INTRODUCTION
Since the 1970’s we have advanced in understanding of bone biology from scarcely appreciating that there are cells in bone to seeing now a wonderful complex of intercellular communication, involving not only the cells of bone but also those of the hemopoietic and immune systems, and control systems far beyond the early simple ideas of hormone regulation. Many cytokines contribute to the balanced outcome of these cell communication processes, the brain and sympathetic nervous systems play central roles, and the skeleton even behaves as an endocrine organ itself.
This chapter consists of reflections on the scientific background and events that set the scene for the eventual discovery of the control of osteoclast formation by members of the tumor necrosis factor (TNF) ligand and receptor families, and of the impact of these discoveries.
THE OSTEOCLAST
From the time of their discovery in 1873[1] as the multinucleated cells responsible for bone resorption, osteoclasts provoked great interest, first in their study for many years by light and electron microscopy, when they were recognised to possess unique ultrastructural characteristics which both distinguished them from other cell types and seemed likely to provide them with an advantage in achieving motility to aid in bone resorption[2]. Apart from their multinuclearity, a striking feature of the osteoclast was noted as the presence of the “ruffled border”, a complex structure of deeply interfolded finger-like projections of the plasma and cytoplasmic membranes adjacent to the bone surface. Adjacent to and surrounding the ruffled border is the clear zone, an area of cytoplasm devoid of cellular organelles except for numerous cytoplasmic actin filaments. The clear zone became known as the “sealing zone”, since the plasma membrane in this region comes into very close apposition with the bone surface to ensure osteoclast attachment, and to separate the bone-resorbing area beneath the ruffled border from the unresorbed area, which maintains in a closed compartment a favourable microenvironment for bone resorption[2].
From the mid - 1970’s the organ culture of fetal and newborn bone provided for the first time means of deducing more of the function of living osteoclasts, and in the 1980’s the first osteoclasts were studied as isolated cells in culture. These approaches began to provide biochemical explanations for the earlier structural observations. Osteoclasts were considered to bring about dissolution of bone mineral by creating an acid microcompartment under the ruffled border, adjacent to the bone surface[3], with acidification achieved by the passage of hydrogen and chlorides ions through the ruffled border[4,5]. The functions of the acid milieu are to promote dissolution of mineral and to provide the appropriate pH for optimal action of the protease, cathepsin K, in the dissolution of bone matrix. Osteoclasts were found to be rich in tartrate-resistant acid phosphatase (TRAP), which is a commonly used histochemical marker for osteoclasts, although not exclusive to those cells. It is nevertheless a convenient marker for in vitro generated cells when combined with identification of calcitonin receptors[6,7] and the ability to form resorption pits when grown on thin slices of cortical bone or dentine. Some other properties include possession of vitronectin receptors, vacuolar ATP-ase, and chloride-7 channels and secretion of cathepsin K. This combination of properties provides the phenotype that equips osteoclasts uniquely to resorb bone.
DEVELOPMENT OF CONCEPTS OF THE ORIGIN OF OSTEOCLASTS
Ideas of the cellular origin and development of osteoclasts were much more contentious, and remained so until the 1980’s. Autoradiographic evidence had led Tonna[8] to conclude that osteoclasts arise from fusion of osteoblasts and that osteoclasts can dissociate again into osteogenic precursor cells. Fornadley et al[9] believed that osteoclasts and osteoblasts originate from a common osteoprogenitor cell, and at a later stage may return to the osteoprogenitor pool. In 1974, Rasmussen et al[10] proposed that endosteal mesenchymal cells differentiate into pre-osteoclasts which may then form an osteoclast by fusion. At a certain time and place the osteoclast then dissociates into pre-osteoblasts, giving rise to osteoblasts and osteocytes. These views of a connective tissue cell origin of osteoclasts were all subsequently superseded in the face of compelling evidence for a hemopoietic origin of osteoclasts.
The first convincing evidence that osteoclasts and osteoblasts came from different lineages came in the mid-1970’s. Studies using a variety of model systems including quail-chick chimera experiments, parabiosis experiments and the restoration of bone resorption in osteopetrosis by bone marrow and spleen cell transplantation, showed that osteoclasts are supplied to bone via the circulatory system, and are formed by fusion of mononucleated precursors derived from hemopoietic progenitor cells[11-13]. The important finding common to these experiments was that the precursors of the osteoclast could travel via the blood to an area where osteoclasts were needed, whereas osteoblasts were recruited from local precursors. This suggested that local precursors could not differentiate into osteoclasts and consequently that the lineages of osteoblasts and osteoclasts are different[13]. Although such experiments did not show definitively that the osteoclast is derived from the hemopoietic stem cell, since bone marrow is diverse and contains stromal cells in addition to hemopoietic cells, the accumulated evidence strongly suggested that the osteoclast is derived from the fusion of mononucleated precursors of hemopoietic origin. It rather fitted with Maureen Owen’s conclusion on the basis of extensive studies in rabbits[14], that osteoclasts are very sparsely distributed in bone - “anyone who has looked at histologic sections of bone will have been struck by the paucity of osteoclasts compared with the number of osteoblasts”. It seemed therefore that osteoclasts only come where and when they are needed, and it made no sense for their development and arrival to be orchestrated by any circulating factors - these would more likely be local. This is consonant with the thinking of Chambers in his considering the osteoclast as a “wandering” cell, whose formation would logically be programmed by genuine bone cells[15].
BIRTH OF BONE CELL BIOLOGY
In 1970 there was little prospect of isolating cells from bone in sufficient numbers and purity to characterize them adequately, although the first enzymatically digested cells from newborn rodent bone had been cultured in the 1960’s[16]. Another possibility was to develop a tumor of bone cell origin and use it to study hormone action. Experimental tumors had been described that retained hormone-responsiveness throughout prolonged animal passage[17], allowing studies of hormone-receptor interactions and effects of hormones on specific cell functions[18], so it seemed logical to develop bone tumors with the aim of learning something of the properties of cells of bone origin.
That was the reasoning behind the decision to induce an osteogenic sarcoma in the rat and investigate the hormone responsiveness of the tumor cells. Tumors were induced in rats by serial injections of 32P-orthophosphate that resulted in a high incidence of osteogenic sarcoma development[19]. The tumors were readily transplantable within the same strain of rats, retaining their phenotypic properties throughout many years of transplantation. Membrane and cell preparations from the tumors showed dose-dependent increases in adenylyl cyclase and cellular cAMP respectively, to parathyroid hormone (PTH) and to prostaglandins of the E series[20-22]. The tumors were rich in alkaline phosphatase, made a bone-like ground substance and mineralized it. The stability of the phenotype was such that a few years later stable clonal cell lines were derived from the osteogenic sarcoma[23,24], one of which, UMR106, has been used extensively by our own and many other laboratories.
What the osteogenic sarcoma provided was a tumor of the osteoblast lineage that could be used for studies of certain aspects of hormone action, particularly PTH, prostaglandins and metabolites and vitamin D. It has always been important to recognize that the cells are not osteoblasts, but can at best be described as malignant cells originating in bone, that possess a number of features in common with cells of the osteoblast lineage. The osteogenic sarcoma and the clonal cells derived from it proved valuable in many ways, since at that time, the early 1970’s, knowledge of the cells of bone was primitive, so much the case that only a little later in the 1970’s was there evidence for the separate developmental origin of the osteoclast and osteoblast lineage (v supra).
The experimental pathways offered by the UMR106 and related clones were matched by the ROS17.2/8 cells[25,26], a further rat osteosarcoma clonal line enriched in a number of osteoblast properties. A major advance though, came when cells were extracted from newborn rodent bone by sequential enzymatic digestion[27,28]. Although such cultures were inevitably heterogeneous, they could be enriched in properties identified with the osteoblast, and could be studied in vitro up to a few subculture passages. The two osteosarcoma approaches and the rodent osteoblast culture methods came together at the same time, the end of the 1970’s, and in many ways signalled the birth of bone cell biology.
The UMR106 and ROS 17.2/8 cells were useful in studying mechanisms of hormone or cytokine action on cells that possessed a number of osteoblast-like properties. Careful comparisons between primary bone cells and clonal osteosarcoma cells that were enriched in a number of the phenotypic features of osteoblasts, allowed general conclusions about osteoblast function to be drawn[23,29,30]. At the simplest level, the hormone and prostaglandin responsiveness of osteosarcoma and calvarially-derived osteoblast cultures were very similar, and we chose to apply this principle of regularly comparing the behaviour of osteosarcoma cells with primary cultured cells. The phenotypic properties of osteoblasts were studied in such rodent cell culture systems, and the observations made in those systems extrapolated to adult bone in vivo, leading to concepts of the “osteoblast phenotype”. It was this work that led to the thoughts that that the osteoblast lineage might control osteoclasts.
COULD OSTEOBLASTS REGULATE OSTEOCLASTS?
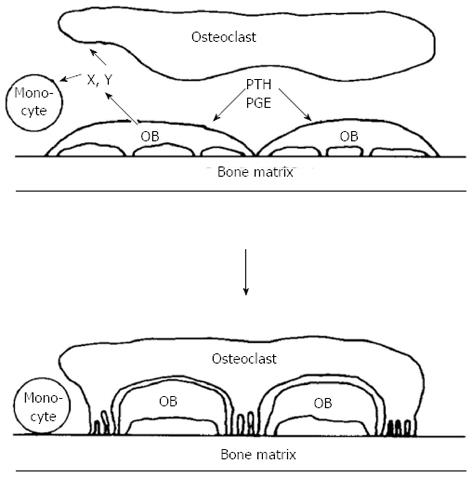
It was reassuring in this work that, when comparing osteogenic sarcoma cells with calvarial osteoblast-rich cultures in their adenylyl cyclase responsiveness to prostaglandins, their metabolites and analogues, their relative efficacies in repeated dose-response studies were very closely similar. At this time also the laboratories of Larry Raisz and Armen Tashjian had shown in two different organ culture systems that prostaglandins were powerful stimulators of bone resorption[31,32]. What was most striking, however, was that the dose responses to prostaglandins, metabolites and analogues in promoting bone resorption in organ culture so very closely mimicked the cyclic AMP response in the cells isolated either from osteosarcoma or calvariae [20,21,33,34]. This was despite the fact that these were such very different assay systems, one requiring the generation in organ culture of osteoclasts that resorbed bone, and with a read-out after 48 h or longer, the other a response within only a few minutes in cells of the osteoblast phenotype. These observations helped lay the foundation for the hypothesis, that in order to generate active osteoclasts, bone-resorbing agents act first on cells of the osteoblast lineage[35] (Figure 1).
Figure 1 Schematic representation of osteoblast involvement in hormone - stimulated bone resorption (reproduced with permission).
OB: Osteoblast; PTH: Parathyroid hormone; PGE: Prostaglandin E.
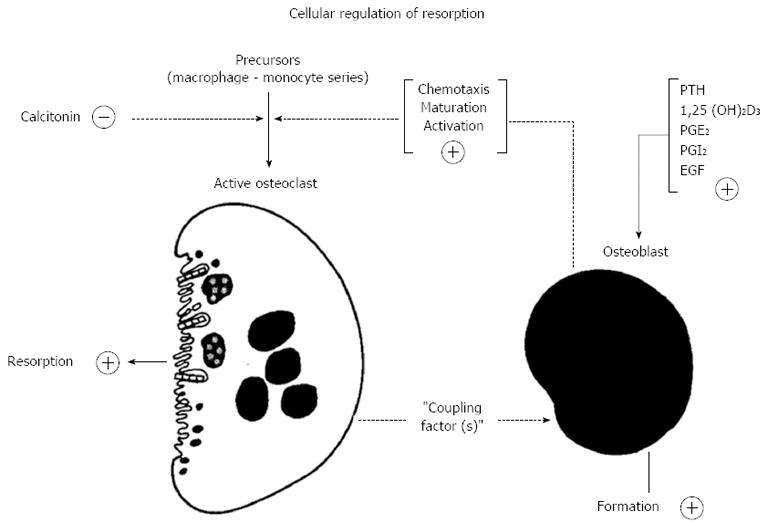
The thinking behind this drew also on observations made shortly before that by Alan Boyde, who showed that PTH had many actions upon osteoblasts or “osteoblast-like cells”, including cell shape changes resulting in less tight packing of cells that were evident in organ cultures[36,37] and isolated cells[38]. This drew attention to “lining” cells - those cells that were by far the most abundant on bone surfaces. The lining cells were envisaged as providing a barrier between osteoclasts and the bone mineral surface[36]. The proposal in the hypothesis was that this was a barrier to be breached when bone resorbing hormones acted upon them[35]. In addition to this physical process, of making the bone available to osteoclasts that would initiate resorption, the concept was that the osteoblastic lineage cells in response to activators, would generate signal(s) leading to the recruitment, maturation and activation of osteoclasts[39] (Figure 2). We considered the lining cells to be the most likely of the osteoblast lineage cells to mediate the actions of resorbing agents in promoting formation of active osteoclasts - with the least likely of all being fully active, synthesizing osteoblasts.
Figure 2 Proposed participation of cells in the osteoblast lineage in the action of the bone - resorbing hormones (reproduced with permission from Ref.
39). PTH: Parathyroid hormone; PGE2: Prostaglandin E2; EGF: Epidermal growth factor.
Chambers[15] came to the same conclusion about osteoblast control of the osteoclast, but arrived at this for other reasons. Coming from work on macrophages, he argued that since the osteoclast derives from a “wandering” cell, it made sense to have its activity programmed by an authentic bone cell, i.e., the osteoblast. He subsequently did much to establish this as fact.
These developments gave rise to a major interest in intercellular communication in bone. The hypothesis of osteoblast lineage control, attractive as it might have seemed to its proponents, was not received with wide enthusiasm by the field, with a common view being that intercellular communication in bone was unlikely. It was testable though, and the next several years would bring much activity along those lines. It was still the case that we were not well educated about the cells of bone, and this applied particularly to osteoclasts. Scarcely a thought was given to osteocytes, because there was simply no way we could approach their study, although there was an old literature on osteocytes that indicated they had an interesting association with lysis around their lacunae[40-42].
OSTEOBLAST REGULATION OF OSTEOCLASTS
Organ cultures of fetal or embryonic bone continued to provide valuable information concerning the actions of hormones, cytokines and drugs on bone resorption, but were not readily adaptable to testing the idea that cells of the osteoblast lineage might control osteoclasts - that was out there to be proved or disproved. Now that it was possible to culture cells with the properties expected of osteoblasts, and with quite an increase in understanding of where osteoclasts come from, the next several years proved to be enlightening.
Regulation of the osteoclast by the osteoblast lineage was still only a hypothesis. Over the next several years methods were developed to address the question, using cells isolated from newborn rat bone and plated onto thin slices of cortical bone, dentine or ivory[43-48]. Resorption by isolated osteoclasts was assessed by measuring the areas or numbers of resorption pits produced by the cells in response to treatment with bone resorbing agents. A number of methods were used to isolate the cells from newborn bone, all of which yielded osteoclasts inevitably mixed with a large excess of other cells, many of them osteoblastic cells and fibroblasts.
Osteoclasts rapidly attached to the bone slice surfaces, and other cells, including osteoblasts, that adhered less tightly, were removed as much as possible by vigorous washing. By limiting the time of cell adherence to less than 15 min, “functionally pure” osteoclast populations could be prepared, i.e., cultures in which treatment overnight with bone-resorbing agents (e.g., PTH, IL-1, TNFα, etc.) resulted in no stimulation of resorption. Alternatively, deliberate contamination with osteoblasts could be achieved, either by allowing long settlement times before washing, or by adding osteoblasts (or surrogate osteoblasts in the form of certain osteogenic sarcoma cells) to the cultures. In the latter conditions the bone-resorbing agents could stimulate resorption that could be quantitated.
These experiments showed convincingly that the bone resorbing agents stimulate resorption by a mechanism which requires the presence of contaminating osteoblasts that made contact with the precursors of osteoclasts. This was true of PTH, 1,25-dihydroxyvitamin D, TNFα and β, IL-1, and thyroid hormone[46-50]. The question whether cell contact was essential was a crucial one. Although some evidence was obtained that the activation of resorption by “functionally pure” osteoclast cultures could be produced by the transfer of medium from osteoblasts that had been treated by resorbing agents[51,52], this was insufficiently consistent to allow purification of such activity. The overall conclusion from the data supported the hypothesis that osteoblastic cells were needed for osteoclast activity.
An important question was whether osteoclast formation from hemopoietic precursors also required participation of accessory cells. The isolated osteoclast assay summarized above was considered to be an assay predominantly of osteoclast activity, as measured by resorption of bone, dentine or ivory. In some circumstances though, osteoclast formation clearly took place, and required cell contact. What was a “functionally pure” culture at the beginning of an experiment was not so after a period of time because of the proliferation of osteoblast lineage and other cells in these crude cultures. For example, when cells were isolated from endosteal surfaces of newborn rat (mouse) bone and grown on bone for several days, resorption that was unresponsive to PTH in the first 24 h became responsive thereafter, because of continuing increases in osteoblast numbers. This was illustrated by results of growing isolated rat osteoclasts on bone for several days[53]. Osteoblast numbers increased 3-fold from 24 h to 48 h, and cultures that were not responsive to PTH in the first 24 h became responsive thereafter. Furthermore the actual numbers of osteoclasts continued to increase beyond 24 h, indicating the generation of new osteoclasts osteoclasts under the culture conditions. Observations such as this were instructive, indicating that osteoblastic cells might indeed be needed for osteoclast formation as well as for their activity. Convincing evidence for that was to come a few years later (v infra). Importantly also, it had become clear that great care and attention to detail should be exerted in attempting to use the isolated newborn bone cell cultures as an osteoclast activity assay - often it was more than that.
CONTROL OF OSTEOCLAST FORMATION
Once it became firmly established that osteoblasts and osteoclasts had entirely different origins, with hemopoietic cells arriving in the bone environment and being used to form osteoclasts where they were needed in bone, generated from “wandering” precursors, how did this happen? Burger et al [54], using a co-culture system in which hemopoietic cells from embryonic mouse liver were co-cultured with fetal long bone rudiments from which the periosteum had been stripped, showed that that living bone cells are required for osteoclast development. However it was the development of murine bone marrow cultures by Naoyuki Takahashi in the group of Tatsuo Suda, that led to major advances, with reproducible assays of osteoclast formation[55,56]. These were used first to show that treatment with bone resorbing agents such as 1,25(OH)2D3 could promote osteoclast formation in a dose-dependent manner, with osteoclast quantitation carried out by counting TRAP positive multinucleated cells that were also CT receptor positive by receptor autoradiography. In the course of these studies, Takahashi et al[55] made an observation that turned out to be a crucial one. They noted consistently that more than 90% of the TRAP-positive mononucleated cell clusters and multinucleated cells formed in mouse marrow cultures in response to bone resorbing stimuli were located near colonies of alkaline phosphatase-positive mononucleated cells (possibly osteoblasts). This was consistent with the idea that osteoblastic cells are involved in osteoclast formation, in addition to the evidence produced in the few earlier years of their influence on osteoclast activity. They set out to determine whether close contact between osteoclast progenitors and osteoblastic cells was necessary in order for osteoclast formation to occur.
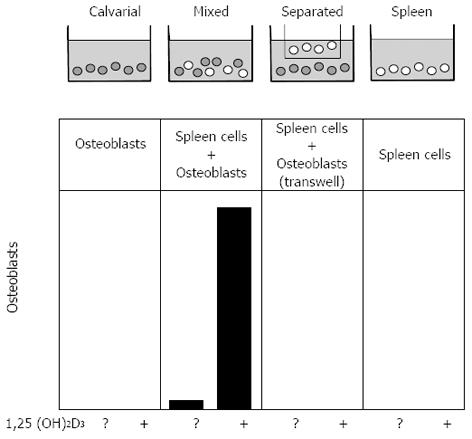
A simple experimental design was developed by Takahashi to examine this possibility further, and this had a major impact on the field. That was the establishment of a co-culture system in which osteoblast-rich cells from mouse calvariae were grown with osteoclast precursors (from spleen), either on the same surface or separated by a 0.45 µm filter (Figure 3)[57]. Osteoclast formation was measured and shown to require direct contact between the participating cells. Similar results were obtained with the bone marrow-derived stromal cell lines[58,59], any of which could be substituted for primary osteoblastic stromal cells in co-cultures with spleen cells, to result in the formation of osteoclast-like cells in the presence of 1,25(OH)2D3. These studies highlighted the fact that the ability to promote osteoclast formation was one distributed within the osteoblast lineage, clearly demonstrable in cells early in the lineage, and later shown to be a property also of fibroblasts[60] and of chondrocytes[61], and even claimed recently for osteocytes[62,63]. On the other hand we did not consider it likely that precursors would be presented to mature, bone-forming osteoblasts in a way that would favour these members of the osteoblast lineage having a role in osteoclast generation. A later illustration of this point came from the finding that genetic ablation of mature osteoblasts, driven through the osteocalcin promoter, had no influence on the ability of mice to form osteoclasts[64].
Figure 3 Representation of the co-culture method that showed the contact-dependent promotion of osteoclast formation by stromal osteoblasts.
HORMONE AND CYTOKINE CONTROL OF OSTEOCLAST FORMATION
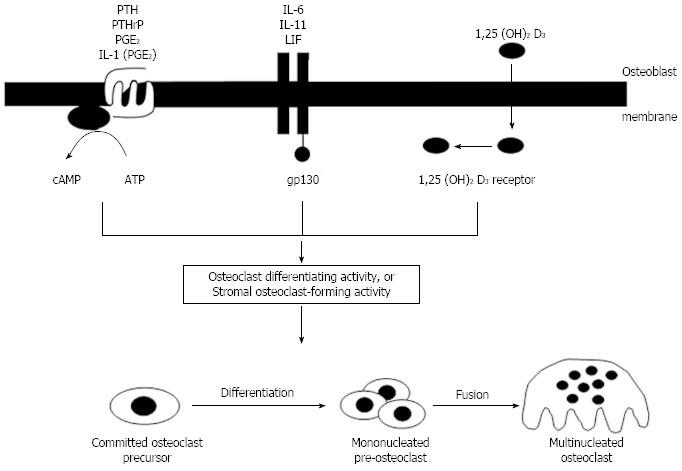
With increasing acceptance of a role for cells of the osteoblast lineage in controlling osteoclast formation and activity by a contact-dependent mechanism, it was important to understand how this process was regulated by hormones and cytokines that stimulate osteoclast formation. PTH and PTHrP, acting through their common receptor, promoted osteoclast formation in marrow cultures by a cAMP-dependent mechanism[48,65] as did PGE2, and the effect of interleukin-1 (IL-1) resulted from the generation of PGE2 as an intermediate effector[66]. It remains the case that there is no evidence for the existence of functional PTH/PTHrP receptors in osteoclasts, capable of supporting a direct stimulatory effect of PTH on the osteoclast. A second signaling mechanism for regulation of osteoclastogenesis by osteoblasts was provided by the steroid hormone, 1,25(OH)2D3 which had very similar effects on osteoclast formation in marrow cultures and in co-cultures of osteoblasts with hemopoietic cells[7]. 1,25(OH)2D3 signals by combining with its receptor and translocating to the nucleus to influence transcriptional events. Finally, a membrane bound receptor complex involving a 130 kDa glycoprotein (gp130)[67] provides for osteoclast formation under the influence of the group of cytokines that use this signalling mechanism [IL-6, IL-11, leukemia inhibitory factor (LIF) and oncostatin M (OSM)].
Thus the concept of stromal/osteoblastic regulation of osteoclastogenesis through local signaling mechanisms was firmly established, along with its regulation by a number of circulating and local factors. Despite the fact that they fell into three main classes with respect to their initial signaling mechanisms, it seemed that they converged in later actions to use a common pathway to promote osteoclast formation. Figure 4 depicts the state of understanding of this process from the late 1980’s until the matter was resolved about ten years later, with the search for the common mechanism occupying many of us in bone cell biology research throughout those years.
Figure 4 Concepts of regulation of osteoclastogenesis from the mid-1980’s until the discovery of the molecular regulation mechanisms.
Three different signaling pathways converged to promote formation of osteoclasts through undefined mechanisms. IL: Interleukin; PTH: Parathyroid hormone; LIF: Leukemia inhibitory factor.
EFFORTS TO IDENTIFY ODF AND SOFA
While understanding of the regulation of osteoclastogenesis had improved somewhat, with signaling through the three pathways as depicted in Figure 4, by the 1990’s there had been no real progress towards identifying the molecular mechanisms by which contact between osteoblast lineage cells and hemopoietic cells could lead to osteoclast formation. The stromal cell-hemopoietic cell co-culture data provided the strongest evidence for the existence of a contact-dependent process, likely a stromal cell membrane molecule requiring contact between the stromal cell and a hemopoietic precursor. This hypothetical substance was termed “stromal osteoclast forming activity” (SOFA)[68], or “osteoclast differentiation factor” (ODF)[56] (Figure 4). Although involvement of matrix factors was not excluded, we favoured the idea that such a membrane molecule existed[56].
It was not surprising that for a time, attention was directed at the colony - stimulating factors as possible candidate specific molecular regulators of osteoclast formation, but they were eventually excluded. A mutation in the coding region of the Macrophage colony-stimulating factor (M-CSF) gene in the mouse impaired the ability to form multinucleated osteoclasts, resulting in one variant of murine osteopetrosis, the op/op mouse. M-CSF appeared to play a role in both proliferation and differentiation of osteoclast progenitors[69,70]. On the other hand, M-CSF inhibited the bone resorbing activity of isolated osteoclasts[71], and osteoclasts were found to be rich in M-CSF receptors[72]. As was the case with M-CSF, both GM-CSF and IL-3 reduced bone resorption in organ culture[73]. All three cytokines inhibit the generation of osteoclasts in mouse bone marrow cultures. However, when marrow hemopoietic cells were pretreated with CSFs before co-culture with osteoblast/stromal cells and 1,25(OH)2D3, each of the CSFs enhanced osteoclast formation, with M-CSF the most effective[69]. The conclusion from these various observationswas that M-CSF, GM-CSF, and IL-3 secreted by cells in the bone marrow (stromal-osteoblasts) contribute to the development of osteoclast-like cells by enhancing proliferation of precursors. In the case of M-CSF, this is also necessary for the differentiation of osteoclasts later in the development pathway. However none of these hemopoietic growth factors fulfilled criteria required of one which is a product of the osteoblast lineage and is specific for osteoclast formation.
A claim was made for identification and isolation of an “osteoclast colony-stimulating factor”[74]. However the biological assay used in that isolation work was the mixed marrow culture system (containing both stromal and hemopoietic elements). Therefore the material isolated had no actions which distinguished it from several cytokines and hormones capable of promoting osteoclast formation with the mediation of stromal cells/osteoblasts. No convincing evidence was produced in that or in subsequent work from the same group that the isolated factor could promote authentic osteoclast formation from purely hemopoietic cells. Lee et al[75] showed that the activity which they had isolated promoted formation of TRAP-positive cells from bone marrow cells cultured in Bacto agar, as did IL-3 and stem cell factor. The results are similar to those of Kurihara et al[76], using spleen cells from 5-FU-treated mice. On the other hand, when strict criteria for osteoclast identification were used, none of the CSFs were able to induce osteoclast differentiation in semi-solid cultures of mouse bone marrow cells[56]. Furthermore, Chambers et al[68] established a number of osteoclastogenic cell lines from the H-2KbtsA58 transgenic mouse, but the osteoclastogenesis with these cell lines still required the presence of stromal cells and 1,25(OH)D.
Some continued to argue that osteoblastic stromal cells are not required for osteoclast differentiation[77]. Much of the lack of agreement is in the interpretation of the data. In experiments in which very strict criteria of osteoclast identification were applied, the need for stromal/osteoblast participation was convincing. This is not so when a single criterion is used, as in the case of TRAP staining in the experiments of Lee et al[75].
A DRAMATIC CONCLUSION
Of the many multifunctional cytokines that had some role in osteoclast formation, none provided an explanation for the molecular regulation of osteoclast formation and activity. Dramatic resolution of the question came in 1997 with the discovery by two groups independently that osteoclast formation is controlled physiologically by regulated interactions among members of the TNF ligand and receptor families. Finally, after many years of hypothesizing, testing those hypotheses, refining them, arguing about what turned out to be false leads, and acquiring useful information along the way, the denouement came in what seemed to be the twinkling of an eye. What made this rapid progress possible was due in no small measure to the fact that the history of research in this area made it obvious what experiments to do, and how to do them, once a really promising candidate molecule came along. These few months surely marked a major point in the history of the study of bone biology - not the end of history though, by any means, because new doors were opened.
The discovery of osteoprotegerin (OPG), a soluble member of the TNF receptor superfamily, revealed it as a powerful inhibitor of osteoclast formation[78,79]. This provided the means of identifying and cloning the elusive stimulator of osteoclast formation, which proved to be a TNF ligand family member that came to be called receptor activator of nuclear factor κB ligand (RANKL), as the common factor mediating osteoclast formation in response to all known stimuli[80,81]. As a membrane protein, RANKL fulfilled the predictions of earlier work, that osteoclast differentiation required contact-dependent activation of hemopoietic precursors. The communication with the hemopoietic lineage results from RANKL binding to its receptor on the osteoclast lineage, RANK, thereby initiating signaling essential for osteoclast differentiation. RANKL was the unknown “osteoclast differentiating activity” of Figure 3. The bone-resorbing cytokines and hormones, with disparate signaling mechanisms, were found to converge in promoting RANKL production[82], just as they had previously been predicted to promote production of “ODF” or “SOFA”[56]. The decoy receptor, OPG, was found to have an essential physiological role as a paracrine regulator of osteoclast formation, produced by the osteoblasts and binding RANKL, with constitutive production of OPG necessary to limit the osteoclast formation resulting from RANKL stimulation[83].
Studies in genetically altered mice established clearly the essential physiological role of these TNF ligand and receptor family members in controlling osteoclast formation and activity, filling in the gaps that had been eluding us for many years[84]. The concepts that drove the research to such outcomes had been developed over years of study of bone cell biology, predominantly with in vitro studies using rodent systems, but drawing on some in vivo observations also. The control of osteoclast formation and activity by the osteoblast lineage predicted a control mechanism that was so important from the evolutionary point of view that it was likely to be highly conserved. That certainly proved to be so, both in respect of the overall mechanism and of the conserved sequences of the central molecules. The physiology of the bone resorption regulatory system was in a short time laid out before us with convincing evidence of the essential regulatory function of RANKL, not only in promoting osteoclast formation, but also their survival and activity[85], as was predicted from the earlier demonstration of activation of osteoclasts through contact with osteoblastic cells[44].
Each of four research groups arrived independently and at about the same time at the identification and cloning of RANKL. Two of these groups in the final stages of their work had the specific aim of identifying the long sought-after membrane promoter of osteoclast formation. The other two, working in immunology, were studying the T cell-dependent immune response, identifying RANKL in the process, and subsequently became aware of its role in bone.
The discovery of the crucial parts played by the members of the TNF ligand and receptor family (RANKL, RANK, OPG) was characterized by a number of outstanding feats of cell and molecular biology and protein chemistry. When reviewing things historically, some partisanship might be expected. This author, who was a close observer of all the related events, and was one of those who would like to have made the discovery, nominates the work carried out at the Snow Brand Milk Products Company, Japan, as the most commendable single scientific achievement. They had found that a human embryonic lung fibroblast cell line IMR90, secreted into the medium an activity that inhibits osteoclast formation in mouse marrow culture. They saw this as an opportunity to identify a key player in osteoclast control, which they began to call “osteoclastogenesis inhibitory factor (OCIF)”, and set out to purify and then sequence it. All such protein purification work is heavily dependent on the robustness and throughput of the biological assay used to monitor purification. The bioassay they used in their work was the one developed originally by Takahashi[57], in which they looked for inhibition of 1,25(OH)2D3 - treated osteoclast formation in mouse bone marrow cultures. The assay is technically demanding and time-consuming, with a very slow turnaround time (greater than 7 d), and indeed with no features favourable for protein purification. The fact that they succeeded in purifying and sequencing the heparin-binding protein that they called osteoclast-inhibitory factor (OCIF)[79], must be regarded as an outstanding technical achievement. Using this sequence they cloned OCIF, showing it to be identical with OPG[81], as a novel member of the TNF receptor family.
Because OCIF/OPG strongly inhibited osteoclast formation in cocultures or marrow cultures treated with 1,25(OH)2D3, PTH, or IL-11, it seemed to them evident that OCIF would achieve its inhibition of osteoclast formation by binding to the responsible effector molecule, i.e., ODF/SOFA. They had the means at their disposal to address this question, knowing that certain mouse marrow stromal cells would be expected to express ODF/SOFA strongly on the cell surface when given appropriate stimuli. They used expression cloning of the ligand for OCIF/OPG with a cDNA library of mouse ST2 cells that had been treated in this way, and identified a cDNA encoding a 316 amino acid type II transmembrane protein of the TNF ligand family[81]. Expression of the protein confirmed its ability to promote osteoclast formation.
A different path was followed by the Amgen group. In the course of a fetal rat intestine cDNA sequencing project they noted an expressed sequence tag (EST) with features suggesting that it might be a member of the TNF receptor family, based on known domain structures. This was confirmed when a full length clone was prepared and sequenced, revealing a 401 amino acid glycoprotein with features of a secreted member of the TNF receptor family[78]. This was at that stage essentially an “orphan” receptor, but they had resources at their disposal that allowed them to pursue molecules of likely interest when they were were discovered. They did this using transgenic mice, and found that hepatic expression of the novel protein yielded mice that survived with profound osteopetrosis. This they showed to be due to inhibition of late stages of osteoclast differentiation, and furthermore, recombinant protein inhibited osteoclast formation in vitro and increased bone density when administered to normal mice. They named the protein “OPG”, and they too recognised that it could provide a crucial approach to unravelling the molecular mechanisms of control of osteoclast formation.
Using recombinant OPG-Fc fusion protein as an immunoprobe, they identified a mouse myelomonocytic line that expressed on its surface a molecule which could be readily detected. An expression library prepared from these cells was constructed and screened for binding in pools of transfected COS7 cells. A single plasmid clone was identified, and when expressed, gave rise to on OPG-binding protein on the surface of the expressing cells. They called this 316 amino-acid protein OPG ligand (OPGL), and showed that there was 87% conservation between mouse and human protein sequences[86]. OPGL was able to promote osteoclast formation from hemopoietic precursors in the presence of M-CSF, and to stimulate bone resorption and elevate the blood calcium levels when administered in vivo.
The publication[78] of identification of OPG (later agreed as RANKL) was a landmark event in the field, but although Tsuda et al[79] published some months later, their independent contributions were equally outstanding. From the ways in which each of these groups discovered OPG/OCIF, and with any appreciation of the concepts that had developed over the previous decade or more of osteoclast control, it was quite apparent that this discovery would prove to be central to completing the picture of the local control of osteoclastogenesis.
Remarkably enough, two other groups were successful in identifying and cloning RANKL[87,88], each of them in fact publishing this work some months before either the Snow Brand or the Amgen groups. Wong et al identified and characterized a TNF-related activation-induced cytokine (TRANCE) during a search for apoptosis-regulatory genes in murine T cell hybridomas, finding it to be predominantly expressed on T cells and in lymphoid organs and controlled by the T cell receptor through a calcineurin-regulated pathway[87]. The putative receptor for TRANCE was detected on mature dendritic cells. They were not aware at that time of any involvement of TRANCE in bone biology, and in their survey of tissue distribution of TRANCE mRNA in mouse tissues, bone was not examined. It might be noted that this omission remains the case almost always, when new molecules of whatever variety are discovered, unless it takes place in the context of investigators who have a direct interest in bone.
In studying the processing and presentation of antigens by dendritic cells to T cells, Anderson et al[88] characterized receptor activator of NF-kB (RANK), a new member of the TNF receptor family derived from dendritic cells, and its ligand RANKL, which they recognized to be identical to TRANCE[68]. A soluble form of RANKL augmented the ability of dendritic cells to stimulate T cell proliferation in a mixed lymphocyte reaction and increased the survival of RANK-positive T cells. Again, these workers were not aware at the time of their first publication of any link between RANK/RANKL and bone. Interestingly though, the type I membrane protein, RANK, contained four extracellular cysteine-rich domains, as was the case with OPG, published earlier that year[78].
OSTEOCLAST REGULATION AND FUNCTION
These discoveries filled in the gaps that had been eluding us for many years[56]. The concepts that drove the research to such outcomes had been developed over years of study of bone cell biology. The ODF/SOFA hypothesis predicted a control mechanism that was sufficiently important from the evolutionary point of view that it was likely to be highly conserved, and that has certainly proven to be so, both in respect of the overall mechanism and of the conserved sequences of the central molecules. By treating with RANKL and M-CSF it was now possible for the first time to prepare osteoclasts in relatively large numbers without the participation of stromal/osteoblastic precursors, including the preparation of human osteoclasts from peripheral blood[89,90]. The physiology of the bone resorption regulatory system was in a short space of time laid out before us with convincing evidence of the essential regulatory function of RANKL, not only in promoting osteoclast formation, but also their survival and activity[85], as was predicted from the earlier demonstration of activation of osteoclasts through contact with osteoblastic cells[44].
The remainder of this Issue is devoted to details of the ways in which this physiological control system functions, how the knowledge has led to drug development, and how this communication system is crucial not only to the control not only of osteoclast biology and hence bone remodeling, but also to other biological systems.