Revised: November 22, 2011
Accepted: February 16, 2012
Published online: February 18, 2012
AIM: To assess the presence of nerves in ventral facet joint capsules as facet capsules are generally implicated in neck pain.
METHODS: Twenty-four ventral cervical facet joint capsules were harvested from 3 unembalmed cadavers. Paraffin sections from these capsules were processed to identify neurofilament and substance P immunoreactive fibers. Nerve fiber presence was also verified by a silver impregnation method.
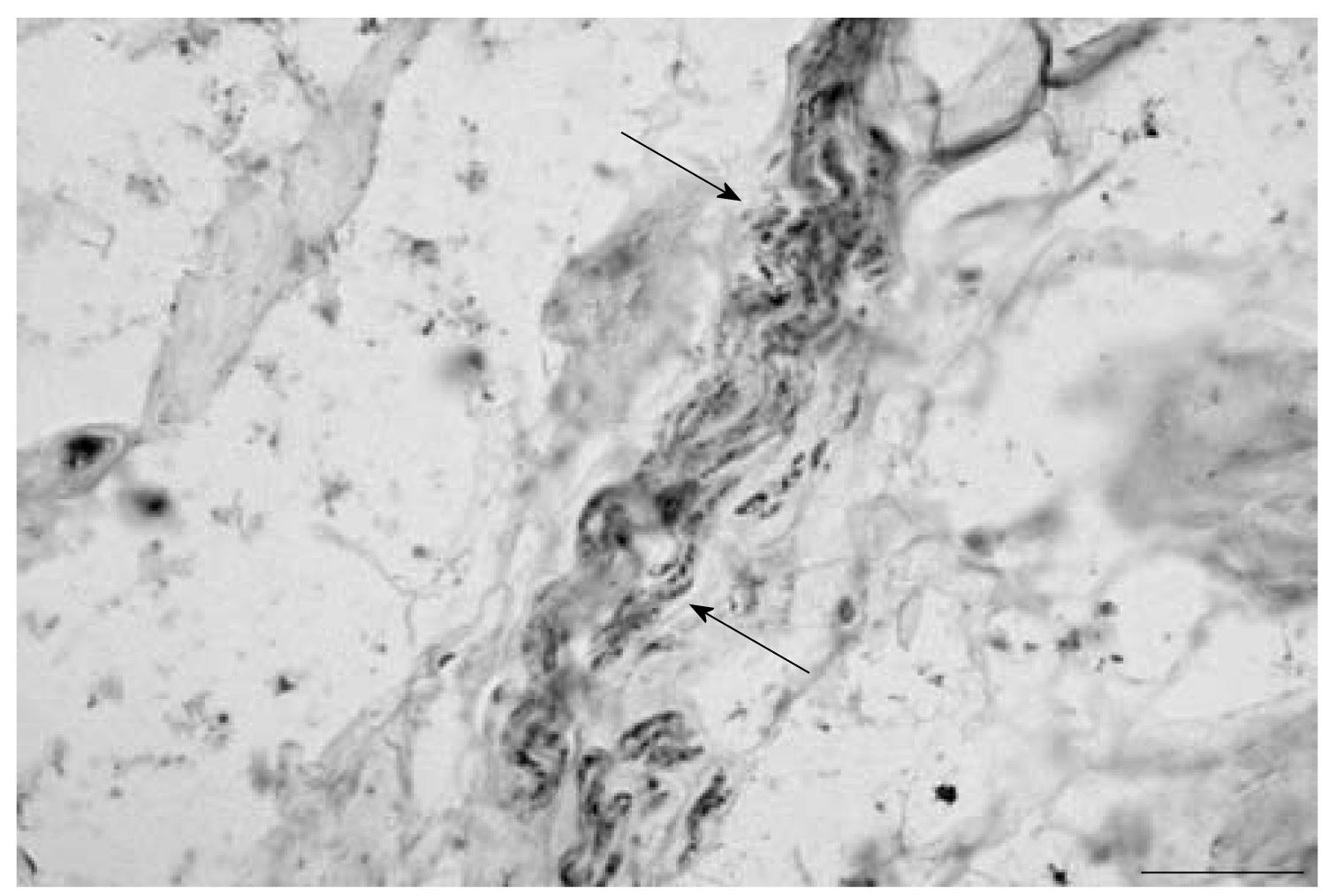
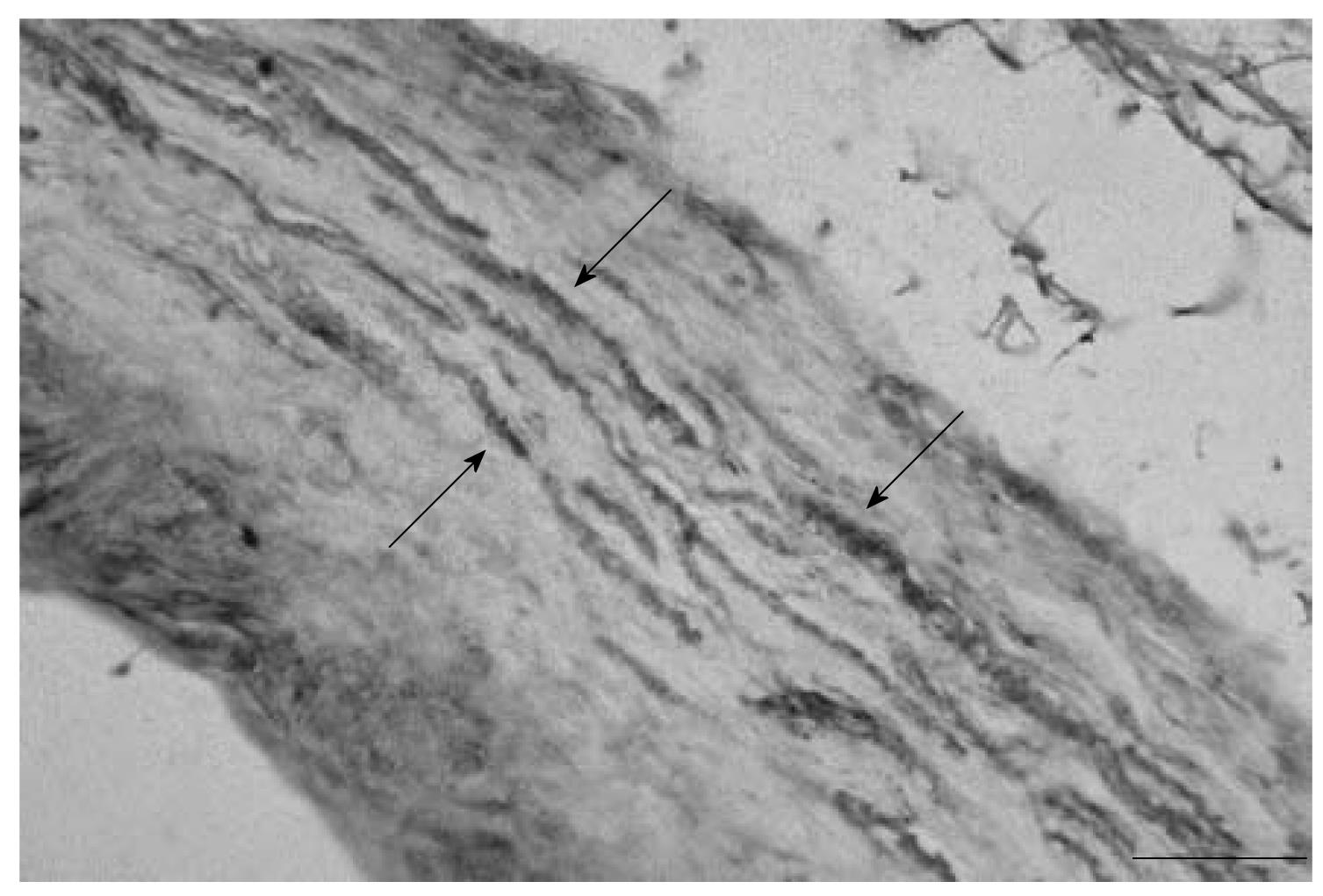
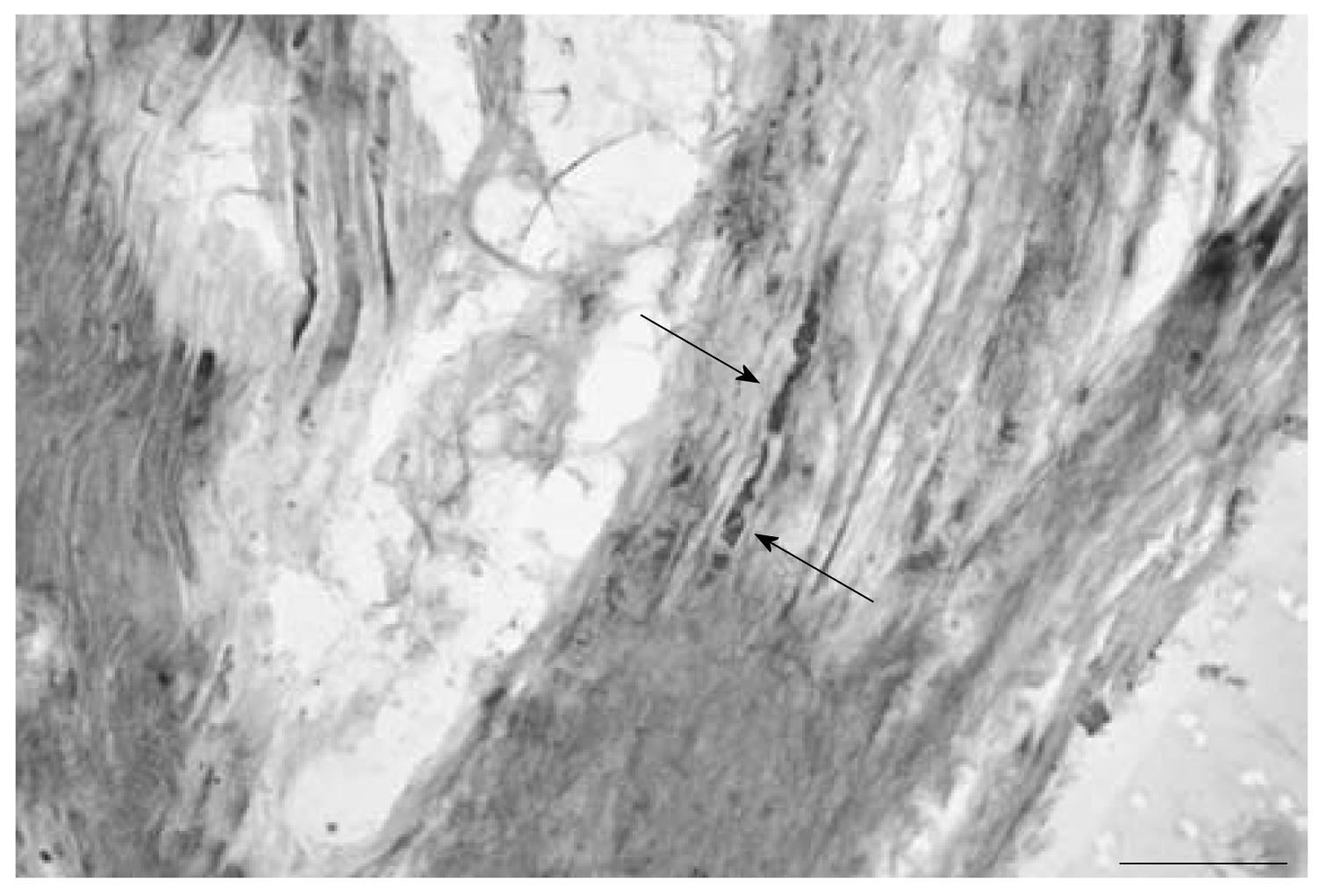
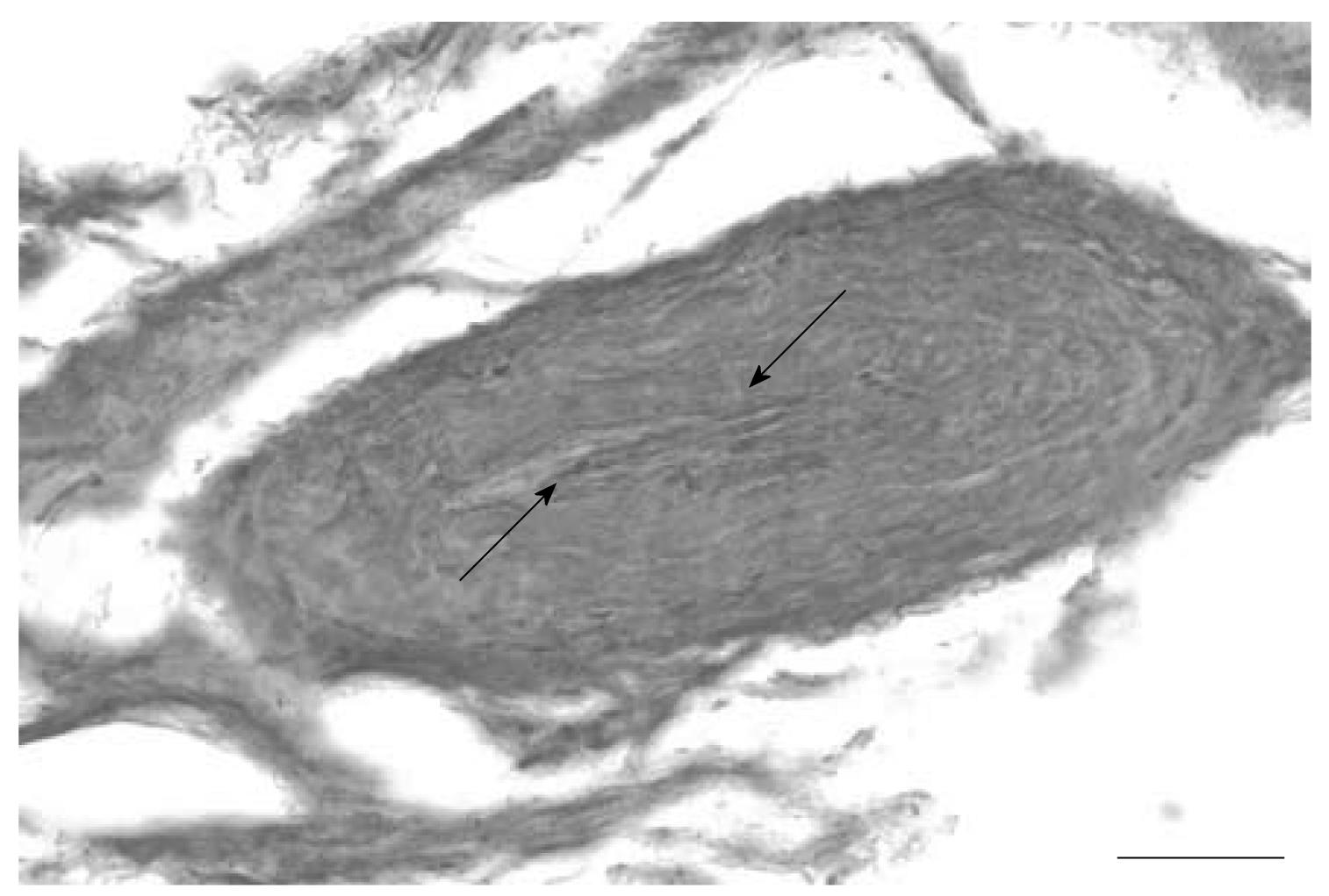
RESULTS: Neurofilament reactive fibers were observed in sections from 9 capsules. They were observed in areas with collagen fibers and areas with irregular connective tissue. Substance P reactive nerve fibers were found in sections from 7 capsules in similar areas. Silver impregnation also revealed the presence of nerve fibers. The nerve fibers were also found as bundles in the lateral margins of the capsule. A Pacinian corpuscle-like ending was also observed in one specimen.
CONCLUSION: Nerve fibers revealed by neurofilament immunoreactivity and silver staining support innervation of the ventral aspect of the facet joint capsule. The presence of substance P reactive fibers supports the potential role of these elements in mediating pain. The presence of a Pacinian-like ending implicates a potential role in joint movement.
- Citation: Kallakuri S, Li Y, Chen C, Cavanaugh JM. Innervation of cervical ventral facet joint capsule: Histological evidence. World J Orthop 2012; 3(2): 10-14
- URL: https://www.wjgnet.com/2218-5836/full/v3/i2/10.htm
- DOI: https://dx.doi.org/10.5312/wjo.v3.i2.10
According to a United Nations Transport division report, the total annual cost of rear impact whiplash injuries has been estimated to be approximately $ 2.7 billion in the United States, with an estimated 272 464 annual injuries. Whiplash associated disorders (WAD) to the neck are common and can occur during frontal, side and rear-end impacts, but most complaints occur after a rear-end collision by another vehicle. Studies by Aprill and Bogduk[1], Barnsley et al[2] and Lord et al[3] provide clinical evidence for the role of cervical facet joints in the etiology of neck pain with several other studies providing biomechanical evidence[4,5]. It has been hypothesized that in a rear impact, the facet joints slide with the posterior-most regions of the joints undergoing compression more than the anterior-most regions, resulting in a pinching or capsular stretch mechanism that may be related to neck pain[5,6].
Additionally, the role of cervical osteoarthritis as a contributing factor to neck pain and other upper extremity painful conditions related to arms and shoulders can not be ignored. An analysis on the progression rates of cervical osteoarthritis from a cohort of 707 subjects showed that women were more likely to experience worsening of their disease compared to men[7], while a recent anatomical and epidemiological study of cadaveric spines revealed concurrent lumbar and cervical arthrosis as a common condition, suggesting an underlying systemic component for spinal osteoarthritis[8].
The cervical facet joint capsules (FJCs) are innervated by medial branches of the dorsal ramus[9]. Igarashi et al[10] suggested that inflammatory cytokines produced in degenerated facet joints may leak into the intra-spinal space through the most lateral part of the ventral FJC. Evidence for the presence of inflammatory cytokines was demonstrated in homogenates of degenerated lumbar facet joint cartilage and synovial tissues harvested from patients undergoing posterior lumbar surgery, while evidence for leakage was shown by stained ventral facet joint capsule and lateral ligamentum flavum following methylene blue injection into the degenerated dorsal facet joint. Therefore degenerative changes in the ventral cervical facet joints capsule may lead to a painful sensation in the neck and surrounding areas.
Thus, previous research indicates that the ventral aspect of the cervical FJC can undergo degenerative and inflammatory changes which can lead to a sensation of pain in the neck. However, studies focused on the innervation of ventral areas of FJC have not been published and the available studies are limited to innervation of the dorsal aspect of the cervical FJC[11] or the synovial fold[12]. Hence, the purpose of this study was to characterize the presence of nerve fibers in the ventral cervical FJCs by immunocytochemical and silver impregnation methods.
A total of 24 ventral facet joint capsules from C1/C2-C6/C7 were harvested from the cervical spines of 3 unembalmed cadavers. One (female; 73 years) was donated as part of the Willed Body Program to the institution for other biomechanical studies and the other two (56 year old female and 44 old male) were procured from the National Disease Research Interchange (NDRI, Philadelphia, PA). All procedures were approved by the institutional Human and Animal Investigation Committee. The harvested capsules were fixed (4% paraformaldehyde) and processed for paraffin infiltration and sectioning (10-15 µm; Reichert Yung, Leica Microsystems Nussloch GmbH, Nussloch). These sections were deparaffinized and cleared in xylene and graded alcohol and washed thoroughly in distilled water and processed for a silver impregnation technique and avidin biotin peroxidase immunocytochemistry.
A Palmgren silver impregnation technique was used to visualize nerve fibers in the ventral FJC sections. The sections were incubated in a solution of 10% silver nitrate for 30 min at 37°C and then were transferred to 2% sodium borate and then thoroughly washed. This was followed by developing for 10 min in 0.05 g hydroquinone and 5.0 g sodium sulphite dissolved in 100 mL of freshly prepared solution of 2% sodium borate. The sections were then rinsed in 50% alcohol, treated for 60 s to 70 s in 0.5% oxalic acid in 50% alcohol, rinsed in distilled water and then fixed for 10 s in 5% sodium thiosulphate. Finally, the sections were rinsed in distilled water, dehydrated in graded alcohol, xylene and cover-slipped.
The sections were incubated in 4% hydrogen peroxide to block endogenous peroxide activity, followed by blocking in 2% normal goat serum (Vector Laboratories, Burlingame, CA). The sections were then incubated overnight in 1:500 diluted antisera to neurofilament light chain (NF; AB9568, Millipore, CA), 1:200 diluted antisera to substance P (SP, Millipore, CA) at 4 °C. This was followed by incubation in biotinylated secondary antibodies before being exposed to Vectastain Elite ABC reagent (Vector Laboratories). After each step, the sections were rinsed three times in phosphate buffered saline (PBS). The reaction product was then developed by brief incubation in 3, 3’-diaminobenzidine and hydrogen peroxide. The sections were then washed, counterstained with hematoxylin and dehydrated through graded alcohol, cleared in xylene, and cover-slipped with permount.
A total of 90 slides were stained for immunocytochemistry and 60 for silver staining, respectively. The sections were analyzed to determine the presence or absence of nerve fibers but not for quantitative comparisons. All the stained sections were observed under a light microscope (Leica DMLB, Leica Microsystems Ltd., Heerburg, Switzerland) attached to a digital camera system (Diagnostic Instruments Inc., Sterling Heights, MI).
Sections of the ventral facet joint capsule have parallel bundles of collagen fibers and areas of irregular connective tissue. Also found were areas with adipose-like connective tissue. Neurofilament (NF) reactive fibers were observed in sections from 9 capsules at levels C2/C3-C5/C6. They were observed as single fibers and in groups of 2 or 3 fibers (Figure 1). The NF-reactive fibers were observed to be in areas of joint capsule with collagen fibers as well as in areas with irregular connective tissue. Some of the NF reactive regions appeared as a chain of beads with intermittent staining.
Substance P (SP) reactive nerve fibers were found in areas with parallel bundles of collagen fibers as well as in areas with irregular connective tissue from 7 capsules at levels C2/3-C5/6. Additionally, SP reactive fibers were observed to be both as single fibers (Figure 2) and in bundles, similar to those found with NF reactivity.
Silver impregnation also revealed the presence of short and long nerve fibers in capsule sections with parallel bundles of collagen fibers, as well as in areas with dense irregular connective tissue (Figure 3). The nerve fibers appeared to be running parallel to the collagen fibers and occasionally showed profiles of dotted staining. The nerve fibers were also found as bundles in the lateral margins of the capsule. There also appeared to be a nerve profile that resembled an encapsulated nerve ending composed of groups of fibers surrounded by a ring-like Pacinian-type end organ (Figure 4).
There is ample clinical[2,3,13,14] and biomechanical[15,16] evidence of the role of cervical FJC in the etiology of neck pain. Neurophysiological studies in goat cervical spine showed activation of low threshold mechanoreceptors at low strains and high threshold mechanoreceptors at high strains[17] on the dorsal aspect of the capsule. While several studies showed innervation of the dorsal aspect of cervical FJC[11,12,18,19] or to the synovial fold[12,19,20], the results of the current study provide histological evidence to the presence of neural elements on the ventral aspect of cervical FJC.
In the current study, no systematic effort was made to study the morphology of cervical FJC. Although the samples were harvested from only 3 cadavers, nerve fibers were identified in the ventral aspect of sections from all three cadavers in a total of 10 facet joints. In the present study, sections of the ventral aspect of the cervical facet joint capsule showed areas of parallel bundles of collagen fibers and areas with irregular connective tissue. This may be similar to the findings of Yamashita et al[21] who described an outer layer of densely packed parallel bundles of collagen fibers and inner layer with loose connective tissue with irregularly oriented fibers[21]. This study also showed areas in some sections where collagen fibers were associated with adipose-like tissue that may be similar to fat filled intra-capsular folds present in the lumbar facet joint capsules[22] or adipose plical tissue.
Bogduk et al[9] provided some of the most detailed descriptions of the anatomy of the dorsal rami and their supply of nerve fibers to the cervical FJC through the medial branch and reports the space ventral to the joint as being free of articular nerves in the cervical region. Bogduk[23] did not report any specific articular branches to the ventral aspect of the lumbar zygapophyseal joint but eludes to the findings of others. Whether these findings from the lumbar region can be extended to the cervical region remains to be investigated. However, our present study provides strong evidence for the presence of nerve fibers in the ventral FJC by the presence of NF immunoreactive nerve fibers and bundles in various layers of the capsule. This was further supported by the silver stained nerve fibers. However, a clear neuroanatomical description on the origin of ventral articular fibers still remains elusive.
Despite these limitations, innervation of the ventral aspect of the capsule has clinical implications. If these nerve fibers do not originate from the dorsal ramus, dorsal ramus rhizotomy would be ineffective in the treatment of pain from this area. In addition, facet injections may not provide anesthesia to the ventral capsule. Finally, effectiveness of epidural steroids may be partially related to its effects on the ventral capsule.
This study also shows SP reactive fibers in the ventral cervical FJC, lending credence to its putative role as a source of pain-related sensation. This may be important in the context of inflammatory pain originating from cervical FJC. In a recent study, Igarashi et al[10] showed higher visual analog and Roland-Morris disability questionnaire scores in patients with lumbar spinal canal stenosis that co-related with high levels of IL1ß. They concluded that inflammatory cytokines produced in a degenerated facet joint may leak into the intraspinal space through the lateral part of the ventral FJC, based on leakage of dorsally injected methylene blue dye that stained the joint cartilage, ventral capsule and other surrounding structures in cadavers.
In a recent study, Ivancic[24] showed that exposing FJC in cervical spine preparations to elongations at a rate of 1 mm/s in increments of 0.5 mm can lead to increased laxity and indicated that this increased laxity may be one of the components perpetuating chronic pain clinical instability in whiplash patients. Biomechanical evidence on ventral capsular kinematics comes from Stemper et al[15] who subjected intact head and neck complexes to whiplash accelerations and analyzed shear and distraction motions in ventral and dorsal joint regions from C4-C7 levels. They showed lower cervical facet joints undergoing dorsally directed shear motion with distraction in the ventral and compression in the dorsal regions of the joint. Facet joint shear and distraction motion increased with impact severity. They suggested that the ventral region responded with greater magnitudes of stretch. Moreover, high levels of capsule stretch may also be related to altered axonal morphological changes (axonal swellings) similar to those reported in cervical dorsal facet joint capsule[25]. Axonal swellings and retraction balls are characteristic of diffuse axonal injury seen in patients of TBI. Finally, a Pacinian-like end organ in some layers of the ventral FJC was also found, which may be similar to the findings of McLain[18] who also reported encapsulated and simple or free nerve endings in the human cervical FJC subsynovial loose areolar and dense connective tissue. Presence of Pacinian-like end organs may indicate that ventral FJC may be involved in responding to high velocity changes in joint position.
Prominent NF and SP reactive fibers in the ventral cervical FJC suggest a nociceptive function and a putative role for ventral FJC in the etiology of neck pain following whiplash injury, inflammatory conditions and spondylosis.
Whiplash associated disorders to the neck are common and can occur during frontal, side and rear-end impacts, but most complaints occur after a rear-end collision by another vehicle. The role of cervical facet joint capsules (FJC) in whiplash has been widely implicated by both clinical and biomechanical studies. Although the presence of nerve fibers on the dorsal capsule has been reported in several studies, histological evidence revealing their presence on the ventral aspect is lacking.
There is extensive clinical and biomechanical evidence on the role of cervical FJC in the etiology of neck pain. Neurophysiological studies in goat cervical spine showed activation of low threshold mechanoreceptors at low strains and high threshold mechanoreceptors at high strains on the dorsal aspect of the capsule. While several studies showed innervation of the dorsal aspect of cervical FJC or to the synovial fold, studies related to the presence of nerve fibers on the ventral aspect are lacking. It is possible that the ventral aspect of the cervical FJC can undergo degenerative and inflammatory changes which can lead to a sensation of pain in the neck.
Previous studies have shown innervation of dorsal facet capsule. This is the first histological study to document ventral capsule innervation. The presence of nerve fibers is supported by neurofilament immunoreactive fibers as well as those shown by silver staining.
This study offers support for the potential role for ventral facet joint capsules in the etiology of neck pain following whiplash injury, inflammatory conditions and spondylosis.
Whiplash: Rapid flexion and extension of the neck during frontal and rear-end impacts. Substance P: A neuropeptide involved in pain signaling.
This is a very interesting topic that has been focused on. It is a very well written manuscript with a good educational point of view and will be a good contribution for publication in the journal.
Peer reviewers: Marco Giuseppe Angelo Teli, MD, Department of Orthopaedics, Policlinico San Marco, Corso Europa 7, 20035 Osio Sotto-Bergamo, Italy; Marios Georgios Lykissas, MD, PhD, Department of Orthopaedic Surgery, University of Ioannina School of Medicine Dourouti, Ioannina, PC 45110, Greece
S- Editor Yang XC L- Editor Roemmele A E- Editor Yang XC
| 1. | Aprill C, Bogduk N. The prevalence of cervical zygapophyseal joint pain. A first approximation. Spine (Phila Pa 1976). 1992;17:744-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 100] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Barnsley L, Lord SM, Wallis BJ, Bogduk N. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine (Phila Pa 1976). 1995;20:20-5; discussion 26. [PubMed] |
| 3. | Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine (Phila Pa 1976). 1996;21:1737-144; discussion 1737-144;. [PubMed] |
| 4. | Deng B, Begeman P, Yang K, Tashman S, King AI. Kinematics of human cadaver cervical spine during low speed rear-end impacts. Proceedings of the 44th Stapp Car Crash Conference, 2000: 171-188. . |
| 5. | Yoganandan N, Pintar FA, Cusick JF. Biomechanical analyses of whiplash injuries using an experimental model. Accid Anal Prev. 2002;34:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Yang KH, Begeman PC, Muser M, Niederer P, Waltz F. On the role of cervical facet joints in rear end impact neck injury mechanisms. Society for Automotive Engineers. 1997;970497. [DOI] [Full Text] |
| 7. | Wilder FV, Fahlman L, Donnelly R. Radiographic cervical spine osteoarthritis progression rates: a longitudinal assessment. Rheumatol Int. 2011;31:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Master DL, Eubanks JD, Ahn NU. Prevalence of concurrent lumbar and cervical arthrosis: an anatomic study of cadaveric specimens. Spine (Phila Pa 1976). 2009;34:E272-E275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine (Phila Pa 1976). 1982;7:319-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 215] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Igarashi A, Kikuchi S, Konno S. Correlation between inflammatory cytokines released from the lumbar facet joint tissue and symptoms in degenerative lumbar spinal disorders. J Orthop Sci. 2007;12:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Kallakuri S, Singh A, Chen C, Cavanaugh JM. Demonstration of substance P, calcitonin gene-related peptide, and protein gene product 9.5 containing nerve fibers in human cervical facet joint capsules. Spine (Phila Pa 1976). 2004;29:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Inami S, Shiga T, Tsujino A, Yabuki T, Okado N, Ochiai N. Immunohistochemical demonstration of nerve fibers in the synovial fold of the human cervical facet joint. J Orthop Res. 2001;19:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Manchikanti L, Boswell MV, Singh V, Pampati V, Damron KS, Beyer CD. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;5:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Manchikanti L, Manchikanti KN, Pampati V, Brandon DE, Giordano J. The prevalence of facet-joint-related chronic neck pain in postsurgical and nonpostsurgical patients: a comparative evaluation. Pain Pract. 2008;8:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Stemper BD, Yoganandan N, Pintar FA. Gender- and region-dependent local facet joint kinematics in rear impact: implications in whiplash injury. Spine (Phila Pa 1976). 2004;29:1764-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Quinn KP, Winkelstein BA. Cervical facet capsular ligament yield defines the threshold for injury and persistent joint-mediated neck pain. J Biomech. 2007;40:2299-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Neural response of cervical facet joint capsule to stretch: a study of whiplash pain mechanism. Stapp Car Crash J. 2005;49:49-65. [PubMed] |
| 18. | McLain RF. Mechanoreceptor endings in human cervical facet joints. Spine (Phila Pa 1976). 1994;19:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 150] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Giles LG, Harvey AR. Immunohistochemical demonstration of nociceptors in the capsule and synovial folds of human zygapophyseal joints. Br J Rheumatol. 1987;26:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Vandenabeele F, Creemers J, Lambrichts I, Robberechts W. Fine structure of vesiculated nerve profiles in the human lumbar facet joint. J Anat. 1995;187:681-692. [PubMed] |
| 21. | Yamashita T, Minaki Y, Ozaktay AC, Cavanaugh JM, King AI. A morphological study of the fibrous capsule of the human lumbar facet joint. Spine (Phila Pa 1976). 1996;21:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Taylor JR, McCormick CC. Lumbar facet joint fat pads: their normal anatomy and their appearance when enlarged. Neuroradiology. 1991;33:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Bogduk N. The innervation of the lumbar spine. Spine (Phila Pa 1976). 1983;8:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 305] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Ivancic PC, Ito S, Tominaga Y, Rubin W, Coe MP, Ndu AB, Carlson EJ, Panjabi MM. Whiplash causes increased laxity of cervical capsular ligament. Clin Biomech (Bristol, Avon). 2008;23:159-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Kallakuri S, Singh A, Lu Y, Chen C, Patwardhan A, Cavanaugh JM. Tensile stretching of cervical facet joint capsule and related axonal changes. Eur Spine J. 2008;17:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |