rim of the humeral cup against the posterior-inferior scapular neck in adduction[9,32,35]. Retrieval of the prosthesis/humeral cup revealed polyethylene wear due to this collision erosive effect[9,35]. The rim rubbed on denuded screw during flexion, extension and rotational movements in adduction[35]. Further to this, there may be detrimental effect of foreign-body reaction as a result of the wear particle to tissue surrounding the joint[35]. Radiolucencies were also seen at the lateral and medial proximal metaphyseal zone of the proximal humerus[24,32].
Identification of the inferior glenoid is also critical to allow inferior placement of the glenoid component. Inferior placement, inferior eccentricity or even overhang of the glenoid component was associated with less common occurrence of scapular notching[9,32,36-38]. The recommended overhang of 5.7 mm in female was predicted to decrease notching rate to 0.9% from 13% and 5.6 mm in male to 8.7% from 13%[39]. Notching seemed to be less common when a lateralised humeral cup was used that resulted in higher tension therefore restricting movement of the humeral component[32]. Notching was seen in 60% of 6 mm lateralised cup compared to 78% using standard cup (Levigne 2008). A larger and lateralised glenosphere allows more degree of adduction and abduction without inferior impingement[37]. Lesser notching was seen when using glenosphere of size 42 mm vs 38 mm and none in 46 mm[39]. Other factors such as less horizontal humeral neck and lesser prosthesis-scapular-neck-angle also contributed towards lesser chance of notching[39]. Lateralised centre of rotation resulted in early failure of the glenoid component therefore it should be used with caution[40]. A prospective randomised study comparing fixation of 36 mm glenosphere in neutral or in an inferiorly tilted position with 3 mm of overhang did not revealed difference in incidence of scapular notching or clinical outcome[41].
Range of movement
Abduction and anterior flexion of the shoulder with reverse replacement is provided mainly by the deltoid muscle. The amount of motion is affected by several factors. In a cadaveric study, Berhouet et al[37] shows that the shortest abduction were achieved using a 36 mm glenosphere. When a larger diameter glenosphere of 42 mm was used and lateralised 10 mm, it allowed the largest range of abduction (97°vs 87°).
External rotation is seen to be better in reverse shoulder replacement with an intact teres minor[6,9] and a less medialised glenoid component[34]. Lateralising the glenoid component alters the center of rotation to the component itself and may cause glenoid loosening without erosion[9,40]. Increasing the humeral retroversion improves the external rotation but at the cost of internal rotation.
Latissimus dorsi transfer can improve external rotation and subsequently function[42-44]. Ortmaier et al[43] reported harvesting the tendon together with a small piece of bone. The effect of latissimus dorsi transfer during reverse shoulder replacement for pseudoparesis on outcome scores and movement was reported to be preserved at 5 year review in 17 patients[44].
Internal rotation was reported to be less satisfactory or not improved after reverse shoulder replacement[9,45]. This is most likely due to insufficient internal rotator not compensated by the anterior deltoid fibers. The design of the prosthesis in lowering the humerus may also weaken the subscapularis by changing the vector of muscle contraction. The best rotationally balanced reverse shoulder replacement in a cadaveric study was native 17.5 degree retroversion[37].
Perioperative fractures
Complication decreases with learning curve[46]. Acromial fracture weakens the deltoid therefore rendering the reverse shoulder replacement non functional and clinically relevant. Fracture at the base of the acromion resulted in the worst outcome[47]. Pain along the acromion or scapular spine should alert the physician to such complication. CT scan may be required to help aid diagnosis where the plain radiographs are not diagnostic[47]. The decision of management needs to be tailored to the individual patient[47,48]. Humeral fracture is less common but perforation or propagation of cracks can occur during cementation or implantation of prosthesis. Fracture of the glenoid at the rim, glenoid surface or glenoid neck can occur during glenoid reaming or tightening of screws[7].