INTRODUCTION
Distal radius fractures (DRFs) are common fractures in children, peaking during the metaphyseal growth spurt typically between ages 10-14[1,2]. These injuries, more prevalent in boys, usually result from a fall on an outstretched hand, leading to rotational and axial loading forces[3]. Pediatric bone differs significantly from adult bone, with a thick periosteum, unique remodeling capacity, and a physis that contributes substantially to longitudinal growth[4].
Pediatric DRFs are anatomically classified as diaphyseal, metaphyseal, or physeal, with metaphyseal injuries being the most common[5]. Physeal fractures are conventionally categorized by the Salter-Harris classification, a system that describes the involvement of the growth plate, metaphysis, and epiphysis. In Type I injuries, the fracture line passes entirely through the physis. Type II fractures extend through physis and into metaphysis, and often display the characteristic metaphyseal fragment known as the Thurston-Holland fragment. Type III fractures extend from physis to epiphysis. Type IV injuries traverse metaphysis, physis, and epiphysis. Type V fractures represent a crush or compression injury to the growth plate and are often diagnosed retrospectively with development of premature physeal closure or growth arrest[6,7].
Given the wide spectrum of fracture patterns and the substantial remodeling capacity of the pediatric distal radius, the indications for operative intervention and the role of routine FU imaging remain subjects of ongoing clinical debate. This editorial seeks to re-examine the necessity and value of routine postoperative radiographs following treatment of pediatric DRFs, considering evolving evidence, radiographic predictors, and the growing emphasis on cost-effective, individualized care.
TREATMENT OPTIONS
Nondisplaced pediatric DRFs are routinely managed with cast immobilization, reflecting the natural stability and excellent remodeling potential of these injuries[8]. However, the management of displaced ones remains a subject of clinical debate, with treatment strategies tailored according to the degree of displacement, fracture configuration, and patient-specific risk factors. Options include non-reduction immobilization, reduction immobilization, and surgical fixation, each with distinct indications and thresholds[9].
Immobilization without reduction is typically reserved for torus/buckle fractures, uni-cortical injuries, or bi-cortical fractures with minimal angulation of less than 10 degrees[10]. When angulation exceeds 10-20 degrees, or when alignment is deemed unacceptable-particularly in Salter-Harris type I and II fractures-closed reduction (CR) under conscious sedation followed by casting is usually recommended[11]. In cases of instability, failed initial reduction, or documented loss of alignment on follow-up (FU) radiographs, percutaneous pinning with K-wires becomes necessary. K-wire fixation is also indicated in the presence of significant edema or early signs of compartment syndrome, where tight casting may pose a risk of neurovascular compromise[12]. Open reduction with internal fixation is reserved for irreducible fractures including most displaced Salter-Harris types III and IV and those obstructed by soft-tissue interposition, such as entrapment of the periosteum or pronator quadratus[13].
RADIOGRAPHIC CRITERIA FOR ACCEPTABLE REDUCTION
The assessment of reduction adequacy in displaced pediatric DRFs must account for multiple factors, including the patient’s age, fracture classification, and the presence of an associated ulnar fracture. In fractures involving distal diaphysis, consideration of the ulna is particularly important, as the radius and ulna function as a single rotational unit. Rotational malalignment, especially in this region, is of particular concern because it does not reliably remodel and may lead to long-term functional impairment. A rotational deformity exceeding 20 degrees in the diaphysis can result in a clinically significant restriction of forearm rotation[14,15].
Traditionally, age-based thresholds have been used to determine acceptable angulation following DRF reduction, with up to 30° considered tolerable in children under 10 years of age and up to 20° in those over 10[16]. However, the scientific basis for these thresholds remains limited. Emerging evidence suggests that even completely displaced metaphyseal fractures in children younger than 10 years can be remodeled successfully without reduction, particularly when managed with simple splint immobilization, and still achieve excellent long-term function[17]. Beyond angulation alone, the quality of reduction can be graded more precisely using the classification proposed by Alemdaroğlu et al[18], which categorizes outcomes as anatomic, good, or fair. An anatomic reduction denotes complete alignment with no residual translation or angulation. A good reduction permits less than 10 degrees dorsal angulation or up to 2 mm of translation. A fair reduction involves either 10-20 degrees of angulation, 2-5 mm of translation, radial deviation under 5 degrees, or a combined deformity with 5-10 degrees of angulation and ≤ 2 mm of translation. This structured classification facilitates objective radiographic evaluation and provides useful thresholds for anticipating the likelihood of fracture stability during FU.
In this evolving context, several radiographic parameters have been developed to objectively evaluate the quality of CR and cast immobilization. Among these, the cast index remains the most widely recognized and clinically applicable. It is calculated by dividing the internal cast width on the lateral view by that on the anteroposterior (AP) view, with a value below 0.8 considered optimal[19]. The padding index similarly evaluates cast adequacy by comparing dorsal soft-tissue space on the lateral view to the maximum interosseous distance on the AP view; a value below 0.3 is associated with greater stability[20]. The Canterbury index, a simple sum of the cast and padding indices, offers a composite assessment, with values below 1.1 suggesting effective molding[21].
Additional indices provide further insights into cast quality and fracture stability. The gap index evaluates fracture site contact by measuring the sum of radial and ulnar gaps on the AP view, along with dorsal and volar gaps on the lateral view, each normalized by the inner cast width in the respective planes. A combined gap index value of less than 0.15 is considered acceptable and has been associated with a lower risk of fracture re-displacement[19]. The three-point index assesses cast contact at three key points along the fracture zone: Proximal, central, and distal on the AP view, and dorsal and volar aspects on the lateral view. A value below 0.8 suggests adequate three-point molding[22]. Lastly, the second metacarpal–radius index estimates the degree of wrist deviation applied during casting by measuring the angle between the longitudinal axes of the radius and the second metacarpal on the AP radiograph. A positive angle, typically greater than 0°, is thought to enhance stability. However, its clinical utility in predicting loss of reduction (LOR) remains inconsistent, particularly in pediatric patients where soft-tissue compliance and remodeling potential may diminish its relevance[23].
Collectively, these indices assess the mechanical efficiency of cast molding in achieving stable three-point fixation. When applied correctly, a well-molded cast exerts consistent compressive forces that maintain fracture alignment and minimize the risk of displacement. These indices have therefore become important adjuncts in judging not only the immediate success of a CR but also its likelihood of remaining stable throughout the healing process.
Beyond those mentioned radiographic thresholds and objective indices, the clinical success of functional reduction in pediatric DRF is well supported by long-term outcomes. Numerous studies have shown that even fractures with mild residual angulation can remodel adequately during growth, especially in younger children with inherent biological plasticity, leading to excellent functional and cosmetic results[24-28]. This remodeling capacity reduces the need for aggressive realignment or serial imaging, particularly for metaphyseal fractures managed conservatively. When an acceptable initial reduction is achieved and maintained, long-term outcomes are often equivalent to those treated surgically, further supporting a conservative and selective FU strategy[28,29]. Clinical judgment remains an indispensable element during FU, as subtle clinical changes may warrant additional imaging even in cases initially deemed low risk. Radiographs should not be omitted reflexively but rather guided by evolving clinical findings and physician experience. Importantly, decisions regarding imaging and FU care should be made collaboratively between orthopedic surgeons and families, balancing patient safety with the need to avoid unnecessary radiation exposure and excessive healthcare utilization. This individualized, family-centered approach embodies the core principles of modern value-based pediatric orthopedic practice.
LOR: DEFINITIONS, INCIDENCE, AND PREDICTIVE FACTORS
LOR refers to the radiographic deterioration of fracture alignment following an initially successful CR. It is observed in both cast immobilization and, less commonly, in cases stabilized with percutaneous K-wire fixation. However, the uniform definition of LOR remains elusive, with thresholds varying across studies. McLauchlan et al[17] defined LOR as a postoperative angulation exceeding 10 degrees combined with more than 50% displacement. In contrast, Miller et al[30] applied a stricter criterion, requiring greater than 25 degrees of angulation and complete displacement to qualify as LOR. LaValva et al[31] and Colaris et al[32] introduced age-dependent parameters, defining LOR as > 10° angulation in children older than 10 years, and > 15°-20° in those younger, in addition to > 50% displacement. Ozcan et al[33] considered multiple parameters in evaluating LOR on FU radiographs. These included > 10° of volar or dorsal angulation, > 5° of radial deviation, > 3 mm of translation, or a combination of 2-3 mm of translation with 5°-10° of angulation[33].
Radiographic re-displacement typically occurs within the first 5 to 10 days post-reduction[34]. The reported incidence of LOR also varies according to treatment modality. Colaris et al[32] and Ozcan et al[33] observed 8.1% and 10% LOR rates following K-wire fixation, while 0% was also reported by others[17,30,35,36]. In a meta-analysis by Sengab et al[9], the incidence of LOR was substantially lower in the pinning group (3.8%: 7/185) compared to the casting group (45.7%: 90/197). These findings are mirrored in the more recent and larger meta-analysis by Alotaibi et al[37], which documented LOR in 10.9% of patients (57/521) treated with CR and pinning, and in 38.4% (302/786) of those treated with casting alone.
Several studies have identified key risk factors for re-displacement after reduction of displaced pediatric DRFs. McQuinn et al[34] emphasized that an initial displacement greater than 50% and the failure to achieve an anatomic reduction were strongly associated with LOR. These findings align with broader literature, which consistently highlights the degree of initial displacement and the quality of reduction as primary predictors of fracture stability[8,9,17,31]. Although several radiographic indices have been developed to predict LOR, no single index parameter has achieved universal consensus. McQuinn et al[34] identified the cast index, specifically a mean value of 0.83, as the most significant predictor of LOR in their cohort. Meanwhile, Ravier et al[38] found that the cast index, padding index, and Canterbury index offered greater predictive value than standard radiographs alone. These findings underscore the importance of high-quality cast molding in maintaining reduction and suggest that objective indices may play a critical role in risk stratification, though further validation is needed before any can be applied universally.
LOR: A DRIVER OF SECONDARY INTERVENTION
The risk of requiring secondary intervention following failed reduction of pediatric DRFs is notably higher in patients treated with cast immobilization compared to those stabilized with K-wire fixation[9,37]. Multiple comparative studies have shown that, although LOR can occur with either treatment method, the likelihood of needing revision is substantially lower after initial pinning[9,17,30,32,33,36,37]. In fact, in most series evaluating K-wire fixation, LOR was either rare or did not necessitate surgical revision. For instance, only isolated cases of secondary intervention were reported by Colaris et al[32] (1.6%: 1 out of 61 cases), McLauchlan et al[17] (2.8%: 1/35), and Ozcan et al[33] (5%: 1/20), despite minor degrees of LOR observed.
In contrast, studies involving cast-only treatment have documented a significantly higher frequency of revision procedures for re-displaced fractures. Colaris et al[32] reported that 25.3% (17/67) of fractures in the casting group required re-intervention). Gibbons et al[36] documented an even more striking rate, with 90.9% (10/11) of re-displaced fractures undergoing revision. Similarly, McLauchlan et al[17] and Van Leemput et al[39] reported secondary interventions in 24.2% (8/33) and 62.5% (15/24) of re-displaced cases, respectively. These revision procedures typically included repeat manipulation followed by casting or conversion to K-wire fixation.
Collectively, these findings suggest that LOR can occur in both treatment pathways. The clinical consequences, particularly the likelihood of revision, are notably more pronounced in the cast immobilization group. This reinforces the role of meticulous reduction, and high-quality casting in avoiding the downstream burden of secondary procedures.
RETHINKING ROUTINE IMAGING: HOW OFTEN ARE FU RADIOGRAPHS TRULY NEEDED?
Historically, postoperative radiographic FU has been standard across various orthopedic procedures particularly in patients with osteoporosis or comorbidities that compromise bone healing[40,41]. In contrast, the necessity of routine radiographic surveillance in pediatric patients, especially following CR of DRFs, has become increasingly questioned[42]. These injuries are known for their excellent remodeling potential and high rates of healing, prompting a reassessment of whether serial imaging is always justified[4].
The current literature offers limited guidance on the optimal number or timing of FU radiographs after CR of pediatric DRFs. Variability in clinical practice stems in part from differing protocols around cast duration, pin removal, and fracture classification. Traditionally, radiographs have been obtained at multiple intervals until radiographic union, particularly in Salter-Harris fractures where concerns about growth arrest, radial shortening, or angular deformity may necessitate long-term surveillance[43]. However, this routine imaging approach has come under scrutiny in recent years.
Bochang et al[44] proposed a more selective strategy, recommending FU radiographs only after two weeks post-casting in greenstick or complete fractures. If clinical examination at the time of cast removal reveals no tenderness, deformity, or functional limitation, they advise that further imaging is unnecessary. Supporting this approach, Perhomaa et al[45] concluded that clinical FU alone is sufficient in most cases of nonoperatively treated pediatric DRFs, with no compromise in outcomes. In a similar vein, Molokwu et al[42] suggested that post–cast removal radiographs and even the second FU appointments rarely alter management of minimally displaced DRFs in children. Recent evidence by Alomran et al[46] further supports this perspective, showing no statistically significant association between the frequency of postoperative radiographs and clinical outcomes in children with DRFs, regardless of the fixation method used.
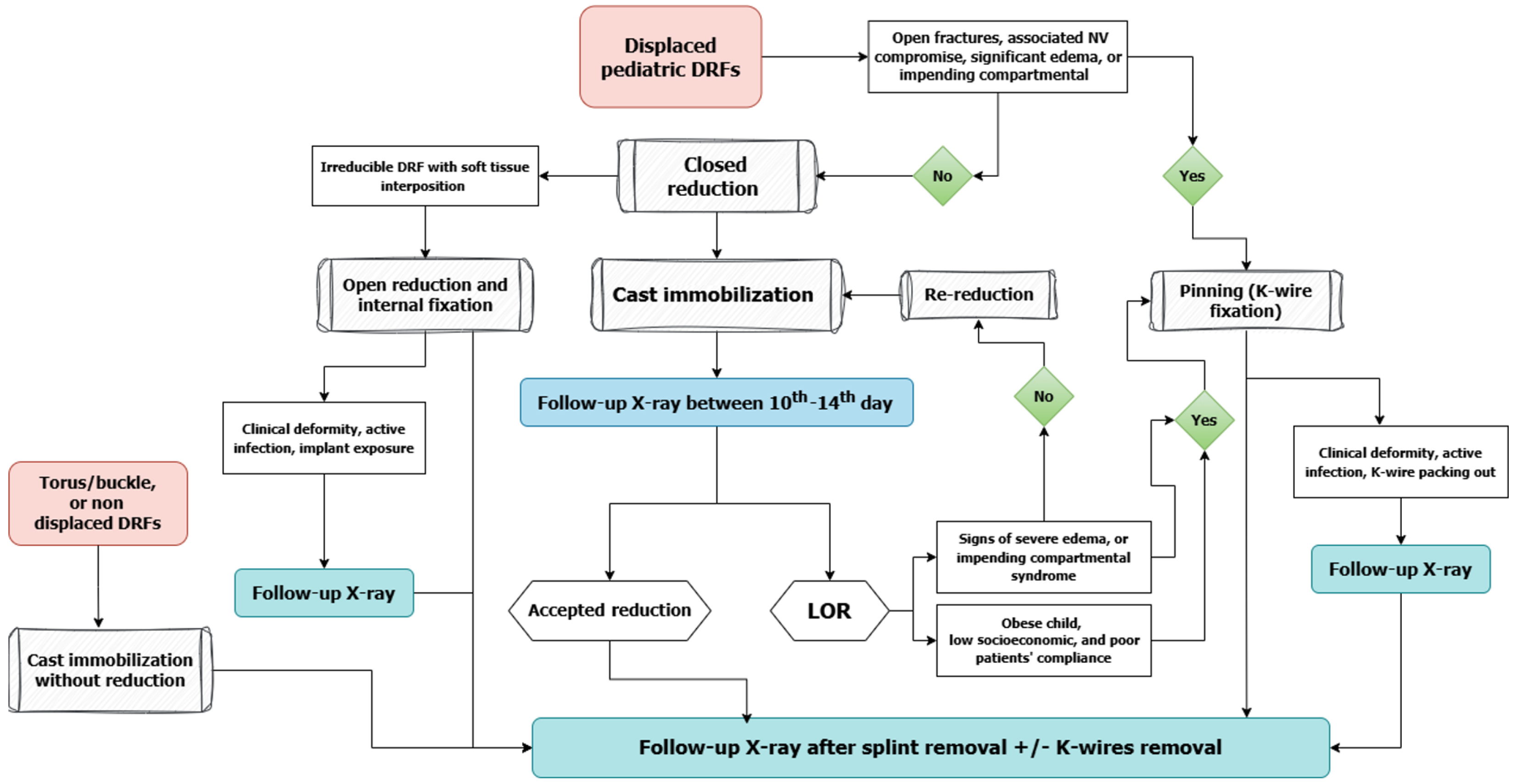
These evolving recommendations underscore the tension between maintaining vigilance for rare complications and reducing the overuse of imaging. Given the high healing potential in children, especially in metaphyseal fractures, routine radiographs may offer little added value when clinical progress is satisfactory. At the same time, minimizing unnecessary imaging reduces radiation exposure, healthcare costs, and logistical burdens on families. This highlights the need for a balanced, individualized approach to FU care, that relies on clinical judgment and evidence-based risk stratification rather than rigid imaging schedules. Figure 1 provides a visual summary of the proposed selective imaging protocol, guiding clinicians through key decision points based on fracture stability, treatment method, and clinical findings.
Figure 1 A decision-making flow chart for follow-up imaging in pediatric distal radius fractures.
This flowchart outlines a risk-based approach to determine the necessity of radiographic follow-up after treatment. Decisions are guided by fracture stability, quality of reduction, treatment method, and clinical findings during follow-up. DRFs: Distal radius fractures; LOR: Loss of reduction.
COST IMPLICATIONS OF TREATMENT STRATEGIES
While CR with pinning generally incurs higher initial costs than CR and casting, the overall economic burden may be greater with casting due to the higher risk of LOR and need for revision. Studies have shown mixed results: Crawford et al[16] reported nearly double the cost for pinning, whereas Miller et al[30] found only a modest difference, which reversed when complication-related costs were included. Godfrey et al[47] demonstrated that operative interventions substantially increase treatment costs, with pinning costing nearly seven times more than conservative care. Moreover, a substantial proportion of reductions may be avoidable, particularly in younger children with high remodeling potential. This eliminated routine FU radiography may yield cost savings of up to 12%[48,49].
A more recent and detailed cost-effectiveness analysis by Franovic et al[50] adds important context to this discussion. Their decision-tree model compared nonoperative treatment, CR with casting, and CR with pinning, incorporating both direct costs and effectiveness measured in quality-adjusted life years (QALYs). While pinning offered a modest improvement in effectiveness (QALY gain of 0.0166), it incurred significantly higher costs. The study used the incremental cost-effectiveness ratio (ICER) formula: [Cost (I)-Cost (II)]/[Effectiveness (I)-Effectiveness (II)], to assess whether these added costs translated into meaningful clinical benefit. In most modeled scenarios, pinning exceeded accepted ICER thresholds unless the failure rate of conservative treatment was high. These findings reinforce that nonoperative management remains the most cost-effective strategy in most pediatric cases, particularly in stable or minimally displaced fractures.
Importantly, Franovic et al’s study[50] also highlights that the selective avoidance of unnecessary FU radiographs, especially after an initially stable reduction, not only minimizes radiation exposure but also contributes to substantial cost savings. These include both direct costs (imaging, sedation, and procedural fees) and indirect costs, including missed school for the child, time away from work for caregivers, and repeated clinic visits. Collectively, the economic evidence supports a shift toward risk-stratified imaging protocols and individualized treatment pathways that prioritize both clinical safety and cost efficiency. Such an approach aligns well with the broader goals of value-based pediatric orthopedic care[47,49,50].
CONCLUSION
Routine postoperative radiographs rarely influence clinical decisions following treatment of pediatric DRF, particularly after stable pin fixation. Emphasis should instead be placed on achieving and assessing high-quality initial reduction and cast molding. When stability is confirmed and risk is low, serial imaging may be unnecessary. This editorial supports a selective, risk-based imaging strategy reserving routine radiographs for high-risk cases such as displaced physeal fractures or suboptimal reductions. In stable fractures, clinical examination combined with objective indices can safely guide FU. Such an approach reduces radiation exposure, lowers costs, and aligns with value-based pediatric orthopedic care.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country of origin: Egypt
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Bai L S-Editor: Qu XL L-Editor: A P-Editor: Zheng XM