Revised: April 19, 2011
Accepted: June 1, 2011
Published online: August 18, 2011
Osteoporosis and age-related bone loss is associated with changes in bone remodeling characterized by decreased bone formation relative to bone resorption, resulting in bone fragility and increased risk of fractures. Stimulating the function of bone-forming osteoblasts, is the preferred pharmacological intervention for osteoporosis. Recombinant parathyroid hormone (PTH), PTH(1-34), is an anabolic agent with proven benefits to bone strength and has been characterized as a potential therapy for skeletal repair. In spite of PTH’s clinical use, safety is a major consideration for long-term treatment. Studies have demonstrated that intermittent PTH treatment enhances and accelerates the skeletal repair process via a number of mechanisms. Recent research into the molecular mechanism of PTH action on bone tissue has led to the development of PTH analogs to control osteoporotic fractures. This review summarizes a number of advances made in the field of PTH and bone fracture to combat these injuries in humans and in animal models. The ultimate goal of providing an alternative to PTH, currently the sole anabolic therapy in clinical use, to promote bone formation and improve bone strength in the aging population is yet to be achieved.
- Citation: Datta NS. Osteoporotic fracture and parathyroid hormone. World J Orthop 2011; 2(8): 67-74
- URL: https://www.wjgnet.com/2218-5836/full/v2/i8/67.htm
- DOI: https://dx.doi.org/10.5312/wjo.v2.i8.67
Osteoporosis is a progressive disorder of aging bone in both men and women, and osteoporotic fractures have become a major public health threat in recent years[1,2]. In spite of widespread research, The lack of reliable and effective drugs to cure osteoporosis related fragility fractures remains an important global issue. Long considered a disease of post-menopausal women, osteoporosis is increasingly being recognized among the growing population of elderly men. New treatments and updates are constantly being recognized for treating osteoporosis in women[3,4]. Although only thirty percent of hip fractures occur in men, the mortality rate during initial hospitalization and the first year after fractures twice as high in men as in women. Nevertheless, osteoporosis in men is under-diagnosed and undertreated, and is an increasingly important clinical issue[5,6]. Osteoporosis in men is a heterogeneous clinical entity. While most men experience bone loss with aging, some develop osteoporosis at a relatively young age, often for unexplained reasons (idiopathic osteoporosis). Declining sex steroid levels and other hormonal changes probably contribute to age-related bone loss, as do impairments in osteoblast number and/or activity[7]. Also, fragility fractures are common in men and are associated with a significant burden in terms of morbidity, mortality and economic cost to the community[7-9].
Intermittent treatment with teriparatide [recombinant human parathyroid hormone (hPTH-(1-34)], the only anabolic hormone, offers the potential to improve skeletal microarchitecture, and is a treatment modality for women with post-menopausal osteoporosis and men at high risk for fractures. Despite its clinical use, PTH has been reported to be associated with incidence of osteosarcoma, and safety is a major consideration for long-term use[10,11]. The molecular mechanisms underlying PTH’s action to evoke increased bone mass are not fully understood. Further elucidation is required using more controlled study designs, to develop an understanding the pathophysiology of bone loss, optimize patient care and to yield novel therapeutic strategies for potentiating bone anabolic agents.
“Osteo” means bone, and “porosis” means porous. Osteoporotic bones become more porous with less solid and less dense bone masses. Bone is an active tissue where new bone is being made continuously by osteoblasts, the bone forming cells, and old bone is removed by osteoclasts, the bone resorbing cells, via a process known as remodeling. In childhood, more bone is built than removed, and so the bones grow in both mass and size. In older age, osteoporosis results from increased bone resorption and decreased bone formation. The cells that build new bone do not keep up with those that remove bone. The total amount of bone mass then decreases, and osteoporosis may develop as a result. This condition finally makes bone thinner, weaker and more fragile, ultimately leading to loss of their structural and functional protein framework.
The human body also needs enough calcium, phosphorus and hormones, including estrogen in women and testosterone in men, to maintain healthy bone. Sufficient vitamin D is required to allow absorption of calcium from food, which is incorporated into bones to maintain their normal function. Osteoporosis exists in both primary or a secondary forms. Primary osteoporosis is the more common form and is due to the typical age-related loss of bone from skeleton. It is classified as type 1 or postmenopausal osteoporosis. Estrogen deficiency is thought to underlie this form of osteoporosis, rendering the skeleton more sensitive to PTH, and resulting in increased calcium resorption from bone. This in turn decreases PTH secretion, 1,25-dihydroxyvitamin D production, and calcium absorption. This ultimately causes loss of trabecular bone, leading to vertebral crush fractures and Colles’ fractures. Primary osteoporosis type 2 or senile osteoporosis occurs in women or men of more than 70 years of age and is usually associated with decreased bone formation along with decreased ability of the kidney to produce 1,25(OH)2D3. Type 3 or secondary osteoporosis results from the presence of other diseases or conditions that predispose to bone loss and occurs equally in men and women and at any age. This type of osteoporosis is associated with a variety of conditions, including hormonal imbalances (e.g. Cushing’s syndrome); cancer (notably multiple myeloma); gastrointestinal disorders (especially inflammatory bowel disease which causes mal-absorption); drug use [e.g. corticosteroids, cancer chemotherapy, anticonvulsants, heparin, barbiturates, valporic acid, gonadotropin-releasing hormone, excessive use of aluminum-containing antacids]; chronic renal failure; hyperthyroidism; hypogonadism in men; immobilization; osteogenesis imperfecta and related disorders; inflammatory arthritis (particularly rheumatoid arthritis); and poor nutrition (including malnutrition due to eating disorders)[12-14]. Thus, osteoporosis is classified as a systematic skeletal disease characterized by low bone strength and increased fracture risk[15]. In this disease spine, hip, wrist and other associated bone joints fracture very easily, leading to serious health problems.
Globally more than 30 million people are affected by osteoporosis with about 1.5-2 million osteoporotic fragility fractures happening in every year[16-18]. This includes more than 700 000 vertebral fractures and over 300 000 hip fractures[19]. The mortality rate following a hip fracture in osteoporotic patients is about 10%-20% within the first year, and less than 50% of survivors regain their pre-fracture level of mobility and independence[16]. Furthermore, mortality within 90 d of an osteoporotic fracture in individuals who are older than 65 years is substantially higher than might be expected, and for a subset of these fractures the risk for early lethality increases approximately sevenfold[20]. In the United States alone, osteoporotic fracture cost exceeds US $17 billion per year[21]. The first critical step in reducing the burden of osteoporotic fractures is to identify individuals at high risk of fracture by skeletal health evaluation and to then determine the appropriate pharmacological therapy, applying anabolic or anti-resorptive medication to reduce fracture risk[22,23]. Over the last decade, the prevention of osteoporotic fractures has been limited to the use of anti-resorptive[24] and anabolic drugs, which have proven to be insufficient for decreasing the mortality and morbidity in this patient population.
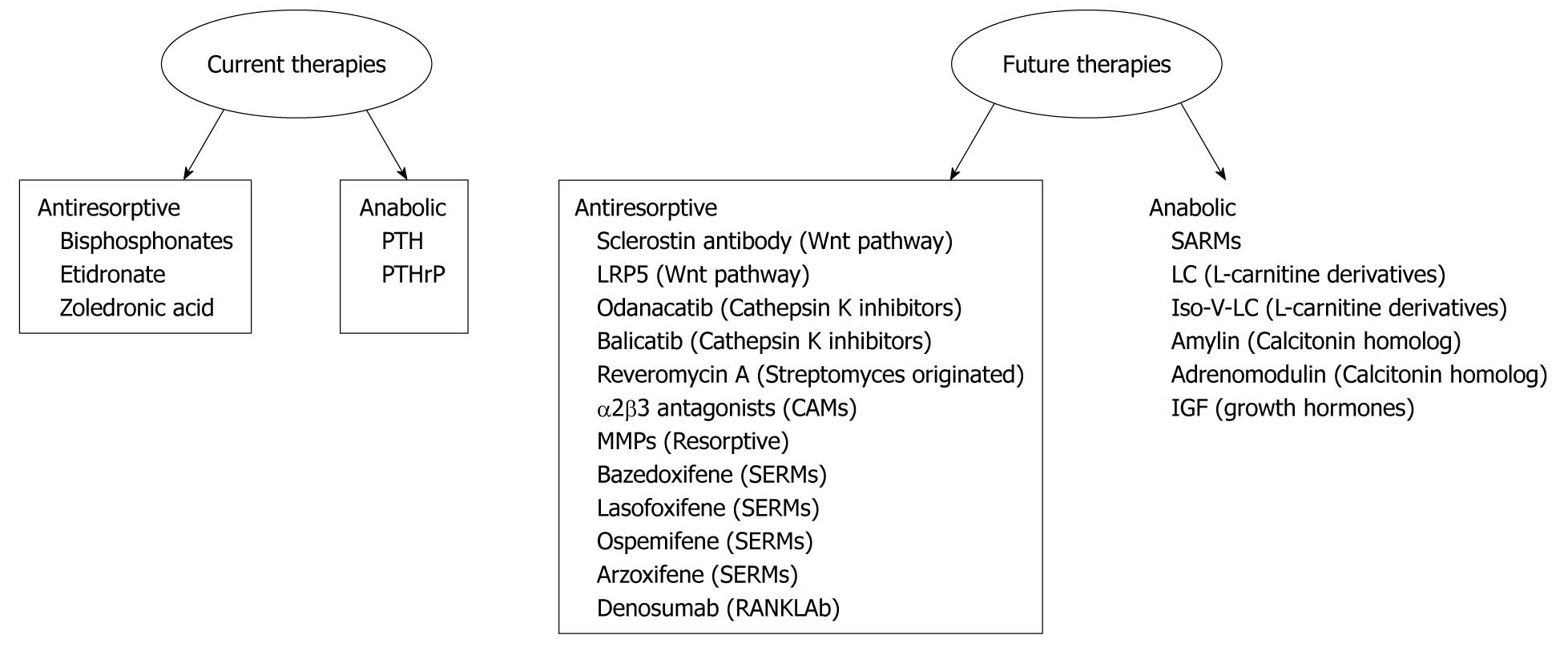
The fracture repairing process is biologically controlled and optimized. In approximately 5% to 10% of the 7.9 million fractures sustained annually it is difficult to achieve union[25]. Hence, there is a compelling need to find novel and effective therapies to enhance fracture repair process. Advances in the understanding of the molecular and cellular signaling pathways of bone biology have led to the development of current and emerging therapeutic agents which are summarized in Figure 1.
The known anabolic effects of PTH on bone formation has led to the development of a human recombinant peptide, teriparatide (1-34 hPTH), corresponding to the first 34 amino acids of PTH. Teriparatide is a drug currently approved for treating patients with osteoporosis who are at high risk for future fracture. Studies have confirmed a striking increase in trabecular bone mass and also showed that an important part of teriparatide’s action is to increase cortical bone. A formal trial in postmenopausal women with osteoporosis was conducted by Eli Lilly and Company in the United States. The unexpected occurrence of osteosarcomas in Fisher 344 rats treated long-term with teriparatide provoked an abrupt cessation of this trial. However, ambiguity concerning the relevance of this rat finding to human disease, combined with significant anti-fracture efficacy, led to FDA approval of teriparatide for men and postmenopausal women with osteoporosis “at high risk for fracture” in 2002. Subsequently, teriparatide has been approved also for treatment of patients with glucocorticoid-associated osteoporosis, and papers indicating the utility of this agent for dental and orthopedic applications have begun to appear[26]. In the treatment of osteoporosis, teriparatide works as an anabolic agent stimulating bone formation throughout the skeleton, principally by enhancing osteoblast-derived bone formation relative to osteoclast-derived bone resorption, resulting in a net increase in bone mass. For patients with a fracture, a similar process of increased bone formation is required transiently at the fracture site for repair. Teriparatide has been investigated in animal models and in patients as a potential agent to enhance fracture repair. Interestingly, in conditions with impaired healing such as aging, estrogen withdrawal, and malnutrition fracture repair is expedited by PTH treatment. Subcutaneous injection of PTH once per day led to increased bone mass in patients with osteoporosis[27] and in ovariectomized monkeys[28]. The capability of PTH to augment bone formation is dependent upon the hormone being administered in a way that yields a transient peak blood level[29,30].
It was initially noted that PTH could increase bone mass in rats[31,32]. Using various animal models, several groups have shown that intermittent exposure to PTH stimulates osteoblast differentiation and function in vivo[33,34]. Evidence that teriparatide enhances chondrogenesis has generated interest in using the agent for articular cartilage repair. Bukata et al[35] and Aleksyniene et al[36] found that treatment with PTH during distraction osteogenesis resulted in substantially higher mineralized tissue volume, mineral content, and bending strength. This suggests that treatment with PTH may benefit new bone formation during distraction osteogenesis and could form the basis for clinical application of this therapy in humans.
Knowledge of the effects of intermittent PTH treatment on newly regenerating bone after distraction osteogenesis is very limited. Seebach et al[37] reported enhanced mechanical strength and density of new bone after distraction osteogenesis in rats. However, no information is available on the effects of intermittent PTH treatment on distraction osteogenesis in larger animals. Recent experiments with rats have demonstrated that treatment with PTH increases mechanical strength and callus formation in normal healing fractures[38-42]. Furthermore, an increased density of regenerated bone and enhanced fixation of steel implants in rats have been shown after PTH treatment[43,44].
In a recent investigation, to evaluate the potential use as a therapeutic agent for osteoporotic fractures, Kim and Jahng examined the effects of intermittent administration of PTH on fracture healing in ovariectomized rats[45]. At 3 mo post-ovariectomy, bilateral tibial shaft fractures were induced and stabilized by intramedullary nailing with Kirschner wires. Saline, 17-estradiol, or recombinant human PTH(1-84) was given once a day for 30 consecutive days during fracture healing. Fracture healing was assessed by morphometric and mechanical analysis of fracture callus. Intermittent PTH administration increased the morphometric and mechanical parameters in a dose-dependent manner. 17-estradiol, a bone-resorption inhibiting agent, showed no benefits in terms of fracture healing in ovariectomized rats. Verhaar et al[46] reported that exogenous PTH analogs, given as daily subcutaneous injections, stimulate bone formation, increase bone mass and bone strength, and improve calcium balance.
Traditionally PTH was thought to be catabolic to the human skeleton as severe osteoporosis and osteitis fibrosacystica may complicate long standing hyperparathyroidism. In 1932 Selye reported the ability of PTH to stimulate osteogenesis[32]. Subsequently, the anabolic effects of PTH have been examined in greater detail[30,47-52]. PTH was initially developed as a drug to treat postmenopausal osteoporotic women, enhancing bone mineral density[29], cortical thickness and trabecular bone volume[53,54] compared to placebo controls. In addition to its anabolic effects on bone turnover[29,47,55] teriparatide was shown in clinical trials to significantly reduce the risk of vertebral and non-vertebral fractures in osteoporotic women[29,56]. In randomized clinical trials PTH was shown to be useful in preventing fracture in osteoporotic subjects[50,57-59]. It is now well accepted that intermittent administration of PTH and PTH-related peptide (PTHrP) has net anabolic effects on bone[47,52,60,61].
Although it is now well established that PTH is a multifunctional molecule with a unique ability to affect the bone metabolism, the biological complexity of bone repair often makes it difficult to specify what events have failed during the repair process. Currently, several PTH analogs are being developed and are under evaluation. In general, PTH analogs are well tolerated and have an acceptable safety profile. They can be used for the prevention and treatment of fractures in postmenopausal women with severe osteoporosis. Thus PTH analogs reduce the risk of vertebral (PTH 1-34 and PTH 1-84) and non-vertebral fractures (only PTH 1-34). In men and women with glucocorticosteroid-induced osteoporosis, PTH 1-34 has been shown to reduce the risk of vertebral fractures[46]. In recent years β-arrestin-based agonists of PTH-1 receptor (PTH1R) have drawn much attention for promoting bone formation independent of G-protein activation[62,63].
It is well established that PTH, secreted from the parathyroid glands, is involved in calcium homeostasis and is a critical mediator of skeletal development and remodeling[64]. There are several reports of the beneficial use of PTH for treating osteoporosis. However, prolonged use of PTH leads to hypercalciurea, hypercalcemia and osteosarcoma[65], thus limiting the safe use of this peptide hormone. Therefore dissecting the molecular mechanisms of PTH actions is essential as this may uncover novel therapeutic targets for the prevention and reversal of osteoporosis and bone-related diseases and allow minimization of the adverse effects of PTH.
Although a large number of in-vitro, in-vivo and human studies have been performed, the mechanisms involved in PTH regulation of osteoblast function is poorly understood and only partly characterized. PTH binds to cells of the osteoblast lineage[66,67] and produces both anabolic and catabolic effects (Figure 2). The fact that PTH has dual effects depending on its administration method raises important questions about its mechanisms of action in bone formation and resorption. It was hypothesized that the anabolic and catabolic effects of PTH and PTHrP on osteoblasts occur through activation of signaling cascades different from PTH1R[64]. The PTH and PTHrP signal via PTH1R which is a G protein-coupled receptor with 7 transmembrane spanning domains. The receptor is encoded by a multi-exon gene, characterized in human, rat and mouse, with potential for alternate splicing and alternate promoter usage[68]. Understanding the physiological roles, molecular and cellular actions of PTH and PTHrP began when PTH1R was first cloned in 1990s[69,70]. PTH1R signaling cascades involve adenylate cyclase/protein kinase A, phospholipase C/protein kinase C, and mitogen activated protein kinases, and lead to various biological effects including both anabolic and catabolic actions in bone[71-75]. Recently, Guo et al[76] further established that phospholipase C signaling via the PTH receptor is essential for normal bone response to PTH. Other studies suggested that FGF2 is equally important for the anabolic action of PTH on bone[77] and a crosstalk between skeletogenesis and FGF receptor was recently highlighted[78]. Among other mechanisms, PTH anabolic action with or without the involvement of Bcl2 has been described[79,80].
Increased bone formation is largely due to a rise in osteoblast number as a result of increased proliferation and differentiation of osteoblasts in vitro and in vivo[52,73,74,81-87], decrease in osteoblast apoptosis[88,89], and activation of bone lining cells[48,90]. A mechanism involving cell-cell contact in PTH-induced osteoblast proliferation has also been suggested[91]. Numerous targets of PTH and PTHrP as mediators of bone tissue regeneration have been suggested[71]. These include local cytokines and growth factors[92,93], transcription factors[94-96], and several genes such as MMP-13, a matrix metalloproteinase/collagenase[97,98], IL-6[99], IL-18[100], macrophage-colony stimulating factor[101], ephrinB2[102], and osteoblast cell cycle regulatory proteins[72,74,103,104].
Intermittent PTH 1-34 treatment stimulates bone formation, but the molecular mechanisms mediating this effect have not previously been studied in humans. A very recent study hypothesized that an inhibition of BMP signaling by PTH may, over time, limit the availability of mature osteoblasts on bone surfaces and thereby contribute to the observed decline in the anabolic response to PTH[105]. Several critical steps in the actions of PTH beyond receptor activation have been identified and more are yet to be discovered.
Fractures usually repair without incident. When fractures associated with osteoporotic bones do not repair in a timely fashion, the result is painful and detrimental to the patient’s quality of life. Treatment, in such cases, is time-consuming and expensive for the health care system 106. Bone repair after orthopedic fracture is a complicated process. PTH is the first bone anabolic drug approved for the treatment of osteoporosis and associated fractures. Intriguingly, a number of animal studies suggest that PTH could be beneficial in the treatment of fractures and could potentially offer a new treatment option for induction of fracture repair in humans. Furthermore, repair of fractures associated with conditions of impaired healing such as aging, estrogen withdrawal, and malnutrition can be expedited by PTH treatment. Although recent advances in molecular bone research using a variety of in vivo and in vitro models have increased our understanding of the role of PTH in osteoporotic the fracture repair process, pharmacological intervention using PTH cannot at present be considered a “gold standard”. Many future therapies are currently under investigation for the management of fractures associated to osteoporosis.
The author was partially supported by the grants from The National Institute of Health, DK087848, during writing of this manuscript.
Peer reviewer: Prachya Kongtawelert, Associate Professor, Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Kook Jin Chung, Professor, Department of Orthopaedic Surgery, HallymUniversity, Kangnam Sacred Heart Hospital, 948-1, Daerim 1-dong, Yeongdeungpo-gu, Seoul 150-950, South Korea; Kemal Nas, Professor, Department of Physical Medicine and Rehabilitation, University of Dicle, Medical School, Diyarbakir 21280,Turkey
S- Editor Lv S L- Editor Hughes D E- Editor Zheng XM
| 1. | Department of Health and Human Services OotSG. Bone health and Osteoporosis. Bone health and osteoporosis: a report of the surgeon general. Rockville: USDHHS 2004; 2004. |
| 2. | Cooper C, Johnell O, Lips P, Melton LJ, Kanis JA. The global burden of vertebral fractures (abstract). J Bone Miner Res. 2002;17:S202. |
| 3. | Vestergaard P, Thomsen SV. Treating postmenopausal osteoporosis in women at increased risk of fracture - critical appraisal of bazedoxifene: a review. Int J Womens Health. 2010;1:97-103. [PubMed] |
| 4. | Lindsay R. Preventing osteoporosis with a tissue selective estrogen complex (TSEC) containing bazedoxifene/conjugated estrogens (BZA/CE). Osteoporos Int. 2011;22:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Wright VJ. Osteoporosis in men. J Am Acad Orthop Surg. 2006;14:347-353. [PubMed] |
| 6. | Nuti R, Merlotti D, Francucci CM, Gennari L. Bone fragility in men: where are we? J Endocrinol Invest. 2010;33:33-38. [PubMed] |
| 7. | Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Boonen S, Kaufman JM, Goemaere S, Bouillon R, Vanderschueren D. The diagnosis and treatment of male osteoporosis: Defining, assessing, and preventing skeletal fragility in men. Eur J Intern Med. 2007;18:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Khosla S, Westendorf JJ, Oursler MJ. Building bone to reverse osteoporosis and repair fractures. J Clin Invest. 2008;118:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Sikon A, Batur P. Profile of teriparatide in the management of postmenopausal osteoporosis. Int J Womens Health. 2010;2:37-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Pietrogrande L. Update on the efficacy, safety, and adherence to treatment of full length parathyroid hormone, PTH (1-84), in the treatment of postmenopausal osteoporosis. Int J Womens Health. 2010;1:193-203. [PubMed] |
| 12. | Alderman CP, Hill CL. Abnormal bone mineral metabolism after long-term anticonvulsant treatment. Ann Pharmacother. 1994;28:47-48. [PubMed] |
| 13. | Praet JP, Peretz A, Rozenberg S, Famaey JP, Bourdoux P. Risk of osteoporosis in men with chronic bronchitis. Osteoporos Int. 1992;2:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Feber J, Cochat P, Braillon P, Castelo F, Martin X, Glastre C, Chapuis F, David L, Meunier PJ. Bone mineral density after renal transplantation in children. J Pediatr. 1994;125:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3072] [Cited by in RCA: 3073] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 16. | Finkelstein JS. Osteoporosis. Cecil textbook of medicine. 21st ed. Philadelphia, PA: WB Saunders Co 2000; 1367-1373. |
| 17. | Gardner MJ, Demetrakopoulos D, Shindle MK, Griffith MH, Lane JM. Osteoporosis and skeletal fractures. HSS J. 2006;2:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Kaback LA, Soung do Y, Naik A, Geneau G, Schwarz EM, Rosier RN, O'Keefe RJ, Drissi H. Teriparatide (1-34 human PTH) regulation of osterix during fracture repair. J Cell Biochem. 2008;105:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Riggs BL, Melton LJ. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17:505S-511S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 866] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 20. | Heaney RP. Advances in therapy for osteoporosis. Clin Med Res. 2003;1:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2687] [Cited by in RCA: 2922] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 22. | van den Bergh JP, van Geel TA, Lems WF, Geusens PP. Assessment of individual fracture risk: FRAX and beyond. Curr Osteoporos Rep. 2010;8:131-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Lewiecki EM. Fracture risk assessment in clinical practice: T-scores, FRAX, and beyond. Clin Rev Bone Miner Metab. 2010;8:101-112[doi: 10.1007/s12018-009-9054-6 ]. |
| 24. | Cranney A, Tugwell P, Zytaruk N, Robinson V, Weaver B, Adachi J, Wells G, Shea B, Guyatt G. Meta-analyses of therapies for postmenopausal osteoporosis. IV. Meta-analysis of raloxifene for the prevention and treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23:524-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Marsell R, Einhorn TA. Emerging bone healing therapies. J Orthop Trauma. 2010;24 Suppl 1:S4-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Marcus R. Present at the beginning: a personal reminiscence on the history of teriparatide. Osteoporos Int. 2011;22:2241-2248. [PubMed] |
| 27. | Sone T, Fukunaga M, Ono S, Nishiyama T. A small dose of human parathyroid hormone(1-34) increased bone mass in the lumbar vertebrae in patients with senile osteoporosis. Miner Electrolyte Metab. 1995;21:232-235. [PubMed] |
| 28. | Brommage R, Hotchkiss CE, Lees CJ, Stancill MW, Hock JM, Jerome CP. Daily treatment with human recombinant parathyroid hormone-(1-34), LY333334, for 1 year increases bone mass in ovariectomized monkeys. J Clin Endocrinol Metab. 1999;84:3757-3763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3315] [Cited by in RCA: 3043] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 30. | Frolik CA, Black EC, Cain RL, Satterwhite JH, Brown-Augsburger PL, Sato M, Hock JM. Anabolic and catabolic bone effects of human parathyroid hormone (1-34) are predicted by duration of hormone exposure. Bone. 2003;33:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Bauer W, Aub JC, Albright F. STUDIES OF CALCIUM AND PHOSPHORUS METABOLISM : V. A STUDY OF THE BONE TRABECULAE AS A READILY AVAILABLE RESERVE SUPPLY OF CALCIUM. J Exp Med. 1929;49:145-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 32. | Selye H. On the stimulation of new bone formation with parathyroid extract and irradiated ergosterol. Endocrinology. 1932;16:547[doi: 10.1210/endo-16-5-547]. |
| 33. | Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res. 1992;7:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 256] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Kulkarni NH, Wei T, Kumar A, Dow ER, Stewart TR, Shou J, N'cho M, Sterchi DL, Gitter BD, Higgs RE. Changes in osteoblast, chondrocyte, and adipocyte lineages mediate the bone anabolic actions of PTH and small molecule GSK-3 inhibitor. J Cell Biochem. 2007;102:1504-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Bukata SV, Puzas JE. Orthopedic uses of teriparatide. Curr Osteoporos Rep. 2010;8:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Aleksyniene R, Thomsen JS, Eckardt H, Bundgaard KG, Lind M, Hvid I. Parathyroid hormone PTH(1-34) increases the volume, mineral content, and mechanical properties of regenerated mineralizing tissue after distraction osteogenesis in rabbits. Acta Orthop. 2009;80:716-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Seebach C, Skripitz R, Andreassen TT, Aspenberg P. Intermittent parathyroid hormone (1-34) enhances mechanical strength and density of new bone after distraction osteogenesis in rats. J Orthop Res. 2004;22:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Holzer G, Majeska RJ, Lundy MW, Hartke JR, Einhorn TA. Parathyroid hormone enhances fracture healing. A preliminary report. Clin Orthop Relat Res. 1999;258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999;14:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 252] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 40. | Andreassen TT, Fledelius C, Ejersted C, Oxlund H. Increases in callus formation and mechanical strength of healing fractures in old rats treated with parathyroid hormone. Acta Orthop Scand. 2001;72:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Alkhiary YM, Gerstenfeld LC, Krall E, Westmore M, Sato M, Mitlak BH, Einhorn TA. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). J Bone Joint Surg Am. 2005;87:731-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Barnes GL, Kakar S, Vora S, Morgan EF, Gerstenfeld LC, Einhorn TA. Stimulation of fracture-healing with systemic intermittent parathyroid hormone treatment. J Bone Joint Surg Am. 2008;90 Suppl 1:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Skripitz R, Andreassen TT, Aspenberg P. Strong effect of PTH (1-34) on regenerating bone: a time sequence study in rats. Acta Orthop Scand. 2000;71:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Skripitz R, Aspenberg P. Implant fixation enhanced by intermittent treatment with parathyroid hormone. J Bone Joint Surg Br. 2001;83:437-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Kim HW, Jahng JS. Effect of intermittent administration of parathyroid hormone on fracture healing in ovariectomized rats. Iowa Orthop J. 1999;19:71-77. [PubMed] |
| 46. | Verhaar HJ, Lems WF. PTH analogues and osteoporotic fractures. Expert Opin Biol Ther. 2010;10:1387-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetić K, Müller R, Bilezikian J. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 418] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 48. | Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632-3638. [PubMed] [DOI] [Full Text] |
| 49. | Cosman F, Nieves J, Woelfert L, Formica C, Gordon S, Shen V, Lindsay R. Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res. 2001;16:925-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Cosman F, Lindsay R. Therapeutic potential of parathyroid hormone. Curr Osteoporos Rep. 2004;2:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Frolik CA, Cain RL, Sato M, Harvey AK, Chandrasekhar S, Black EC, Tashjian AH, Hock JM. Comparison of recombinant human PTH(1-34) (LY333334) with a C-terminally substituted analog of human PTH-related protein(1-34) (RS-66271): In vitro activity and in vivo pharmacological effects in rats. J Bone Miner Res. 1999;14:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;22:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 53. | Jiang Y, Zhao J, Liao EY, Dai RC, Wu XP, Genant HK. Application of micro-CT assessment of 3-D bone microstructure in preclinical and clinical studies. J Bone Miner Metab. 2005;23 Suppl:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85:3069-3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1325] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 56. | Bauer DC, Garnero P, Bilezikian JP, Greenspan SL, Ensrud KE, Rosen CJ, Palermo L, Black DM. Short-term changes in bone turnover markers and bone mineral density response to parathyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2006;91:1370-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Cosman F, Lindsay R. Is parathyroid hormone a therapeutic option for osteoporosis? A review of the clinical evidence. Calcif Tissue Int. 1998;62:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Cosman F, Nieves J, Woelfert L, Gordon S, Shen V, Lindsay R. Parathyroid responsivity in postmenopausal women with osteoporosis during treatment with parathyroid hormone. J Clin Endocrinol Metab. 1998;83:788-790. [PubMed] [DOI] [Full Text] |
| 59. | Cosman F, Nieves J, Woelfert L, Shen V, Lindsay R. Alendronate does not block the anabolic effect of PTH in postmenopausal osteoporotic women. J Bone Miner Res. 1998;13:1051-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Misof BM, Roschger P, Cosman F, Kurland ES, Tesch W, Messmer P, Dempster DW, Nieves J, Shane E, Fratzl P. Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab. 2003;88:1150-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 61. | Zhou H, Iida-Klein A, Lu SS, Ducayen-Knowles M, Levine LR, Dempster DW, Lindsay R. Anabolic action of parathyroid hormone on cortical and cancellous bone differs between axial and appendicular skeletal sites in mice. Bone. 2003;32:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, Luttrell LM. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med. 2009;1:1ra1. [PubMed] |
| 63. | Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62:305-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 64. | Hock JM, Fitzpatrick LA, Bilezikian JP. Actions of Parathyroid Hormone. Principles of Bone Biology. 2nd ed. San Diego: Academic Press. In: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of Bone Biology. 2nd ed. San Diego: Academic Press; 2002; 463-482. |
| 65. | Alves de Oliveira EC, Szejnfeld VL, Pereira da Silva N, Coelho Andrade LE, Heldan de Moura Castro C. Intermittent PTH1-34 causes DNA and chromosome breaks in osteoblastic and nonosteoblastic cells. Calcif Tissue Int. 2010;87:424-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Rouleau MF, Mitchell J, Goltzman D. Characterization of the major parathyroid hormone target cell in the endosteal metaphysis of rat long bones. J Bone Miner Res. 1990;5:1043-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Rouleau MF, Warshawsky H, Goltzman D. Parathyroid hormone binding in vivo to renal, hepatic, and skeletal tissues of the rat using a radioautographic approach. Endocrinology. 1986;118:919-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Kong XF, Schipani E, Lanske B, Joun H, Karperien M, Defize LH, Jüppner H, Potts JT, Segre GV, Kronenberg HM. The rat, mouse, and human genes encoding the receptor for parathyroid hormone and parathyroid hormone-related peptide are highly homologous. Biochem Biophys Res Commun. 1994;201:1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 69. | Jüppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF, Hock J, Potts JT, Kronenberg HM. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 832] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 70. | Abou-Samra AB, Jüppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci U S A. 1992;89:2732-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 657] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 71. | Datta NS, Abou-Samra AB. PTH and PTHrP signaling in osteoblasts. Cell Signal. 2009;21:1245-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 220] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 72. | Datta NS, Chen C, Berry JE, McCauley LK. PTHrP signaling targets cyclin D1 and induces osteoblastic cell growth arrest. J Bone Miner Res. 2005;20:1051-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Datta NS, Kolailat R, Fite A, Pettway G, Abou-Samra AB. Distinct roles for mitogen-activated protein kinase phosphatase-1 (MKP-1) and ERK-MAPK in PTH1R signaling during osteoblast proliferation and differentiation. Cell Signal. 2010;22:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Datta NS, Pettway GJ, Chen C, Koh AJ, McCauley LK. Cyclin D1 as a target for the proliferative effects of PTH and PTHrP in early osteoblastic cells. J Bone Miner Res. 2007;22:951-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Datta NS, Samra TA, Mahalingam CD, Datta T, Abou-Samra AB. Role of PTH1R internalization in osteoblasts and bone mass using a phosphorylation-deficient knock-in mouse model. J Endocrinol. 2010;207:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Guo J, Liu M, Yang D, Bouxsein ML, Thomas CC, Schipani E, Bringhurst FR, Kronenberg HM. Phospholipase C signaling via the parathyroid hormone (PTH)/PTH-related peptide receptor is essential for normal bone responses to PTH. Endocrinology. 2010;151:3502-3513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Sabbieti MG, Agas D, Xiao L, Marchetti L, Coffin JD, Doetschman T, Hurley MM. Endogenous FGF-2 is critically important in PTH anabolic effects on bone. J Cell Physiol. 2009;219:143-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Miraoui H, Marie PJ. Fibroblast growth factor receptor signaling crosstalk in skeletogenesis. Sci Signal. 2010;3:re9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 79. | Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 504] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 80. | Yamashita J, Datta NS, Chun YH, Yang DY, Carey AA, Kreider JM, Goldstein SA, McCauley LK. Role of Bcl2 in osteoclastogenesis and PTH anabolic actions in bone. J Bone Miner Res. 2008;23:621-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | Herrmann-Erlee MP, Heersche JN, Hekkelman JW, Gaillard PJ, Tregear GW, Parsons JA, Potts JT. Effects of bone in vitro of bovine parathyroid hormone and synthetic fragments representing residues 1-34, 2-34 and 3-34. Endocr Res Commun. 1976;3:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | DeBartolo TF, Pegg LE, Shasserre C, Hahn TJ. Comparison of parathyroid hormone and calcium ionophore A23187 effects on bone resorption and nucleic acid synthesis in cultured fetal rat bone. Calcif Tissue Int. 1982;34:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 83. | MacDonald BR, Gallagher JA, Russell RG. Parathyroid hormone stimulates the proliferation of cells derived from human bone. Endocrinology. 1986;118:2445-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 84. | Canalis E, Centrella M, Burch W, McCarthy TL. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest. 1989;83:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 340] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 85. | Nishida S, Yamaguchi A, Tanizawa T, Endo N, Mashiba T, Uchiyama Y, Suda T, Yoshiki S, Takahashi HE. Increased bone formation by intermittent parathyroid hormone administration is due to the stimulation of proliferation and differentiation of osteoprogenitor cells in bone marrow. Bone. 1994;15:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 169] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | Partridge NC, Li X, Qin L. Understanding parathyroid hormone action. Ann N Y Acad Sci. 2006;1068:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2008;42:806-818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 88. | Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 748] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 89. | Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 460] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 90. | Leaffer D, Sweeney M, Kellerman LA, Avnur Z, Krstenansky JL, Vickery BH, Caulfield JP. Modulation of osteogenic cell ultrastructure by RS-23581, an analog of human parathyroid hormone (PTH)-related peptide-(1-34), and bovine PTH-(1-34). Endocrinology. 1995;136:3624-3631. [PubMed] [DOI] [Full Text] |
| 91. | van der Plas A, Nijweide PJ. Cell-cell interactions in the osteogenic compartment of bone. Bone. 1988;9:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17:1570-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 178] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 93. | Sowa H, Kaji H, Iu MF, Tsukamoto T, Sugimoto T, Chihara K. Parathyroid hormone-Smad3 axis exerts anti-apoptotic action and augments anabolic action of transforming growth factor beta in osteoblasts. J Biol Chem. 2003;278:52240-52252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | Tyson DR, Swarthout JT, Jefcoat SC, Partridge NC. PTH induction of transcriptional activity of the cAMP response element-binding protein requires the serine 129 site and glycogen synthase kinase-3 activity, but not casein kinase II sites. Endocrinology. 2002;143:674-682. [PubMed] [DOI] [Full Text] |
| 95. | McCauley LK, Koh-Paige AJ, Chen H, Chen C, Ontiveros C, Irwin R, McCabe LR. Parathyroid hormone stimulates fra-2 expression in osteoblastic cells in vitro and in vivo. Endocrinology. 2001;142:1975-1981. [PubMed] [DOI] [Full Text] |
| 96. | Krishnan V, Moore TL, Ma YL, Helvering LM, Frolik CA, Valasek KM, Ducy P, Geiser AG. Parathyroid hormone bone anabolic action requires Cbfa1/Runx2-dependent signaling. Mol Endocrinol. 2003;17:423-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 97. | Scott DK, Brakenhoff KD, Clohisy JC, Quinn CO, Partridge NC. Parathyroid hormone induces transcription of collagenase in rat osteoblastic cells by a mechanism using cyclic adenosine 3',5'-monophosphate and requiring protein synthesis. Mol Endocrinol. 1992;6:2153-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Chiusaroli R, Maier A, Knight MC, Byrne M, Calvi LM, Baron R, Krane SM, Schipani E. Collagenase cleavage of type I collagen is essential for both basal and parathyroid hormone (PTH)/PTH-related peptide receptor-induced osteoclast activation and has differential effects on discrete bone compartments. Endocrinology. 2003;144:4106-4116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Chen C, Koh AJ, Datta NS, Zhang J, Keller ET, Xiao G, Franceschi RT, D'Silva NJ, McCauley LK. Impact of the mitogen-activated protein kinase pathway on parathyroid hormone-related protein actions in osteoblasts. J Biol Chem. 2004;279:29121-29129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 100. | Raggatt LJ, Qin L, Tamasi J, Jefcoat SC, Shimizu E, Selvamurugan N, Liew FY, Bevelock L, Feyen JH, Partridge NC. Interleukin-18 is regulated by parathyroid hormone and is required for its bone anabolic actions. J Biol Chem. 2008;283:6790-6798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | Weir EC, Lowik CW, Paliwal I, Insogna KL. Colony stimulating factor-1 plays a role in osteoclast formation and function in bone resorption induced by parathyroid hormone and parathyroid hormone-related protein. J Bone Miner Res. 1996;11:1474-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Allan EH, Häusler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B, Pompolo S, Sims NA, Gillespie MT, Onyia JE. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008;23:1170-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 103. | Onishi T, Zhang W, Cao X, Hruska K. The mitogenic effect of parathyroid hormone is associated with E2F-dependent activation of cyclin-dependent kinase 1 (cdc2) in osteoblast precursors. J Bone Miner Res. 1997;12:1596-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 104. | Onyia JE, Miller B, Hulman J, Liang J, Galvin R, Frolik C, Chandrasekhar S, Harvey AK, Bidwell J, Herring J. Proliferating cells in the primary spongiosa express osteoblastic phenotype in vitro. Bone. 1997;20:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Drake MT, Srinivasan B, Mödder UI, Ng AC, Undale AH, Roforth MM, Peterson JM, McCready LK, Riggs BL, Khosla S. Effects of intermittent parathyroid hormone treatment on osteoprogenitor cells in postmenopausal women. Bone. 2011;49:349-355. [PubMed] |
| 106. | Kanakaris NK, Giannoudis PV. The health economics of the treatment of long-bone non-unions. Injury. 2007;38 Suppl 2:S77-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 107. | Honig S. Osteoporosis - new treatments and updates. Bull NYU Hosp Jt Dis. 2010;68:166-170. [PubMed] |
| 108. | Silva BC, Bilezikian JP. New approaches to the treatment of osteoporosis. Annu Rev Med. 2011;62:307-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Salari Sharif P, Abdollahi M, Larijani B. Current, new and future treatments of osteoporosis. Rheumatol Int. 2011;31:289-300. [PubMed] |