Revised: March 19, 2013
Accepted: April 10, 2013
Published online: April 18, 2013
Processing time: 107 Days and 23 Hours
Discogenic low back pain is a serious medical and social problem, and accounts for 26%-42% of the patients with chronic low back pain. Recent studies found that the pathologic features of discs obtained from the patients with discogenic low back pain were the formation of the zones of vascularized granulation tissue, with extensive innervation in fissures extending from the outer part of the annulus into the nucleus pulposus. Studies suggested that the degeneration of the painful disc might originate from the injury and subsequent repair of annulus fibrosus. Growth factors such as basic fibroblast growth factor, transforming growth factor β1, and connective tissue growth factor, macrophages and mast cells might play a key role in the repair of the injured annulus fibrosus and subsequent disc degeneration. Although there exist controversies about the role of discography as a diagnostic test, provocation discography still is the only available means by which to identify a painful disc. A recent study has classified discogenic low back pain into two types that were annular disruption-induced low back pain and internal endplate disruption-induced low back pain, which have been fully supported by clinical and theoretical bases. Current treatment options for discogenic back pain range from medicinal anti-inflammation strategy to invasive procedures including spine fusion and recently spinal arthroplasty. However, these treatments are limited to relieving symptoms, with no attempt to restore the disc’s structure. Recently, there has been a growing interest in developing strategies that aim to repair or regenerate the degenerated disc biologically.
Core tip: Discogenic low back pain is the most common type of chronic low back pain. Why lumbar disc degeneration leads to pain is one of the most important topics in medical field. Studies have revealed that pathologic features of painful discs were the formation of the zones of vascularized granulation tissue, with extensive innervation in annular fissures. Provocation discography now still is the only available means by which to identify a painful disc. There are a multitude of treatments used in clinical practice to treat chronic low back pain, with little consensus amongst clinicians as to which is the best approach.
- Citation: Peng BG. Pathophysiology, diagnosis, and treatment of discogenic low back pain. World J Orthop 2013; 4(2): 42-52
- URL: https://www.wjgnet.com/2218-5836/full/v4/i2/42.htm
- DOI: https://dx.doi.org/10.5312/wjo.v4.i2.42
Chronic low back pain is a serious medical and social problem, and one of the common causes responsible for disability. It is estimated that, in all populations, an individual has an 80% probability of having low back pain at some period during their life time, and about 18% of the population experiences low back pain at any given moment[1,2]. According to US National Center for Health Statistics reports, 14% of new patients that went to a hospital for treatment were patients with low back pain, which represents 13 million people. About 3% of all patients discharged from hospitals have symptomatic low back pain. The expense of treating low back pain is higher than $100 billion each year[3].
The prerequisite for successfully treating low back pain is to make an accurate pathological diagnosis. Despite the inherent challenge in elucidating the specific etiology of chronic low back pain, diagnostic procedures can reveal its source in 90% of patients. DePalma et al[4] found that the prevalence of zygapophysial joints, sacroiliac joints, and lumbar discs was 31%, 18%, and 42%, respectively. They confirmed the disc as the most common etiology of chronic low back pain in adults. Crock[5] first proposed the concept of internal disc disruption (IDD), which indicated the discogenic pain syndrome caused by disc degeneration and non-nerve root referred pain. IDD causing discogenic low back pain accounts for 26%-42% of chronic low back pain patients[4,6,7]. IDD had been assigned as a separate clinical entity to differentiate it from other types of disc degenerative low back pain, such as lumbar disc herniation, degenerative disc disease (DDD) and lumbar segment instability[8]. Lumbar X-ray images of IDD patients show no characteristic changes in degenerative disc diseases such as intervertebral space narrowing, osteophyte formation, endplate sclerosis, and gas formation within disc space[8].
This paper reviews the pathophysiology, diagnosis, and treatment of discogenic low back pain according to the existing literature.
The intervertebral disc is the main joint between two consecutive vertebrae in the vertebral column. Each disc consists of three different structures: an inner gelatinous nucleus pulposus, an outer annulus fibrosus that surrounds the nucleus pulposus, and two cartilage endplates that cover the upper and lower surfaces of vertebral bodies. The cells that form the annulus fibrosus, particularly in the outer region, are fibroblast-like and arranged parallel to the collagen fibers, whereas those in the inner annulus fibrosus are chondrocyte-like. The nucleus pulposus contains collagen fibers that are randomly distributed and elastin fibers that are radially organized embedded in a highly hydrated aggrecan-containing gel. Chondrocyte-like cells synthesize type II collagen, proteoglycans, and non-collagenous proteins that form the matrix of the nucleus pulposus and the cartilage endplate. Fibroblast-like cells synthesize type I and type II collagen for the annulus fibrosus[9]. Proteoglycans consist of a core protein from which radiate chains of glycosaminoglycans containing keratin sulphate and chondroitin sulphate. Multiple proteoglycans are joined to a hyaluronic acid chain to form aggrecan. Aggrecans are held together by type II collagen, which is cross-linked by type IX collagen. Aggrecan is the most common proteoglycan in the disc, and comprises approximately 70% of the nucleus pulposus and 25% of the annulus fibrosus. Aggrecan provides a high level charge density, which creates a high osmotic pressure for retaining water within the nucleus pulposus[10]. A young healthy disc behaves like a water bed, with the high water content of the nucleus and inner annulus enabling the tissue to act like a fluid. Only the outermost annulus acts as a tensile “skin” to restrain the nucleus.
Disc cells synthesize their matrix and break down existing matrix by producing and activating degradative enzymes, including matrix metalloproteinases (MMPs) and “a disintegrin and metalloproteinase” (ADAMS). Degradation of the matrix allows it to be refreshed by newly-synthesized components. Several growth factors, such as bone morphogenetic protein-2 (BMP-2), BMP-7 (also known as osteogenic protein-1; OP-1), growth differentiation factor-5 (GDF-5), transforming growth factor-β (TGF-β), insulin-like growth factor-1 (IGF-1), and others have been found to stimulate matrix production, while interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) inhibit the synthesis of matrix by enhancing its catabolism[9,10].
Disc degeneration will occur if the matrix is not normal. At a molecular level, degeneration will be expressed by the production of abnormal components of the matrix or by an increase in the mediators of matrix degradation, such as IL-1 and TNF-α, and of MMPs and a reduction in the levels of tissue inhibitors of metalloproteinases (TIMPs). Several factors have been considered to cause disc degeneration. Genetic predisposition, mechanical load, and nutritional factors are widely regarded as important contributors to the degenerative process[11]. However; detailed characterization of this complex interplay remains elusive. With the disc degeneration, there is a net loss of proteoglycans and water from the nucleus, leading to poor hydrodynamic transfer of axial stresses to the outer anulus fibrosus. The disc degeneration may result from an imbalance between the anabolic and catabolic processes or the loss of steady state metabolism that is maintained in the normal disc. Alterations in both anabolic and catabolic processes are thought to play key roles in the onset and progression of disc degeneration.
Disc degeneration usually appears in magnetic resonance imaging (MRI) T2-weighted images as a decline in signal intensity, i.e., the so-called “black” disc. MRI may identify a degenerative disc and an annular tear, but it will not help differentiate between a disc which is pathologically painful and one which is physiologically aging[12]. Disc degeneration is a very complicated biological process. Previous views on disc degeneration and the mechanism underlying it were mainly based on histological and biochemical studies using human disc herniation specimens from surgery and animal models of aging and degenerative discs[13,14]. However, the main histological changes and the exact molecular mechanisms underlying the painful pathological disc remain unknown.
With the development and popularization of lumbar fusion, a greater number of painful pathological disc specimens can be obtained, which are beneficial for studies regarding the pathogenesis of painful disc degeneration. Based on our previous histological studies[15-17], we found that the composition and structure of painful disc differed from those of non-painful degenerative disc. Specifically, normal fibroblasts in the annulus fibrosus were replaced by cartilage-like cells. The annulus fibrosus lamellar structure was disordered and fractured. The normal highly hydrated gelatin-like nucleus pulposus, whose matrices showed obvious fibrosis, and cartilage-like cells, were completely replaced with fibroblasts, was substituted by fibrous tissues. The histological changes in the nucleus pulposus were divided into 3 major types: obvious fibrosis, vascular invasion, and inflammatory granulation tissue formation. In addition, we found that the characteristic change in painful pathological discs was the formation of inflammatory vascular granulation tissues with extensive innervation along the tears in the posterior annulus fibrosus, along with mass expression of some growth factors such as basic fibroblast growth factor (bFGF), TGF-β1, and connective tissue growth factor (CTGF). Vascular granulation tissue was not formed in asymptomatic degenerative discs, and only a few growth factors were expressed. Asymptomatic degenerative discs with tears are not painful, because these discs have not been innervated[15].
Blood vessels only exist in the longitudinal ligaments and the outermost layers of the annulus fibrosus in a normal disc. The ingrowth of vascularized granulation tissue along the tear deep into the inner annulus and nucleus pulposus in the painful disc probably begins soon after the injury when repair of the tear starts from the margin of the annulus fibrosus[15]. Owing to the absence of blood vessels in the inner annulus fibrosus and nucleus pulposus, it is unlikely that vascularized granulation tissue which is induced by the tear should originate from there. Different animal models of outer annular injury have proved that the healing of the annulus might initiate a progressive degeneration of the disc[18-24]. In addition, the whole process of healing of annulus fibrosus injury, including inflammatory reaction, formation of granulation tissue, and tissue reconstruction had been observed, implying that the disc has actually been torn and there has been a process of healing in progress[16].
According to recent researches on injury and repair, growth factors have been considered to be essential to regulate and control the whole process of repair of an injury. Some growth factors, such as bFGF, TGF-β, and CTGF, may be important as promoters in tissue repair. Growth factors that control cellular proliferation and differentiation in vitro have been identified. These factors mediate cellular interactions in vivo, which not only contribute to development and growth, regeneration, and wound healing, but also may incite abnormal changes[16]. Growth factors through their each receptor signal transduction pathway, promote cellular proliferation and collagen synthesis of matrix cells such as fibroblast and vascular endothelial cells, which exert a strong effect on adjustment and control of wound and repair[16]. Previous studies have indicated that bFGF as an important mitogen accelerator may directly act on the mitotic cycle of tissue repair cells (for example fibroblast), resulting in shortening of G1 phase, prolongation of G2 and M phases, thus mitotic cycle is shortened, and cell division and proliferation accelerates. TGF-β, as a multi-functional growth factor, not only can attract inflammatory cells and tissue repair cells to aggregate in the wound region, but also directly act on fibroblasts to stimulate synthesis of type I procollagen, formation of granulation tissue, and tissue reconstruction in the later stage of repair[25-27]. Nagano et al[28] in an animal model of disc degeneration found that bFGF was a proliferation stimulating factor promoting proliferation of chondrocytes to replace normal annular cells in degenerated discs in an autocrine or paracrine manner. Tolonen et al[29] studied expression of bFGF and TGF-β in painful degenerative discs, and found that growth factors strongly express in both the annulus fibrosus and the nucleus pulposus. Their study suggests that these growth factors promote cellular remodeling, and create a cascade in the process of disc degenerateion.
Disc tissues are different from other tissues because they comprise the largest avascular tissue. In other tissues, injury healing proceeds from the inside to the outside. On the contrary, healing in disc tissues proceeds from the outside to inside[16]. When the annulus fibrosus is lacerated or injured, vascular tissues can only gradually develop from the outer to the inner annulus fibrosus. Endothelial cells migrating into discs form the principal parts of a new capillary vessel. With the help of various growth factors, endothelial cells migrating into the avascular disc tissues differentiate, proliferate, and gradually form complicated capillary networks. Our studies[15-17] suggested that as annulus fibrosus injuries stimulated local vascular inflammatory reactions, cells including macrophages and mast cells in inflammatory regions produce a large number of growth factors such as bFGF, TGF-β1, and CTGF. The cells in normal disc are separated from the circulatory system. These increased growth factors acted on the intervertebral disc cells, and promoted disc cell dedifferentiation and proliferation, as well as large-scale extracellular matrix synthesis via signal transduction. This may be the main cause of painful disc fibrosis and degeneration. The strong expression of proliferating cell nuclear antigen (PCNA) in painful discs seemed to be an evidence of this hypothesis. PCNA, a nucleoprotein of nonhistone, is an essential auxiliary protein of DNA polymerase-δ[16]. It can markedly increase activity of DNA polymerase-δ, and its expression level is believed to be an important measure of cell proliferation activity[30].
The normal disc is believed to be an organ that is poorly innervated supplied only by sensory and sympathetic perivascular nerve fibers. In the early 1980s, Bogduk[31] clarified the innervation of the outer layers of the annulus. The posterior part of the human disc was supplied not only from the sinuvertebral nerve but also received direct branches in its posterolateral aspect from the ramus communicans or the ventral ramus. Branches from the grey ramus communicans also supplied the lateral aspect of the disc. Anterior discal nerves were observed to arise solely from the sympathetic plexus surrounding the anterior longitudinal ligament. The sensory fibers that innervated the disc are mainly nociceptive and, to a lesser extent, proprioceptive. The sympathetic fibers are considered vasomotor efferents, and also sympathetic afferents conveying pain impulses[32]. The close association of the postganglionic efferent and sympathetic afferent fibers reflected a similar pattern to that seen in certain enteric organs, leading them to suggest that low back pain is a kind of visceral pain[33-35]. In human degenerated disc, as well as in animal models of disc degeneration, the number of nerve fibers in the disc increases[15,36,37]. Furthermore, the nociceptive nerve fibers grow into what are usually aneural inner parts of the annulus and even into the nucleus. In addition to the sensory nerve fibers, there is growing evidence that sympathetic afferents are also increased in degenerated disc and that they play a significant role in low back pain[38-40]. In human normal disc, protein gene product 9.5-positive nerve fibers, either associated with blood vessels or distant from them, innervate the outer layers of the annulus. These nerve fibers are also positive for acetylcholinesterasem NFP, SP, CGRP, VIP, neuropeptide Y, C-flanking peptide and synaptophysin. The nerves entering the rat disc have an identical expression pattern[32]. Mechanical stimuli which are normally innocuous to disc nociceptors can, in certain circumstances, generate an amplified response which has been termed ‘peripheral sensitization’. This may explain why some degenerative discs are painful and others not. There is growing evidence that these pain receptors in painful disc are peripherally sensitized by the activity of sympathetic efferents which may initiate a pain impulse in response to ischaemia, pressure changes or inflammatory irritation[32].
It is accepted that the lumbar disc, which are the main source of discogenic back pain in humans, are innervated segmentally. However, the ventral portions of the rat lower lumbar discs are innervated by upper (L1-L2) dorsal root ganglion neurons and the nerve fibers innervating the posterolateral portion of the disc come from the upper and lower dorsal root ganglion (L3-L6)[38,39]. Nerve fibers reach the lumbar disc through the sinuvertebral nerves or from branches of the paravertebral sympathetic trunks[40]. Clinical studies have indicated those local anaesthetic blocks of L2 nerve root can relief discogenic low back pain[41].
The diagnostic criteria for IDD established by the International Association for the Study of Pain (IASP) are emergence of a concordant pain response during discography, internal annular disruption shown by CT after discography (CTD) and at least one adjacent disc without concordant pain[42]. The term IDD was first coined by Crock[5] on the basis of a large group of patients whose disabling back and leg pain became worse after operation for suspected disc prolapse. He reported this condition, characterizing it by disruption of the internal architecture of the disc, discogenic back pain in the absence of peripheral disc shape abnormality, and the absence of nerve root compression. At present, IDD has been described as a distinct clinical entity to be distinguished from other painful processes such as degenerative disc disease and segmental instability[8]. In our a previous study, according to discography, we classified discogenic low back pain into two types that were annular disruption-induced low back pain (IAD) and internal endplate disruption-induced low back pain (IED), which have been fully supported by clinical and theoretical bases[43]. The term IAD should be more reasonable than the term IDD clinically and pathologically. Clinically, these two types of low back pain should be confirmed by lumbar discography. The diagnostic processes, radial tear and pain responses are identical. During the process of contrast medium injection, the contrast medium was either flowing to the outside of disc through a radial annular tear, or flowing to the vertebral body through the radial endplate tear. The concordant pain responses would be induced in either way.
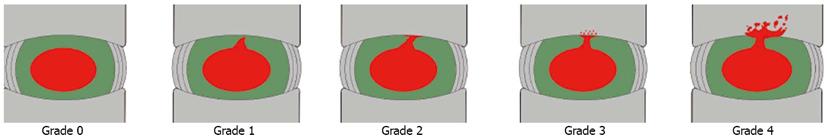
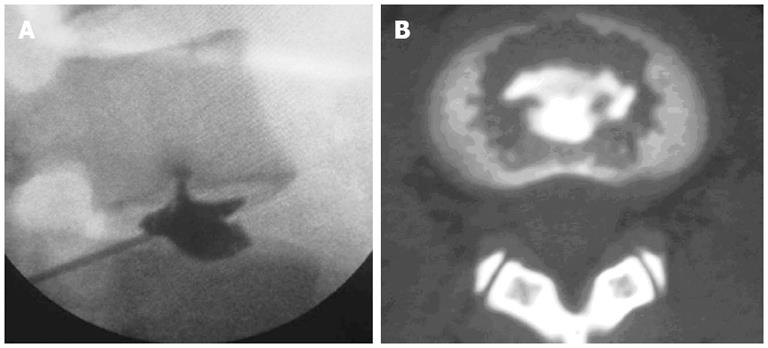
According to the “Modified Dallas Discogram Description” method[44,45], the degrees of annular disruption could be classified into four grades. The definitions are Grade 0: the contrast medium is confined within the normal nucleus pulposus; Grade 1: the contrast medium flows into the inner third of the annulus through annular fissure; Grade 2: the contrast medium flows into the middle third of the annulus; Grade 3: the contrast medium flows into the outer third of the annulus, and extends circumferentially less than 30° arc at the disk center; Grade 4: the contrast medium flows into the outer third of the annulus, and extends circumferentially more than 30° arc at the disk center; and Grade 5: the contrast medium leakage into the outer space. Grades 0, 1 and 2 are normal, while Grades 3 and above are indicative of annular disruption. We combined the discogram and CT scan after discography to evaluate the degree of endplate disruption in IED patients. The disruptive degrees were classified into four grades (Figure 1): Grade 0 (no disruption), Grade 1 (contrast medium flows into the cartilage endplate through tear), Grade 2 (contrast medium flows into the bony endplate), Grade 3 (contrast medium flows into the cancellous bone of vertebra under endplate, showing local dispersion) and Grade 4 (contrast medium disperses extensively in the cancellous bone)[43]. In this group of patients with IED, all intervertebral discs that showed concordant pain responses had endplate disruptions more severe than Grade 3, which was consistent with the distributions of blood vessels and nerves in the endplate (Figure 2)[43].
Theoretically, any innervated vertebra and its peripheral structures might be the source of low back pain. An intervertebral disc has such a structure that, except for the peripheral parts around annulus fibrosus, the endplate also has nerve supplies. Normally, one vertebral endplate has two nerve supplies: one enters the endplate along with perivertebral blood vessels, while the other that belongs to the sinuvertebral nerve branch that enters the endplate through the intervertebral foramen. The nerve density within the endplate is similar to that of the annulus, indicating that the endplate is also an important source of discogenic low back pain[46]. Recently, we published a clinical study article[47], 21 patients with chronic back pain originating from the endplate injuries were selected to explore the methods of diagnosis and surgical treatment. Pain level of disc was determined through discography in each patient. All 21 patients with a diagnosis of back pain originating from endplate injuries according to discography were treated with anterior or posterior fusion surgery. After operation, through a mean follow-up of three years and five months, we found that in all the 21 patients, 20 (20/21) reported a disappearance or marked alleviation of low back pain and experienced a definite improvement in physical function. The study suggests that discography and fusion surgery may be very effective methods for the diagnosis and treatment, respectively, of chronic back pain originating from the endplate injuries. In fact, endplate damage-induced low back pain occurs quite often clinically. In clinical research, we found that endplate damage-induced low back pain accounted for 16.7% of chronic discogenic low back pain. Epidemiological investigation showed that the incidence of endplate damage among populations without low back pain was 30%[48].
Theoretically, the pathogenesis of endplate disruption-induced discogenic low back pain is presumed to be consistent with that of annular disruption. A large number of animal experiments have indicated that damage to the outer layer of the annulus could induce a progressive degeneration of the entire disc[19-23]. Similarly, animal models have indicated that needle punctures from the vertebral side all the way through the endplate into the disc could induce a progressive degeneration of the entire disc[49]. It was found that the apoptosis of nucleus pulposus cells increased and the proteoglycan content decreased after endplate injury in the endplate damage animal model[50]. The ingrowth of nerves and blood vessels is a characteristic of tear discs, and is also directly correlated with discogenic low back pain. Freemont et al[51] found that blood capillaries grew in companion with nerve endings into the painful discs through endplates.
Basic and clinical studies have overwhelmingly illustrated the nerve supply of the disc and pathomorphologic correlates[6-9,15,18,36,37,52-58]. Based on controlled evaluations, the lumbar intervertebral discs have been shown to be sources of chronic low back pain without disc herniation in 26% to 42%[4,6,7]. Because of the variety of anatomic and pathophysiologic causes of chronic low back pain, it is a difficult diagnosis for clinicians to make. Clinicians primarily use advanced imaging techniques, such as MRI to diagnosis low back pain. Studies show that MRI findings such as disc degeneration do not correlate with the presence or severity of low back symptoms. Lumbar provocation discography is a procedure that is used to characterize the pathoanatomy and architecture of the disc and to determine if the disc is a source of chronic low back pain. Recently, the American Pain Society developed and published multiple guidelines[59,60] in managing low back pain which did not recommend discography as a diagnostic test because of poor evidence for its sensitivity, specificity, and predictive value. However, subsequently, these guidelines were severely criticized[52]. There were deficiencies and inappropriate evaluation in almost all areas; inappropriate studies were included and appropriate studies were excluded. The basic deficiency of these guidelines by Chou and Huffman[59] was their failure to recognize the discography must not be performed in asymptomatic volunteers or patients with mild low back pain. They also utilized outdated guidelines from AHCPR and European COST guidelines[52]. In the interim, questioning the validity of discography warrants questioning the role of the disc as a discrete pain generator, or more specifically, challenges the concept of symptomatic internal disc disruption. If one considers discography to be a useless test, then one may have to abandon the concept of the disc as a discrete pain generator and abandon the pursuit of intradiscal therapies, whether surgical or non-surgical[52]. Recent systematic reviews have concluded that there is strong evidence that lumbar discography can identify the subset of patients with chronic discogenic pain[61,62].
Treatment for discogenic low back pain has traditionally been limited to either conservative management or surgical fusion. However, to accurately assess the effect of any therapy for treating discogenic low back pain, the natural history of such pain should be known beforehand. Recently, our a clinical study indicated that the natural history of discogenic low back pain was continuous and chronic[63]. This result indicates that most patients are expected to experience low back pain after a longer time interval, and their pain severity is expected to remain nearly the same. The elucidation of natural history of discogenic low back pain has important clinical significances for decision-making of treatments.
There are a multitude of treatments used in clinical practice to treat chronic low back pain, with little consensus amongst clinicians as to which is the best approach. Pharmacologic treatment usually includes analgesics, nonsteroidal anti-inflammatory drugs, and muscle relaxants, but the evidence for their efficacy is not compelling. In randomized trials, the differences in pain after a patient has taken nonsteroidal anti-inflammatory agents as compared with placebo have generally been in the minimally detectable range[64]. A meta-analysis revealed that opioids seem to have a small effect in improving function and relieving pain for the patients with chronic low back pain[65]. Long-term treatment with narcotics is generally discouraged, given the associated risks of tolerance and side effects. Physical therapy, exercise, manipulation, and back school seem to have some effects, but it is unknown if effects are sustained for the long term[64]. Exercise therapy by the McKenzie method is a popular treatment for low back pain among physical therapists. Clinical studies have indicated that the McKenzie method is slightly more effective than manipulation or is equal to strengthening training for patients with chronic low back pain[66,67].
If conservative treatment fails, then epidural injections are commonly performed for chronic discogenic pain. Epidural injections are administered by accessing the lumbar epidural space by multiple routes including interlaminar, caudal, and transforaminal[68-79]. Epidural procedures continue to be debated regarding their effectiveness, indications, and medical necessity. Recent systematic reviews indicated that effectiveness of epidural injections for treatment of discogenic low back pain was fair[80]. The underlying mechanism of action of epidurally administered steroid and local anesthetic injection is still not well understood. It is believed that the achieved neural blockade alters or interrupts nociceptive input, the reflex mechanism of the afferent fibers, self-sustaining activity of the neurons, and the pattern of central neuronal activities[80]. Further, corticosteroids have been shown to reduce inflammation by inhibiting either the synthesis or release of a number of pro-inflammatory mediators and by causing a reversible local anesthetic effect[81-85].
As alternative treatments, percutaneous treatments directed at altering the internal mechanics or innervation of the disc by heat (intradiscal electrothermal annuloplasty, IDET, and biacuplasty) have recently been advocated[7,86,87], but data supporting their use are controversial[86]. IDET was first used to treat discogenic low back pain in 1996, using a concection technology with a 5 cm active tip placed at the uncleoannuar junction. Two randomised trials have shown either no effect or benefit in only a small number of highly selected subjects[88-90]. Further, of the 6 observational studies[91-96], 4 studies showed positive results, one study showed negative results, and one study showed undermined results. Recent a systematic review evaluated these studied, and concluded that the evidence is fair for IDET[97]. Biacuplasty is one of the minimally invasive treatment methods. It creates heat across the posterior annulus using a cooled bipolar radiofrequency device[98]. The initial study results are promising[99,100], but the effectiveness needs to be evaluated further to use randomized controlled trials.
During recent decades, surgical fusion of the lumbar spine has been performed in increasing number on patients with chronic low back pain[4]. However, the reported results vary considerably in different studies, and the complication rate after fusion surgery in the lumbar spine is not negligible[101-105]. Consequently, artificial disc replacement has been proposed as a substitute for spinal fusion with the aim of treating back pain while preserving vertebral motion at the operated levels and protecting adjacent levels from undergoing degenerative changes, but so far, only several studies have been reported on the results of lumbar disc prosthesis[106-108]. Recent a systematic review suggested that the spine surgery community should be prudent to adopt this technology on a large scale because harm and complications may occur after some years[109]. The results with longer follow-up need to be observed further.
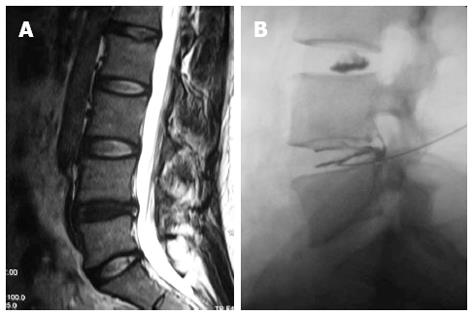
Based on the recent insights into signal transduction mechanisms that might lead to the induction of pain by degenerative discs, it is conceivable that therapies aiming at disrupting pro-inflammatory signaling pathways and the pathway of nerve conduction might be successful in the foreseeable future. Such therapies might not have the ability to reverse the progressing tissue destruction which occurs with aging but may transform a symptomatic to asymptomatic disc degeneration and thereby greatly improve life quality of the affected patients[10]. Recently, a minimally invasive method, intradiscal methylene blue injection for the treatment of painful disc degeneration, had been reported (Figure 3)[110,111]. This successful outcome subsequently was demonstrated by the animal experiments which indicated that methylene blue indeed had destroyed the nerve endings or nociceptors and alleviated inflammatory response in the degenerated discs[112,113].
Recently, there has been a growing interest in developing strategies that aim to repair or regenerate the degenerated disc biologically. Treatments for degenerated discs have two main objectives: restoration of the disc’s structure and elimination of pain[114]. The benefits of biologically based treatments appear to be limited to restoring disc structure. Whether disc regeneration would result in pain relief remains unclear. That said recent data from animal studies have shown changes in cytokine expression following growth factor injection, indicating a possible mechanism for pain relief. Further, the first human clinical trial for growth factor injection therapy is currently underway and may shed light on the clinical outcome. Mesenchymal stem cells (MSCs) may also help relieve pain by reducing inflammation. A recent study indicates that MSCs can induce the production of anti-inflammatory cytokines[115]. However; additional studies are needed to elucidate the underlying mechanisms of pain relief.
P- Reviewers Magalhães E, Manchikanti L, Helm S S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2033] [Cited by in RCA: 2000] [Article Influence: 76.9] [Reference Citation Analysis (1)] |
| 2. | Mooney V. Presidential address. International Society for the Study of the Lumbar Spine. Dallas, 1986. Where is the pain coming from? Spine (Phila Pa 1976). 1987;12:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 323] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 5. | Crock HV. A reappraisal of intervertebral disc lesions. Med J Aust. 1970;1:983-989. [PubMed] |
| 6. | Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976). 1995;20:1878-1883. [PubMed] |
| 7. | Manchikanti L, Singh V, Pampati V, Damron KS, Barnhill RC, Beyer C, Cash KA. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician. 2001;4:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Singh K, Ledet E, Carl A. Intradiscal therapy: a review of current treatment modalities. Spine (Phila Pa 1976). 2005;30:S20-S26. [PubMed] |
| 9. | Hadjipavlou AG, Tzermiadianos MN, Bogduk N, Zindrick MR. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg Br. 2008;90:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 242] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Choi YS. Pathophysiology of degenerative disc disease. Asian Spine J. 2009;3:39-44. [PubMed] |
| 11. | Peng B. Issues concerning the biological repair of intervertebral disc degeneration. Nat Clin Pract Rheumatol. 2008;4:226-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8:18-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 440] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 13. | Hoogendoorn RJ, Helder MN, Kroeze RJ, Bank RA, Smit TH, Wuisman PI. Reproducible long-term disc degeneration in a large animal model. Spine (Phila Pa 1976). 2008;33:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Beattie PF. Current understanding of lumbar intervertebral disc degeneration: a review with emphasis upon etiology, pathophysiology, and lumbar magnetic resonance imaging findings. J Orthop Sports Phys Ther. 2008;38:329-340. [PubMed] |
| 15. | Peng B, Wu W, Hou S, Li P, Zhang C, Yang Y. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br. 2005;87:62-67. [PubMed] |
| 16. | Peng B, Hao J, Hou S, Wu W, Jiang D, Fu X, Yang Y. Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976). 2006;31:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Peng B, Chen J, Kuang Z, Li D, Pang X, Zhang X. Expression and role of connective tissue growth factor in painful disc fibrosis and degeneration. Spine (Phila Pa 1976). 2009;34:E178-E182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Freemont AJ, Jeziorska M, Hoyland JA, Rooney P, Kumar S. Mast cells in the pathogenesis of chronic back pain: a hypothesis. J Pathol. 2002;197:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine (Phila Pa 1976). 1990;15:762-767. [PubMed] |
| 20. | Hampton D, Laros G, McCarron R, Franks D. Healing potential of the anulus fibrosus. Spine (Phila Pa 1976). 1989;14:398-401. [PubMed] |
| 21. | Kääpä E, Han X, Holm S, Peltonen J, Takala T, Vanharanta H. Collagen synthesis and types I, III, IV, and VI collagens in an animal model of disc degeneration. Spine (Phila Pa 1976). 1995;20:59-66; discussion 66-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Ulrich JA, Liebenberg EC, Thuillier DU, Lotz JC. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine (Phila Pa 1976). 2007;32:2812-2819. [PubMed] |
| 23. | Holm S, Holm AK, Ekström L, Karladani A, Hansson T. Experimental disc degeneration due to endplate injury. J Spinal Disord Tech. 2004;17:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Rousseau MA, Ulrich JA, Bass EC, Rodriguez AG, Liu JJ, Lotz JC. Stab incision for inducing intervertebral disc degeneration in the rat. Spine (Phila Pa 1976). 2007;32:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Lee TY, Chin GS, Kim WJ, Chau D, Gittes GK, Longaker MT. Expression of transforming growth factor beta 1, 2, and 3 proteins in keloids. Ann Plast Surg. 1999;43:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Fu X, Shen Z, Chen Y, Xie J, Guo Z, Zhang M, Sheng Z. Randomised placebo-controlled trial of use of topical recombinant bovine basic fibroblast growth factor for second-degree burns. Lancet. 1998;352:1661-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3466] [Cited by in RCA: 3334] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 28. | Nagano T, Yonenobu K, Miyamoto S, Tohyama M, Ono K. Distribution of the basic fibroblast growth factor and its receptor gene expression in normal and degenerated rat intervertebral discs. Spine (Phila Pa 1976). 1995;20:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Tolonen J, Grönblad M, Virri J, Seitsalo S, Rytömaa T, Karaharju E. Basic fibroblast growth factor immunoreactivity in blood vessels and cells of disc herniations. Spine (Phila Pa 1976). 1995;20:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Prelich G, Tan CK, Kostura M, Mathews MB, So AG, Downey KM, Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987;326:517-520. [PubMed] |
| 31. | Bogduk N. The innervation of the lumbar spine. Spine (Phila Pa 1976). 1983;8:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 305] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Edgar MA. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg Br. 2007;89:1135-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | García-Cosamalón J, del Valle ME, Calavia MG, García-Suárez O, López-Muñiz A, Otero J, Vega JA. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217:1-15. [PubMed] |
| 34. | Gillette RG, Kramis RC, Roberts WJ. Sympathetic activation of cat spinal neurons responsive to noxious stimulation of deep tissues in the low back. Pain. 1994;56:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | McMahon SB. Mechanisms of sympathetic pain. Br Med Bull. 1991;47:584-600. [PubMed] |
| 36. | Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O’Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 641] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 37. | Coppes MH, Marani E, Thomeer RT, Oudega M, Groen GJ. Innervation of annulus fibrosis in low back pain. Lancet. 1990;336:189-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 91] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Morinaga T, Takahashi K, Yamagata M, Chiba T, Tanaka K, Takahashi Y, Nakamura S, Suseki K, Moriya H. Sensory innervation to the anterior portion of lumbar intervertebral disc. Spine (Phila Pa 1976). 1996;21:1848-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Nakamura S, Takahashi K, Takahashi Y, Morinaga T, Shimada Y, Moriya H. Origin of nerves supplying the posterior portion of lumbar intervertebral discs in rats. Spine (Phila Pa 1976). 1996;21:917-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Suseki K, Takahashi Y, Takahashi K, Chiba T, Yamagata M, Moriya H. Sensory nerve fibres from lumbar intervertebral discs pass through rami communicantes. A possible pathway for discogenic low back pain. J Bone Joint Surg Br. 1998;80:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Nakamura SI, Takahashi K, Takahashi Y, Yamagata M, Moriya H. The afferent pathways of discogenic low-back pain. Evaluation of L2 spinal nerve infiltration. J Bone Joint Surg Br. 1996;78:606-612. [PubMed] |
| 42. | Merskey H, Bogduk N. Classification of Chronic Pain. Descriptions of Chronic Pain Syndrome and Definitions of Pain Terms. Seattle: IASP Press. 1994;180-181. |
| 43. | Peng BG, Pang XD, Li DM, Zhang XY, Kuang ZD, Du MK, Gao CH. Typing of discogenic low back pain. Zhonghua Guke Zazhi. 2009;31:801-805. [DOI] [Full Text] |
| 44. | Sachs BL, Vanharanta H, Spivey MA, Guyer RD, Videman T, Rashbaum RF, Johnson RG, Hochschuler SH, Mooney V. Dallas discogram description. A new classification of CT/discography in low-back disorders. Spine (Phila Pa 1976). 1987;12:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 185] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Zhou Y, Abdi S. Diagnosis and minimally invasive treatment of lumbar discogenic pain--a review of the literature. Clin J Pain. 2006;22:468-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J Bone Joint Surg Am. 2006;88 Suppl 2:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Peng B, Chen J, Kuang Z, Li D, Pang X, Zhang X. Diagnosis and surgical treatment of back pain originating from endplate. Eur Spine J. 2009;18:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Wagner AL, Murtagh FR, Arrington JA, Stallworth D. Relationship of Schmorl’s nodes to vertebral body endplate fractures and acute endplate disk extrusions. AJNR Am J Neuroradiol. 2000;21:276-281. [PubMed] |
| 49. | Holm S, Ekström L, Kaigle Holm A, Hansson T. Intradiscal pressure in the degenerated porcine intervertebral disc. Vet Comp Orthop Traumatol. 2007;20:29-33. [PubMed] |
| 50. | Haschtmann D, Stoyanov JV, Gédet P, Ferguson SJ. Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J. 2008;17:289-299. [PubMed] |
| 51. | Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MT, Ross ER, O’Brien JP, Hoyland JA. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 336] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 52. | Manchikanti L, Datta S, Gupta S, Munglani R, Bryce DA, Ward SP, Benyamin RM, Sharma ML, Helm S, Fellows B. A critical review of the American Pain Society clinical practice guidelines for interventional techniques: part 2. Therapeutic interventions. Pain Physician. 2010;13:E215-E264. [PubMed] |
| 53. | Ito M, Incorvaia KM, Yu SF, Fredrickson BE, Yuan HA, Rosenbaum AE. Predictive signs of discogenic lumbar pain on magnetic resonance imaging with discography correlation. Spine (Phila Pa 1976). 1998;23:1252-128; discussion 1252-128;. [PubMed] |
| 54. | Moneta GB, Videman T, Kaivanto K, Aprill C, Spivey M, Vanharanta H, Sachs BL, Guyer RD, Hochschuler SH, Raschbaum RF. Reported pain during lumbar discography as a function of anular ruptures and disc degeneration. A re-analysis of 833 discograms. Spine (Phila Pa 1976). 1994;19:1968-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 157] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Walsh TR, Weinstein JN, Spratt KF, Lehmann TR, Aprill C, Sayre H. Lumbar discography in normal subjects. A controlled, prospective study. J Bone Joint Surg Am. 1990;72:1081-1088. [PubMed] |
| 56. | Derby R, Howard MW, Grant JM, Lettice JJ, Van Peteghem PK, Ryan DP. The ability of pressure-controlled discography to predict surgical and nonsurgical outcomes. Spine (Phila Pa 1976). 1999;24:364-71; discussion 371-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 57. | Derby R, Kim BJ, Lee SH, Chen Y, Seo KS, Aprill C. Comparison of discographic findings in asymptomatic subject discs and the negative discs of chronic LBP patients: can discography distinguish asymptomatic discs among morphologically abnormal discs? Spine J. 2005;5:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Laplante BL, Ketchum JM, Saullo TR, DePalma MJ. Multivariable analysis of the relationship between pain referral patterns and the source of chronic low back pain. Pain Physician. 2012;15:171-178. [PubMed] |
| 59. | Chou R, Loeser JD, Owens DK, Rosenquist RW, Atlas SJ, Baisden J, Carragee EJ, Grabois M, Murphy DR, Resnick DK. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 60. | Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976). 2009;34:1078-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 61. | Buenaventura RM, Shah RV, Patel V, Benyamin R, Singh V. Systematic review of discography as a diagnostic test for spinal pain: an update. Pain Physician. 2007;10:147-164. [PubMed] |
| 62. | Willems PC, Staal JB, Walenkamp GH, de Bie RA. Spinal fusion for chronic low back pain: systematic review on the accuracy of tests for patient selection. Spine J. 2013;13:99-109. [PubMed] |
| 63. | Peng B, Fu X, Pang X, Li D, Liu W, Gao C, Yang H. Prospective clinical study on natural history of discogenic low back pain at 4 years of follow-up. Pain Physician. 2012;15:525-532. [PubMed] |
| 64. | Carragee EJ. Clinical practice. Persistent low back pain. N Engl J Med. 2005;352:1891-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Kuijpers T, van Middelkoop M, Rubinstein SM, Ostelo R, Verhagen A, Koes BW, van Tulder MW. A systematic review on the effectiveness of pharmacological interventions for chronic non-specific low-back pain. Eur Spine J. 2011;20:40-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 66. | Petersen T, Larsen K, Jacobsen S. One-year follow-up comparison of the effectiveness of McKenzie treatment and strengthening training for patients with chronic low back pain: outcome and prognostic factors. Spine (Phila Pa 1976). 2007;32:2948-2956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Manchikanti L, Glaser SE, Wolfer L, Derby R, Cohen SP. Systematic review of lumbar discography as a diagnostic test for chronic low back pain. Pain Physician. 2009;12:541-559. [PubMed] |
| 68. | Manchikanti L, Pampati V, Boswell MV, Smith HS, Hirsch JA. Analysis of the growth of epidural injections and costs in the Medicare population: a comparative evaluation of 1997, 2002, and 2006 data. Pain Physician. 2010;13:199-212. [PubMed] |
| 69. | Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2009;34:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 70. | Manchikanti L, Datta S, Derby R, Wolfer LR, Benyamin RM, Hirsch JA. A critical review of the American Pain Society clinical practice guidelines for interventional techniques: part 1. Diagnostic interventions. Pain Physician. 2010;13:E141-E174. [PubMed] |
| 71. | Parr AT, Diwan S, Abdi S. Lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain: a systematic review. Pain Physician. 2009;12:163-188. [PubMed] |
| 72. | Conn A, Buenaventura RM, Datta S, Abdi S, Diwan S. Systematic review of caudal epidural injections in the management of chronic low back pain. Pain Physician. 2009;12:109-135. [PubMed] |
| 73. | Buenaventura RM, Datta S, Abdi S, Smith HS. Systematic review of therapeutic lumbar transforaminal epidural steroid injections. Pain Physician. 2009;12:233-251. [PubMed] |
| 74. | Manchikanti L, Singh V, Derby R, Schultz DM, Benyamin RM, Prager JP, Hirsch JA. Reassessment of evidence synthesis of occupational medicine practice guidelines for interventional pain management. Pain Physician. 2008;11:393-482. [PubMed] |
| 75. | Bogduk N. Epidural steroids for low back pain and sciatica. Pain Digest. 1999;9:226-227. |
| 76. | Kepes ER, Duncalf D. Treatment of backache with spinal injections of local anesthetics, spinal and systemic steroids. A review. Pain. 1985;22:33-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Koes BW, Scholten RJ, Mens JM, Bouter LM. Efficacy of epidural steroid injections for low-back pain and sciatica: a systematic review of randomized clinical trials. Pain. 1995;63:279-288. [PubMed] |
| 78. | Rozenberg S, Dubourg G, Khalifa P, Paolozzi L, Maheu E, Ravaud P. Efficacy of epidural steroids in low back pain and sciatica. A critical appraisal by a French Task Force of randomized trials. Critical Analysis Group of the French Society for Rheumatology. Rev Rhum Engl Ed. 1999;66:79-85. [PubMed] |
| 79. | Watts RW, Silagy CA. A meta-analysis on the efficacy of epidural corticosteroids in the treatment of sciatica. Anaesth Intensive Care. 1995;23:564-569. [PubMed] |
| 80. | Benyamin RM, Manchikanti L, Parr AT, Diwan S, Singh V, Falco FJ, Datta S, Abdi S, Hirsch JA. The effectiveness of lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain. Pain Physician. 2012;15:E363-E404. [PubMed] |
| 81. | Pasqualucci A, Varrassi G, Braschi A, Peduto VA, Brunelli A, Marinangeli F, Gori F, Colò F, Paladini A, Mojoli F. Epidural local anesthetic plus corticosteroid for the treatment of cervical brachial radicular pain: single injection versus continuous infusion. Clin J Pain. 2007;23:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Byröd G, Otani K, Brisby H, Rydevik B, Olmarker K. Methylprednisolone reduces the early vascular permeability increase in spinal nerve roots induced by epidural nucleus pulposus application. J Orthop Res. 2000;18:983-987. [PubMed] |
| 83. | Hayashi N, Weinstein JN, Meller ST, Lee HM, Spratt KF, Gebhart GF. The effect of epidural injection of betamethasone or bupivacaine in a rat model of lumbar radiculopathy. Spine (Phila Pa 1976). 1998;23:877-885. [PubMed] |
| 84. | Lee HM, Weinstein JN, Meller ST, Hayashi N, Spratt KF, Gebhart GF. The role of steroids and their effects on phospholipase A2. An animal model of radiculopathy. Spine (Phila Pa 1976). 1998;23:1191-1196. [PubMed] |
| 85. | Minamide A, Tamaki T, Hashizume H, Yoshida M, Kawakami M, Hayashi N. Effects of steroid and lipopolysaccharide on spontaneous resorption of herniated intervertebral discs. An experimental study in the rabbit. Spine (Phila Pa 1976). 1998;23:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain: a prospective outcome study with minimum 1-year follow-up. Spine (Phila Pa 1976). 2000;25:2622-2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 87. | Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain: prospective outcome study with a minimum 2-year follow-up. Spine (Phila Pa 1976). 2002;27:966-73; discussion 973-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Freeman BJ, Walters RM, Moore RJ, Fraser RD. Does intradiscal electrothermal therapy denervate and repair experimentally induced posterolateral annular tears in an animal model? Spine (Phila Pa 1976). 2003;28:2602-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Freeman BJ, Fraser RD, Cain CM, Hall DJ, Chapple DC. A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine (Phila Pa 1976). 2005;30:2369-277; discussion 2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 90. | Pauza KJ, Howell S, Dreyfuss P, Peloza JH, Dawson K, Bogduk N. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 91. | Derby R, Eek B, Lee SH, Seo KS, Kim BJ. Comparison of intradiscal restorative injections and intradiscal electrothermal treatment (IDET) in the treatment of low back pain. Pain Physician. 2004;7:63-66. [PubMed] |
| 92. | Tsou HK, Chao SC, Kao TH, Yiin JJ, Hsu HC, Shen CC, Chen HT. Intradiscal electrothermal therapy in the treatment of chronic low back pain: experience with 93 patients. Surg Neurol Int. 2010;1:37. [PubMed] |
| 93. | Assietti R, Morosi M, Migliaccio G, Meani L, Block JE. Treatment of discogenic low back pain with Intradiscal Electrothermal Therapy (IDET): 24 months follow-up in 50 consecutive patients. Acta Neurochir Suppl. 2011;108:103-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 94. | Davis TT, Delamarter RB, Sra P, Goldstein TB. The IDET procedure for chronic discogenic low back pain. Spine (Phila Pa 1976). 2004;29:752-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Derby R, Lee SH, Seo KS, Kazala K, Kim BJ, Kim MJ. Efficacy of IDET for relief of leg pain associated with discogenic low back pain. Pain Pract. 2004;4:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 96. | Nunley PD, Jawahar A, Brandao SM, Wilkinson KM. Intradiscal electrothermal therapy (IDET) for low back pain in worker’s compensation patients: can it provide a potential answer? Long-term results. J Spinal Disord Tech. 2008;21:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Helm S, Hayek SM, Benyamin RM, Manchikanti L. Systematic review of the effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician. 2009;12:207-232. [PubMed] |
| 98. | Kapural L, Mekhail N, Hicks D, Kapural M, Sloan S, Moghal N, Ross J, Petrinec D. Histological changes and temperature distribution studies of a novel bipolar radiofrequency heating system in degenerated and nondegenerated human cadaver lumbar discs. Pain Med. 2008;9:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Kapural L, Ng A, Dalton J, Mascha E, Kapural M, de la Garza M, Mekhail N. Intervertebral disc biacuplasty for the treatment of lumbar discogenic pain: results of a six-month follow-up. Pain Med. 2008;9:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Karaman H, Tüfek A, Kavak GÖ, Kaya S, Yildirim ZB, Uysal E, Celik F. 6-month results of TransDiscal Biacuplasty on patients with discogenic low back pain: preliminary findings. Int J Med Sci. 2010;8:1-8. [PubMed] |
| 101. | Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine (Phila Pa 1976). 1995;20:356-361. [PubMed] |
| 102. | Ohtori S, Koshi T, Yamashita M, Yamauchi K, Inoue G, Suzuki M, Orita S, Eguchi Y, Ochiai N, Kishida S. Surgical versus nonsurgical treatment of selected patients with discogenic low back pain: a small-sized randomized trial. Spine (Phila Pa 1976). 2011;36:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 103. | Fritzell P, Hägg O, Wessberg P, Nordwall A. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine (Phila Pa 1976). 2002;27:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 355] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 104. | Fritzell P, Hägg O, Wessberg P, Nordwall A. 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976). 2001;26:2521-2532; discussion 2521-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 598] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 105. | Hanley EN, David SM. Lumbar arthrodesis for the treatment of back pain. J Bone Joint Surg Am. 1999;81:716-730. [PubMed] |
| 106. | Bertagnoli R, Yue JJ, Shah RV, Nanieva R, Pfeiffer F, Fenk-Mayer A, Kershaw T, Husted DS. The treatment of disabling single-level lumbar discogenic low back pain with total disc arthroplasty utilizing the Prodisc prosthesis: a prospective study with 2-year minimum follow-up. Spine (Phila Pa 1976). 2005;30:2230-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Bertagnoli R, Yue JJ, Shah RV, Nanieva R, Pfeiffer F, Fenk-Mayer A, Kershaw T, Husted DS. The treatment of disabling multilevel lumbar discogenic low back pain with total disc arthroplasty utilizing the ProDisc prosthesis: a prospective study with 2-year minimum follow-up. Spine (Phila Pa 1976). 2005;30:2192-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 108. | Hellum C, Johnsen LG, Storheim K, Nygaard OP, Brox JI, Rossvoll I, Rø M, Sandvik L, Grundnes O. Surgery with disc prosthesis versus rehabilitation in patients with low back pain and degenerative disc: two year follow-up of randomised study. BMJ. 2011;342:d2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 109. | Jacobs WC, van der Gaag NA, Kruyt MC, Tuschel A, de Kleuver M, Peul WC, Verbout AJ, Oner FC. Total disc replacement for chronic discogenic low back pain: a cochrane review. Spine (Phila Pa 1976). 2013;38:24-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 110. | Peng B, Zhang Y, Hou S, Wu W, Fu X. Intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Eur Spine J. 2007;16:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Peng B, Pang X, Wu Y, Zhao C, Song X. A randomized placebo-controlled trial of intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Pain. 2010;149:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 535] [Reference Citation Analysis (0)] |
| 112. | Pang X, Xu Z, Peng B, Yang H, Li D, Gao C. The experimental study on the mechanisms of methylene blue injection for treatment of discogenic low back pain in the animal model. Zhongguo Tengtong Yixue Zazhi. 2011;17:274-279. |
| 113. | Kang X, Peng B. The experimental study on the NO content in the degenerated disc induced by needle puncture animal model. Zhongguo Tengtong Yixue Zazhi. 2011;17:266-273. |
| 114. | Peng B, Pang X. Regeneration and repair of intervertebral disc degeneration. Regenerative medicine in China. Washington: Science/AAAS 2012; 52-53. |
| 115. | Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 344] [Article Influence: 24.6] [Reference Citation Analysis (0)] |