Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.279
Revised: June 16, 2013
Accepted: June 19, 2013
Published online: October 18, 2013
Processing time: 239 Days and 13.2 Hours
AIM: To describe the surgical technique of and indications for percutaneous pelvic osteotomy in patients with severe cerebral palsy.
METHODS: Twenty-one non-ambulatory children and adolescents (22 hips) were consecutively treated with percutaneous pelvic osteotomy, which was used in conjunction with varus, derotational, shortening femoral osteotomy and soft tissue release, to correct progressive hip subluxation and acetabular dysplasia. The age, gender, Gross Motor Function Classification System level, side(s) of operated hip, total time of follow-up, immediate post-operative immobilization, complications, and the need for revision surgery were recorded for all patients.
RESULTS: Seventeen patients (81%) were classified as GMFCS level IV, and 4 (19%) patients were classified as GMFCS level V. At the time of surgery, the mean age was 10.3 years (range: 4-15 years). The mean Reimers’ migration percentage improved from 63% (range: 3%-100%) pre-operatively to 6.5% (range: 0%-70%) at the final follow-up (P < 0.05). The mean acetabular angle (AA) improved from 34.1° (range: 19°-50°) pre-operatively to 14.1° (range: 5°-27°) (P < 0.05). Surgical correction of MP and AA was comparable in hips with open (n = 14) or closed (n = 8) triradiate cartilage (P < 0.05). All operated hips were pain-free at the time of the final follow-up visit, although one patient had pain for 6 mo after surgery. We did not observe any cases of bone graft dislodgement or avascular necrosis of the femoral head.
CONCLUSION: Pelvic osteotomy through a less invasive surgical approach appears to be a valid alternative with similar outcomes to those of standard techniques. This method allows for less muscle stripping and blood loss and a shorter operating time.
Core tip: In severe non-ambulatory, Gross Motor Function Classification System IV and V cerebral palsy patients with acetabular dysplasia and progressive hip subluxation or dislocation, most patients can achieve a painless and stable hip when a pelvic osteotomy through a minimally invasive surgical approach is performed in conjunction with a varus, derotational, shortening femoral osteotomy and soft tissue release surgery. Pelvic osteotomy through a less invasive surgical approach appears to be a valid alternative with an outcome similar to that of standard techniques and allows for less muscle stripping and blood loss and a shorter operating time.
- Citation: Canavese F, Rousset M, Samba A, Coulon G. Percutaneous pelvic osteotomy in cerebral palsy patients: Surgical technique and indications. World J Orthop 2013; 4(4): 279-286
- URL: https://www.wjgnet.com/2218-5836/full/v4/i4/279.htm
- DOI: https://dx.doi.org/10.5312/wjo.v4.i4.279
Hip subluxation and dislocation are common in children with cerebral palsy (CP) and have been reported in up to 45% of these patients[1-4]. The risk of hip displacement is related to the gross motor functional level as graded by the Gross Motor Function Classification System (GMFCS)[5-8]. In this system, children can be divided into five groups (Level I through V). Level I and II children have good walking abilities. Level III children can walk with the help of an assistive device. Level IV and V children are unable to walk[6]. The risk of progressive hip subluxation and/or dislocation is higher in patients with GMFCS IV and V ratings[5]. In 50%-70% of CP patients, hip displacement can make perineal care difficult[2,3,9-13], alter sitting balance[13-15], and be a source of pain[2,3,14-18]. Most clinicians agree that surgical treatment is indicated for progressive hip subluxation in this patient population[3,4,18-21].
The goal of any treatment is to create a reduced, stable, mobile hip with reduction of existing pain. Once a hip develops significant subluxation, reduction and stabilization can be achieved with a varus, derotational, shortening femoral osteotomy (VDRSO), which decreases anteversion and the tension on the surrounding hip musculature, including the hamstrings[14]. If acetabular dysplasia is present, acetabular osteotomy may also be needed. Acetabular dysplasia can be addressed with surgical procedures redirecting (e.g., Salter osteotomy), reshaping (e.g., Albee, Dega, Pemberton, or San Diego osteotomy) and salvaging/augmenting the depth of the acetabulum, such as with the shelf and Chiari osteotomies. The Dega osteotomy is an incomplete transiliac osteotomy and takes advantage of the inherent flexibility of the posterior column of bone in the pelvises of young children to reshape the acetabulum[22]. The Pemberton osteotomy extends directly to the triradiate cartilage[16]. In 1915, Albee described a semicircular osteotomy of the lateral part of the acetabular rim that is directed from lateral to medial into the ilium; the osteotomy is just cephalad to the attachment of the hip capsule to the ilium[23]. The San Diego osteotomy hinges symmetrically on or slightly above the triradiate cartilage, with care taken to use bone grafts that provide posterior coverage equal to the anterior coverage[10].
The Albee, Dega, Pemberton, and San Diego osteotomies reduce the volume and shape of the acetabulum by increasing its lateral coverage without a significant reduction in the posterior coverage[8-10,24-26]. Using a standard technique to reshape the acetabulum, Roposch et al[25], Robb et al[26], and Inan et al[27] showed that a stable, painless, and concentric reduction of the hip could be achieved in the majority of their patients. All the authors reported good results, with improvements in migration percentage and acetabular angle[28-30].
More recently, Canavese et al[24] described a minimally invasive, Albee-like percutaneous technique, i.e., percutaneous pelvic osteotomy, to correct acetabular dysplasia in non-ambulatory GMFCS IV and V patients with severe cerebral palsy. In their pilot study, approximately 28 hips treated with this minimally invasive technique, Canavese et al[24] reported similar radiological and clinical results with less muscle stripping and less bleeding and a shorter operating time compared to traditional surgical techniques[26,27,29].
The aim of this project is to describe the minimally invasive surgical technique, i.e., percutaneous pelvic osteotomy, used in conjunction with VDRSO and soft tissue release to correct hip subluxation or dislocation and acetabular dysplasia in non-ambulatory, GMFCS IV and V cerebral palsy patients. A clinical and radiological analysis of all the patients treated surgically with this technique is provided.
Twenty-one non-ambulatory children and adolescents (12 boys and 9 girls) with CP, GMFCS level IV and V, were consecutively treated, regardless of the age at presentation, with the percutaneous pelvic osteotomy technique in conjunction with VDRSO and soft tissue release.
Of these, 17 (81%) patients were classified as GMFCS level IV, and 4 (19%) patients were classified as GMFCS level V. All the patients were available for follow-up (Table 1). The mean age at the time of surgery was 10.3 years (range: 4-15), and the mean follow-up period was 16 mo (range: 9-28). The demographics, orthopedic manifestations, and age at surgery are shown in Table 1. The age, gender, GMFCS level, side(s) of operated hip, total time of follow-up, immediate post-operative immobilization, complications, and the need for revision surgery were recorded for all patients. Overall, 22 consecutive hips (9 right and 13 left) of non-ambulatory patients with severe CP were treated by this technique. All surgical procedures were performed by the first author of this work (FC) at one institution. The data collection and analysis were performed by a confirmed pediatric orthopedic surgeon not involved in the surgery (MR).
| Pt. | Sex | GMFCS | Triradiate | Age at | Percutaneous pelvic | VDRSO | Soft tissue release | Post-operative |
| cartilage | surgery (yr) | osteotomy (Side) | immobilization (Spica cast) | |||||
| 1 | M | IV | Open | 8 | Right | Unilateral (Right) | ||
| 2 | M | IV | Closed | 15 | Left | Bilateral | ||
| 3 | M | V | Open | 12 | Right | Unilateral (Right) | ||
| 4 | M | V | Open | 6 | Left | Bilateral | No | |
| 5 | F | IV | Open | 10 | Left | Bilateral | Yes | |
| 6 | F | V | Open | 8 | Right | Unilateral (Right) | ||
| 7 | M | IV | Open | 11 | Left | Bilateral | ||
| 8 | M | IV | Open | 7 | Left | Bilateral | ||
| 9 | M | IV | Open | 15 | Right | Bilateral | ||
| 10 | M | IV | Closed | 15 | Bilateral | Bilateral | Yes | |
| 11 | F | IV | Open | 8 | Left | Bilateral | ||
| 12 | F | IV | Open | 4 | Left | Bilateral | Yes | |
| 13 | M | IV | Open | 15 | Left | Bilateral | No | |
| 14 | M | IV | Open | 6 | Left | Bilateral | No | Yes |
| 15 | F | IV | Closed | 12 | Left | Bilateral | ||
| 161 | F | V | Closed | 14 | Left | Bilateral | ||
| 17 | M | IV | Closed | 14 | Left | Bilateral | ||
| 18 | F | IV | Closed | 12 | Right | Bilateral | Yes | |
| 19 | F | IV | Open | 10 | Right | Bilateral | No | Yes |
| 20 | M | IV | Open | 5 | Right | Bilateral | Yes | |
| 21 | M | IV | Open | 8 | Right | Bilateral | No | Yes |
The percutaneous pelvic osteotomy is performed as part of a combined procedure that includes femoral VDRSO and soft-tissue release.
Position of the patient: The patient was placed in a supine position on the operating table. A small sand bag must be placed under the gluteal area of the operated side to push the affected hemipelvis forward. First, the VDRSO is carried out through a standard lateral approach to the proximal femur. Subsequently, without changing the position of the patient, the reference points for the percutaneous pelvic osteotomy are identified under an image intensifier. The image intensifier is placed in front of the surgeon
Landmarks and skin incision: Two lines must be drawn on the patient’s skin to identify the correct site of incision. Under an image intensifier, a straight vertical line, corresponding to the axis of the acetabular roof, is drawn 5-10 mm proximal to the roof of the acetabulum. A second horizontal line starting at the tip of the greater trochanter is traced between the anterior superior iliac spine (ASIS) and the posterior iliac spine. The intersection between the first and the second line indicates where to make the skin incision. The skin incision should measure approximately 2 to 3.5 centimeters in length and be parallel to the femoral shaft.
Superficial and deep surgical dissection: After the skin incision is made, dissection down through the subcutaneous fat can be performed with scissors; electrocautery can be used if needed. During dissection, the proximal portion of the tensor fascia latae muscle must be opened to reach the gluteus medius and the gluteus minimus muscles. The gluteus minimus muscle must be dissected bluntly to reach the outer table of the iliac bone. At this stage, an image intensifier must be used to verify that the instrument used for the dissection has reached the planned point for the osteotomy. Using a Cobb dissector, the muscle tissue is scraped off the outer table of the iliac bone from the sciatic notch to the ASIS. By sliding the index finger under the muscles, it is possible to feel the smooth surface of the outer table of the iliac crest, the prominence of the ASIS anteriorly, and the curved most lateral portion of the sciatic notch posteriorly. At this point, a smooth dissector can be slid under the periosteum to reach the sciatic notch and used to protect the nervous structures when performing the osteotomy.
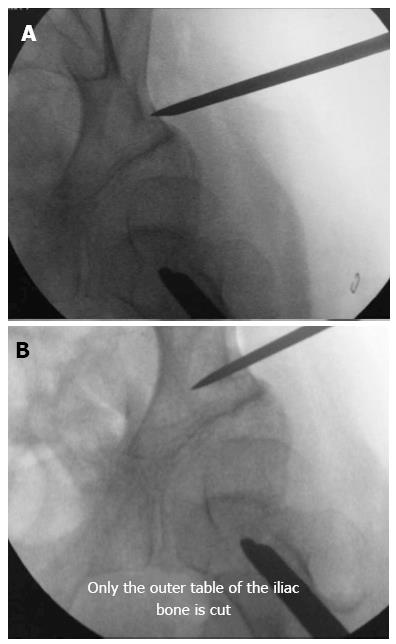
Pelvic osteotomy: The pelvic osteotomy should be performed between 5 and 10 mm proximal to the acetabular roof[10]. The pelvic osteotomy must be performed under an image intensifier, and the osteotome should always appear as a straight line during the entire procedure, indicating that the osteotome is perpendicular to the bone and parallel to the source of radiation. Only the outer table of the iliac bone from the ASIS to the sciatic notch must be cut (Figure 1).
The osteotomy is first performed with a straight osteotome, and then, a curved osteotome is used to complete the osteotomy. The osteotomes must always be directed towards the triradiate cartilage. The skin incision must be wide enough to allow the straight and curved osteotomes to perform the pelvic osteotomy without damaging the skin and the subcutaneous tissues. The osteotome can be displaced upwards and downwards toward the ASIS and the sciatic notch, respectively. In patients with closed triradiate cartilage, the osteotomy must be extended to the original site of the cartilage and takes advantage of the reduced resistance of the porous iliac bone[24]. In this subgroup of patients, broader osteotomes should be used to open the osteotomy to avoid collapse of the porotic iliac bone under the pressure exerted by the osteotome during the opening maneuver[22,24].
Bone graft: Once the pelvic osteotomy is completed, two straight osteotomes are inserted and used to lever open the osteotomy. The maximum opening of the osteotomy is assessed under an image intensifier. The graft obtained from the femoral shortening must be wedged on the basis of this measurement. A 2 mm Kirschner wire is inserted into the graft to help push it into the opened space. Spreading the two osteotomes, or, using a bone distractor, opens the space created by the osteotomy and allows the bone graft to slide into it, without having the graft rotate around the wire. As soon as approximately 40% of the length of the graft passes the outer table, the upper osteotome can be removed. At this point, the graft can be advanced further by hitting the tip of the Kirschener wire with an adequately sized hammer. Counter pressure must be applied on the Kirschener wire when removing the second osteotome. At this stage, the Kirschener wire can be removed and, if needed, a bone impactor can be used to impact the graft further. There is no need to fix the bone graft with metal hardware because soft tissues contribute toward keeping the bone graft in place by pushing it against the iliac bone, as the surgical dissection is reduced compared with standard techniques[24].
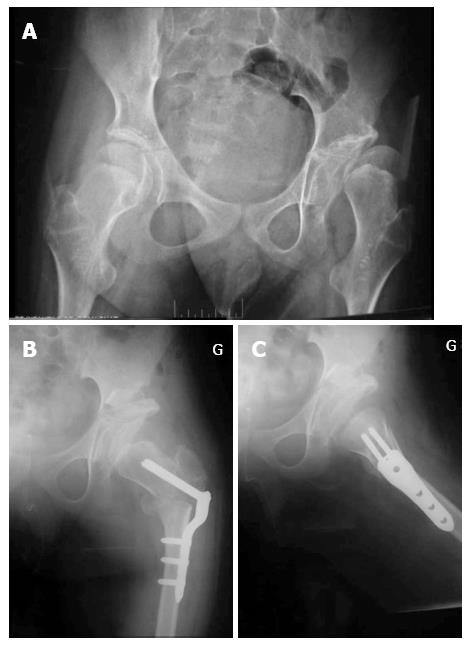
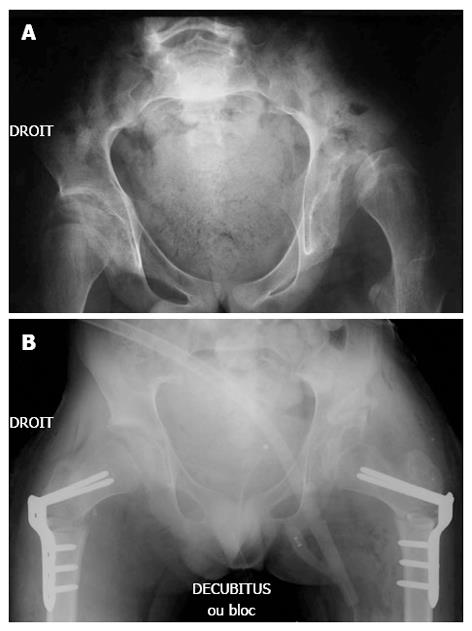
Radiographic assessment: An anterior-posterior radiograph of the pelvis and a lateral radiograph of the operated hip must be performed to assess the coverage of the femoral head and verify good positioning of the bone graft (Figures 2 and 3).
Length of surgery: Surgical time is reduced with this procedure compared with standard techniques. The procedure lasts between 15 and 25 min, from skin incision to skin closure. Longer operating times (from 30 to 40 min) were observed at the beginning of our experience.
Radiation exposure: Because the osteotomy is performed under image intensifier guidance, the amount of exposure may be higher compared with standard open techniques.
One possible complication, although not yet encountered by the authors of this study, is a lesion of the superior gluteal artery and/or the superior gluteal nerve, which run close to each other approximately 3-4 cm proximal to the skin incision. We recommend ensuring that the skin incision is not excessively proximal and that the incision is made only after the identification of adequate reference points. In order not to damage the sciatic nerve, a smooth dissector can be placed under the periosteum to reach the sciatic notch. The osteotome can be used safely above the dissector, which protects the nerve running below it. No cases of sciatic nerve damage or transection have been reported[24].
This procedure is indicated for non-ambulatory, GMFCS IV and V, patients with severe CP who have unilateral or bilateral hip subluxation or dislocation and acetabular dysplasia. If the hip does not reduce after soft-tissue release and VDRSO, an open reduction should be considered. Hips that have been displaced for several years are most likely to be associated with capsular retraction, and percutaneous pelvic osteotomy should not be performed. This surgical procedure should not be performed in ambulatory patients because the effect of cutting through the abductor muscle mass is not known.
Study ethics approval was obtained (CECIC Rhône-Alpes-Auvergne, Grenoble, France IRB 5921).
The results were analyzed using a paired Student’s t test to assess the pre- and post-operative differences. The level of significance was set at P < 0.05.
The mean Reimers’ migration percentage (MP) improved from 63% (range: 33%-100%) pre-operatively to 6.5% (range: 0%-70%) at the final follow-up (P < 0.05). The MP remained stable during the follow-up period; it was 14%, 15%, 13.6% and 12.4% at 3, 6, 12 and 18 mo after surgery, respectively.
The mean acetabular angle (AA) improved from 34.1° (range: 19-50°) pre-operatively to 14.1° (range: 5-27°) (P < 0.05). The AA remained stable during the follow up period; it was 8.0°, 6.9°, 6.4° and 8.0° at 3, 6, 12 and 18 mo after surgery, respectively.
At 18 mo average follow-up (range: 9-28), all hips remained located and the MP and AA improved from 63% to 6.5% and from 34.1° to 14.1°, respectively (P < 0.05). The MP and AA remained stable between the index surgery and the last follow up visit. We did not detect radiographic evidence of premature closure of the triradiate cartilage in any patient with open triradiate cartilage in our series. The surgical correction of the MP and AA was comparable in hips with open (n = 14) or closed (n = 8) triradiate cartilage (P < 0.05). All operated hips were pain-free at the time of the last follow-up visit, although one patient had pain for 6 months after surgery. We did not observe any cases of bone graft dislodgement or avascular necrosis of the femoral head.
Immobilization was accomplished postoperatively with a spica cast for 10 wk in 9 out of 21 patients (42.8%) because of abnormal and/or dystonic movements.
One GMFCS V patient died approximately three months after surgery because of a severe respiratory tract infection. Two patients had unilateral femur fractures. One femur fracture occurred at the distal metaphysis and was not directly related to the surgical procedure (osteoporosis). The other fracture occurred 18 mo after surgery at the distal end of the metal hardware (trauma). No cases of avascular necrosis of the femoral head, bone graft dislodgment, hip dislocation and premature closure of triradiate cartilage were recorded (Table 2).
Most non-ambulatory, GMFCS IV and V patients with severe CP that present with progressive hip subluxation or dislocation and acetabular dysplasia can achieve a painless and stable hip when a pelvic osteotomy is performed through a minimally invasive surgical approach in conjunction with VDRSO and soft tissue release surgery.
The skin incision, 2-3 cm in length, is wide enough to allow the straight and curved osteotomes to perform the osteotomy. One to two and a half centimeter osteotomes can be used without damaging the skin and subcutaneous soft tissues. The osteotomes can be displaced easily upwards and downwards towards the anterior iliac spine and the sciatic notch, respectively. Using a standard technique to reshape the acetabulum, Roposch et al[25], Robb et al[26], and Inan et al[27] demonstrated that a stable, painless, and concentric reduction of the hip could be achieved in most of their patients. All authors reported good results with improvement of the MP and AA[25-30].
More recently, Canavese et al[24] described a minimally invasive, pelvic osteotomy technique to correct acetabular dysplasia in severely involved CP patients. Canavese et al[24] undertook a retrospective review of 28 patients (17 boys and 11 girls) with the diagnoses of CP, GMFCS level IV and V, progressive hip subluxation and acetabular dysplasia who were treated surgically by simultaneous percutaneous pelvic osteotomy, VDRSO and soft tissue release. The authors reported favorable mid to long term results and concluded that similar radiological and clinical results with less muscle stripping, less bleeding and a shorter operating time compared to traditional surgical techniques can be achieved with a percutaneous pelvic osteotomy in conjunction with VDRSO and soft tissue release[24].
We did not detect radiographic evidence of premature closure of the triradiate cartilage in any patients with open triradiate cartilage in our series. Image intensifier guidance and adequate osteotomes are needed to prevent physeal damage and avoid this complication. The osteotomy extends down to the triradiate cartilage, but does not cross it (Figure 2). Bucholz et al[31] found that premature closure of the triradiate cartilage did not affect the stability of the hip if the closure occurred after 10 years of age. Twelve patients (5%) in our group were 10 years or older at the time of surgery.
We found that this technique could also be successfully performed in patients with severe CP that have closed triradiate cartilage (Figure 3). Surgical correction of the MP and AA was comparable in hips with open or closed triradiate cartilage. This is most likely related to the porous bone quality of non-ambulatory CP patients, which allows the surgeon to open the osteotomy site and pack in the graft. Osteoporosis is a common finding in patients with severe neuromuscular conditions and in non-ambulatory CP patients[32]. In particular, patients with severe CP suffer from osteoporosis, which contributes to poor bone condition, pathological fractures, and discomfort[32-35].
In skeletally mature patients, the osteotomy must be extended to the original site of the triradiate cartilage to take advantage of the reduced resistance of the porous iliac bone. In this subgroup of patients, broader osteotomes should be used to open the osteotomy. We believe that this procedure is necessary to avoid collapse of the porotic iliac bone under the pressure exerted by the osteotome during the opening maneuver.
We did not observe any cases of bone graft dislodgement. The bone graft comes from the femoral shortening and is reshaped immediately before insertion. Our hypothesis is that soft tissues play an important role in keeping the graft in place, as the surgical dissection is reduced compared to classic techniques. Soft tissue may contribute to keeping the bone graft in place by pushing it against the iliac bone. This hypothesis is further supported by the findings that approximately one-half of our patients did not require cast immobilization, regardless of the age at surgery. In our series, all hips remained located, and no one developed avascular necrosis.
Blood loss is reduced with this technique compared to standard techniques, and the surgical time is shorter. Because the osteotomy is performed under image intensifier guidance, the amount of exposure may be higher compared to standard open techniques.
There is some evidence that VDROs are highly effective in younger patients, before significant acetabular dysplasia develops, but are less effective in older patients[14,29,31,36]. In their long-term follow-up study, Song and Carroll reported hip dislocation or subluxation rates of 26% after VDRSO alone and 12% after VDRSO and pelvic osteotomy[29]. Khalife et al[30] reported a 13.5% redislocation rate after VDRSO. They concluded that the major risk factors for secondary dislocation appear to be insufficient correction of preexisting valgus and uncorrected acetabular dysplasia. In their group of patients with severe CP that underwent unilateral hip surgery, Canavese et al[14] found that greater than 50% had an MP over 50%, or had redislocation of the operated hip or displacement of the contralateral hip at skeletal maturity. Mid- and long-term follow-up studies are required to assess redislocation and/or revision surgery rates and to draw a definitive conclusion. During the follow-up period, none of the patients required additional surgery because all hips remained located.
Although longer follow-up studies are required to draw definitive conclusions, our findings indicate that a combined approach of percutaneous pelvic osteotomy, VDRSO and soft-tissue release is an effective, reliable, and minimally invasive method for the treatment of spastic dislocated hips in patients with severe CP. Patients with relative incongruity, closed triradiate cartilage, and some deformity of the femoral head can benefit from this combined approach. If the hip does not reduce after soft-tissue release and VDRSO, an open reduction should be considered. Pelvic osteotomy through a less invasive surgical approach appears to be a valid alternative with outcomes similar to standard techniques. This method allows for less muscle stripping and blood loss and a shorter operating time. Recent studies suggest that soft-tissue procedures for the management of hip displacement in children at GMFCS levels IV and V have a high failure rate, but the best form of bony reconstruction is yet to be determined.
Acetabular dysplasia and hip subluxation and dislocation are common findings in children with cerebral palsy. The risk of hip displacement is related to the gross motor functional level as graded by the Gross Motor Function Classification System (GMFCS). The risk of progressive hip subluxation and/or dislocation is higher in GMFCS IV and V patients.
The goal of any treatment is to create a reduced, stable, mobile hip with reduction of existing pain. Once a hip develops significant subluxation, reduction and stabilization can be achieved with a varus, derotational shortening femoral osteotomy (VDRSO) to decrease anteversion and the tension on the surrounding hip musculature, including the hamstrings. If acetabular dysplasia is present, acetabular osteotomy may be needed.In this study, authors demonstrate that the correction of hip dysplasia can be safely achieved with a minimally invasive technique, i.e., percutaneous pelvic osteotomy.
Although longer follow-up studies are required to draw definitive conclusions, a combined approach of percutaneous pelvic osteotomy, VDRSO and soft-tissue release is an effective, reliable, and minimally invasive method for the treatment of spastic dislocated hips in patients with severe cerebral palsy. Patients with relative incongruity, closed triradiate cartilage, and some deformity of the femoral head can also benefit from this combined approach.
This procedure is indicated for non-ambulatory, GMFCS IV and V, severe cerebral palsy patients with unilateral or bilateral hip subluxation or dislocation and acetabular dysplasia. If the hip does not reduce after soft-tissue release and VDRSO, an open reduction should be considered. This surgical procedure should not be performed in ambulatory patients because the effect of cutting through the abductor muscle mass is not known.
Cerebral palsy indicates a group of non-progressive disorders of movement and posture caused by abnormal development of, or damage to, the motor control centers of the brain. The condition can be caused by events before, during, or after birth. Acetabular dysplasia, hip subluxation and dislocation, lower limb abnormalities, tendon contractures and scoliosis are common orthopedic disorders in cerebral palsy patients.
The authors presented a new surgical technique. The pelvic osteotomy can be performed through a 2 to 3 cm skin incision. The clinical and radiological outcomes are good. The results are interesting, and this approach can be used in patients with open and closed triradiate cartilage. The percutaneous pelvic osteotomy seems to be an effective, reliable, and minimally invasive method for the treatment of acetabular dysplasia in patients with severe cerebral palsy.
P- Reviewer Willis-Owen C S- Editor Zhai HH L- Editor A E- Editor Wang CH
| 1. | Howard CB, McKibbin B, Williams LA, Mackie I. Factors affecting the incidence of hip dislocation in cerebral palsy. J Bone Joint Surg Br. 1985;67:530-532. [PubMed] |
| 2. | Lonstein JE, Beck K. Hip dislocation and subluxation in cerebral palsy. J Pediatr Orthop. 1986;6:521-526. [PubMed] |
| 3. | Spencer JD. Reconstruction of dislocated hips in children with cerebral palsy. BMJ. 1999;318:1021-1022. [PubMed] |
| 4. | Scrutton D, Baird G, Smeeton N. Hip dysplasia in bilateral cerebral palsy: incidence and natural history in children aged 18 months to 5 years. Dev Med Child Neurol. 2001;43:586-600. [PubMed] |
| 5. | Soo B, Howard JJ, Boyd RN, Reid SM, Lanigan A, Wolfe R, Reddihough D, Graham HK. Hip displacement in cerebral palsy. J Bone Joint Surg Am. 2006;88:121-129. [PubMed] |
| 6. | Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214-223. [PubMed] |
| 7. | Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1229] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 9. | Miller F, Girardi H, Lipton G, Ponzio R, Klaumann M, Dabney KW. Reconstruction of the dysplastic spastic hip with peri-ilial pelvic and femoral osteotomy followed by immediate mobilization. J Pediatr Orthop. 1997;17:592-602. [PubMed] |
| 10. | McNerney NP, Mubarak SJ, Wenger DR. One-stage correction of the dysplastic hip in cerebral palsy with the San Diego acetabuloplasty: results and complications in 104 hips. J Pediatr Orthop. 2000;20:93-103. [PubMed] |
| 11. | Song HR, Carroll NC. Femoral varus derotation osteotomy with or without acetabuloplasty for unstable hips in cerebral palsy. J Pediatr Orthop. 1998;18:62-68. [PubMed] |
| 12. | Oh CW, Presedo A, Dabney KW, Miller F. Factors affecting femoral varus osteotomy in cerebral palsy: a long-term result over 10 years. J Pediatr Orthop B. 2007;16:23-30. [PubMed] |
| 13. | Cooperman DR, Bartucci E, Dietrick E, Millar EA. Hip dislocation in spastic cerebral palsy: long-term consequences. J Pediatr Orthop. 1987;7:268-276. [PubMed] |
| 14. | Canavese F, Emara K, Sembrano JN, Bialik V, Aiona MD, Sussman MD. Varus derotation osteotomy for the treatment of hip subluxation and dislocation in GMFCS level III to V patients with unilateral hip involvement. Follow-up at skeletal maturity. J Pediatr Orthop. 2010;30:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Graham HK, Boyd R, Carlin JB, Dobson F, Lowe K, Nattrass G, Thomason P, Wolfe R, Reddihough D. Does botulinum toxin a combined with bracing prevent hip displacement in children with cerebral palsy and "hips at risk"? A randomized, controlled trial. J Bone Joint Surg Am. 2008;90:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Pemberton PA. Pericapsular osteotomy of the ilium for treatment of congenital subluxation and dislocation of the hip. J Bone Joint Surg Am. 1965;47:65-86. [PubMed] |
| 17. | Shea KG, Coleman SS, Carroll K, Stevens P, Van Boerum DH. Pemberton pericapsular osteotomy to treat a dysplastic hip in cerebral palsy. J Bone Joint Surg Am. 1997;79:1342-1351. [PubMed] |
| 18. | Zuckerman JD, Staheli LT, McLaughlin JF. Acetabular augmentation for progressive hip subluxation in cerebral palsy. J Pediatr Orthop. 1984;4:436-442. [PubMed] |
| 19. | Gordon JE, Capelli AM, Strecker WB, Delgado ED, Schoenecker PL. Pemberton pelvic osteotomy and varus rotational osteotomy in the treatment of acetabular dysplasia in patients who have static encephalopathy. J Bone Joint Surg Am. 1996;78:1863-1871. [PubMed] |
| 20. | Sankar WN, Spiegel DA, Gregg JR, Sennett BJ. Long-term follow-up after one-stage reconstruction of dislocated hips in patients with cerebral palsy. J Pediatr Orthop. 2006;26:1-7. [PubMed] |
| 21. | Al-Ghadir M, Masquijo JJ, Guerra LA, Willis B. Combined femoral and pelvic osteotomies versus femoral osteotomy alone in the treatment of hip dysplasia in children with cerebral palsy. J Pediatr Orthop. 2009;29:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Dega W. Selection of surgical methods in the treatment of congenital dislocation of the hip in children. Chir Narzadow Ruchu Ortop Pol. 1969;34:357-366. [PubMed] |
| 23. | Albee FH. The bone graft wedge. Its use in the treatment of relapsing, acquired, and congenital dislocation of the hip. New York Med J. 1915;102:433-435. |
| 24. | Canavese F, Gomez H, Kaelin A, Ceroni D, de Coulon G. Percutaneous pelvic osteotomy and intertrochanteric varus shortening osteotomy in nonambulatory GMFCS level IV and V cerebral palsy patients: preliminary report on 30 operated hips. J Pediatr Orthop B. 2013;22:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Roposch A, Wedge JH. An incomplete periacetabular osteotomy for treatment of neuromuscular hip dysplasia. Clin Orthop Relat Res. 2005;435:166-175. [PubMed] |
| 26. | Robb JE, Brunner R. A Dega-type osteotomy after closure of the triradiate cartilage in non-walking patients with severe cerebral palsy. J Bone Joint Surg Br. 2006;88:933-937. [PubMed] |
| 27. | Inan M, Gabos PG, Domzalski M, Miller F, Dabney KW. Incomplete transiliac osteotomy in skeletally mature adolescents with cerebral palsy. Clin Orthop Relat Res. 2007;462:169-174. [PubMed] |
| 28. | Reimers J. The stability of the hip in children. A radiological study of the results of muscle surgery in cerebral palsy. Acta Orthop Scand Suppl. 1980;184:1-100. [PubMed] |
| 29. | Sharp IK. Acetabular dysplasia: the acetabular angle. J Bone Joint Surg Br. 1961;43:268-272. |
| 30. | Khalife R, Ghanem I, El Hage S, Dagher F, Kharrat K. Risk of recurrent dislocation and avascular necrosis after proximal femoral varus osteotomy in children with cerebral palsy. J Pediatr Orthop B. 2010;19:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Bucholz RW, Ezaki M, Ogden JA. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg Am. 1982;64:600-609. [PubMed] |
| 32. | Allington N, Vivegnis D, Gerard P. Cyclic administration of pamidronate to treat osteoporosis in children with cerebral palsy or a neuromuscular disorder: a clinical study. Acta Orthop Belg. 2005;71:91-97. [PubMed] |
| 33. | Henderson RC. Bone density and other possible predictors of fracture risk in children and adolescents with spastic quadriplegia. Dev Med Child Neurol. 1997;39:224-227. [PubMed] |
| 34. | Henderson RC, Lark RK, Gurka MJ, Worley G, Fung EB, Conaway M, Stallings VA, Stevenson RD. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110:e5. [PubMed] |
| 35. | Henderson RC, Lin PP, Greene WB. Bone-mineral density in children and adolescents who have spastic cerebral palsy. J Bone Joint Surg Am. 1995;77:1671-1681. [PubMed] |
| 36. | Huh K, Rethlefsen SA, Wren TA, Kay RM. Surgical management of hip subluxation and dislocation in children with cerebral palsy: isolated VDRO or combined surgery? J Pediatr Orthop. 2011;31:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |