Published online Aug 18, 2025. doi: 10.5312/wjo.v16.i8.107083
Revised: May 5, 2025
Accepted: July 23, 2025
Published online: August 18, 2025
Processing time: 146 Days and 21.8 Hours
Aneurysmal bone cysts (ABCs) are usually treated with curettage or various minimally invasive percutaneous procedures. Patient refractory to these treat
To test the hypothesis that the sequential use of BPs after denosumab induction improves treatment outcomes in surgically unsalvageable ABCs.
Using data from five electronic databases (Scopus, MEDLINE, EMBASE, PubMed, Web of Science), we aimed to identify all patients who received denosumab therapy (DT) for unresectable ABCs. Among published case reports and case series, we identified patients who discontinued denosumab for various reasons and divided them into two groups: Group 1 included 31 patients without further anti-resorptive therapy and Group 2 included 12 patients who received BPs in the context of rebound hypercalcemia. Local control rates in both groups were analyzed.
As of December 2024, 43 patients have been reported in the literature who received DT for locally advanced/unresectable ABCs. There were 27 males and 16 females with a mean age of 15.8 years. At a median follow-up time of 15.5 months, there were 10 confirmed and two pathologically unconfirmed relapses after de
BPs used in post-denosumab ossifying ABCs appear to improve treatment outcomes, presumably by targeting residual tumor cells. Prospective clinical studies are warranted to validate this promising two-stage conceptual strategy in difficult-to-treat ABC.
Core Tip: Benign bone tumors containing giant cells undergo significant ossification after denosumab treatment. In these conditions, sequentially administered bisphosphonates (BPs) accumulate better in newly formed bone, which may lead to long-term local control, presumably due to a pro-apoptotic effect on residual tumor cells. In this context, we studied a group of patients with inoperable aneurysmal bone cysts (ABCs) who were treated with denosumab and found that among those patients who received BPs for post-denosumab rebound hypercalcemia, there were no local relapses. We assume that BPs could have the same irreversible effect on residual tumor cells in ABCs and propose to continue experimental and prospective clinical studies to confirm this hypothesis.
- Citation: Machak GN, Bruland ØS, Kovalev AV, Rodionova SS. Rethinking the role of bisphosphonates after denosumab treatment in locally advanced or unresectable aneurysmal bone cysts: A meta-analysis. World J Orthop 2025; 16(8): 107083
- URL: https://www.wjgnet.com/2218-5836/full/v16/i8/107083.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i8.107083
Aneurysmal bone cyst (ABC) is a rare benign tumor of bone with abundant blood-filled spaces in its classical form, belonging to the group of osteoclastic giant cell-rich tumors[1,2]. ABCs occurs most frequently in patients under 20 years of age, with the metaphysis of long tubular bones and vertebrae being anatomic predilection sites.
Knowledge about ABCs has evolved, especially regarding the molecular biology and neoplastic nature of this lesion[3-5]. A translocation t (16;17) (q22; p13) involving multiple fusion partners[6-11] results in the activation of the USP6 on
Standard treatment involves curettage with or without local adjuvants and bone grafting[38]. In rapidly growing, locally advanced, and axial ABC, surgical treatment may be associated with severe intraoperative complications and long-term morbidity[39-41]. Multiple less invasive approaches have been investigated as preoperative or definitive treatment including sclerotherapy (ST)[42], selective arterial embolization (SAE)[43], cryotherapy[44], “curopsy”[37], and bone marrow injection[45]. A small proportion of patients with ABC are refractory to these treatments, necessitating new approaches targeting other pathogenetic mechanisms.
Two major classes of drugs targeting osteoclastogenesis are used for the treatment of locally advanced and inoperable ABC: Bisphosphonates (BPs) and RANKL inhibitors such as denosumab[46,47]. In most cases, such anti-resorptive therapy (ART) results in clinical improvement and tumor ossification. However, several issues are associated with this strategy, i.e. relapses after treatment cessation, acute side effects in children, and long-term toxicity in skeletally mature patients. Additionally, there are no generally accepted standards regarding the optimal management of patients after discontinuation of denosumab therapy (DT).
In a recent paper, we hypothesized that the use of BPs as maintenance following DT induction may have a protective role in giant cell tumor of bone (GCTB) due to the direct antitumor effect on mutated cells[48], and we formulated the concept of a two-stage treatment for locally advanced or inoperable GCTB. In light of the similarities in pathogenesis between GCTB and ABCs, and the frequent use of BPs for rebound hypercalcemia (RH) observed after discontinuation of DT in children, we were intrigued to explore the effect of sequential use of these two drugs to achieve long-term local control of ABC as well. In this paper, we have reviewed the existing literature on DT in ABC, focusing on the potential benefit of the additional use of BPs. The concept is further illustrated by two cases from our own clinical practice.
A systematic literature search was conducted across five electronic databases: Scopus, MEDLINE, EMBASE, PubMed, and Web of Science, from inception to December 2024. The Medical Subject Headings (MeSH) and keywords used were “Aneurysmal bone cyst” and “Denosumab”.
The systematic searches revealed 29 peer-reviewed articles describing the use of DT in ABCs. Due to the rarity of ABCs and the extreme rarity of their treatment with Denosumab[47], and recognizing the possible impact of this fact on the quality of the analysis, we included all reported cases regardless of patient age, disease stage, and treatment regimen. Among the 68 patients with pathologically confirmed ABC, DT was used in a neoadjuvant setting for 12 patients (17.6%). Nine patients (13%) were reported to have continued DT. In 46 cases (67.6%), DT was discontinued for different reasons, mainly due to stable clinical and imaging findings. Four patients had a progression-free follow-up time of < 6 months and were excluded from the analysis. Hence, 43 patients met the above inclusion criteria and were selected for review (Table 1)[49-64]. Extracted data included age, gender, tumor site, disease status (primary/refractory or recurrent), as well as treatments prior to DT. Upon initiation of denosumab, the following data were extracted: Duration of DT, clinical and radiological response, outcome after DT cessation, relapse-free survival after DT discontinuation, follow-up time, adverse effects in the form of RH, and treatment of this complication.
| Ref. | Age | Gender | Localization | Status | Pre-denosumab treatment | DT duration | Response to DT | Group | Outcome after DTS | RFS | REC | Follow-up |
| Ghermandi et al[49], 2016 | 42 | Male | Spine | PD | SAE | 9 | CR IR | 1 | SD | 20 | 0 | 20 |
| Ghermandi et al[49], 2016 | 16 | Male | Spine | PD | SAE | 7 | CR IR | 1 | SD | 16 | 0 | 16 |
| Ntalos et al[50], 2017 | 35 | Female | Pelvis-sacrum | R | SAE SURG DT 60 mg | 17 | CR IR | 1 | SD | 15 | 0 | 15 |
| Kurucu et al[51], 2017 | 16 | Male | mandible | R | ST IT | 11 | CR IR | 1 | SD | 20 | 0 | 20 |
| Kurucu et al[51], 2017 | 17 | Female | Pelvis | P | 14 | CR IR | 1 | REC CLIN ST | 3 | 1 | 3 | |
| Kurucu et al[51], 2017 | 5 | Female | Spine | P | 12 | CR IR | 1 | SD | 12 | 0 | 12 | |
| Kurucu et al[51], 2017 | 6 | Male | Pelvis | P | 9 | CR IR | 1 | REC CLIN HYST negative SD | 6 | 0 | 20 | |
| Kurucu et al[51], 2017 | 8 | Male | Humerus | R | SURG | 12 | CR | 1 | SD | 12 | 0 | 12 |
| Kurucu et al[51], 2017 | 16 | Female | Spine | P | 12 | CR IR | 1 | REC DRCH | 17 | 1 | 21 | |
| Kurucu et al[51], 2017 | 10 | Female | Spine | P | 6 | CR PIR | 1 | REC SURGERY | 6 | 1 | 24 | |
| Patel et al[52], 2018 | 16 | Male | Spine | R | ST | 12 | CR IR | 1 | SD | 12 | 0 | 12 |
| Palmerini et al[53], 2018, Evangelisti et al[54], 2024 | 16 | Male | L5-S1 | P | SAE 5 | 41 | CR IR | 1 | SD | 24 | 0 | 24 |
| Palmerini et al[53], 2018, Evangelisti et al[54], 2024 | 42 | Male | Spine C7 | P | SURG SAE 2 | 26 | CR IR | 1 | REC DRCH ongoing SD 24 mos. | 15 | 1 | 103 |
| Palmerini et al[53], 2018, Evangelisti et al[54], 2024 | 25 | Male | Spine Th10 | P | ND | 36 | CR IR | 1 | REC MSC SD 26 mos. | 20 | 1 | 56 |
| Palmerini et al[53], 2018, Evangelisti et al[54], 2024 | 19 | Male | Spine L3-L4 | P | ND | 10 | CR IR | 1 | SD | 33 | 0 | 33 |
| Palmerini et al[53], 2018 | 12 | Male | Proximal ulna | P | 8 | CR PIR | 1 | SD | 12 | 0 | 12 | |
| Raux et al[55], 2019 | 8 | Male | Spine | P | 12 | CR IR | 1 | REC DRCH ongoing SD 12 mos. | 10 | 1 | 10 | |
| Raux et al[55], 2019 | 7 | Female | Femur | R | SURG | 4 | CR IR | 1 | SD | 32 | 0 | 32 |
| Dürr et al[56], 2019 | 6 | Male | Sacrum | P | 12 | CR IR | 1 | SD | 6 | 0 | 6 | |
| Dürr et al[56], 2019 | 15 | Female | Distal radius | PD | SURG ST | 6 | CR IR | 1 | SD | 36 | 0 | 36 |
| Dürr et al[56], 2019 | 16 | Female | Distal femur | P | SURG SAE | 24 | CR IR | 1 | SD | 24 | 0 | 24 |
| Dürr et al[56], 2019 | 30 | Female | Pelvis | R | SURG DT SURG | 8 | CR IR | 1 | REC DRCH ongoing SD | 36 | 1 | 18 |
| Dürr et al[56], 2019 | 18 | Male | Sacrum | R | ST SAE | 24 | ND | 1 | REC DRCH ongoing SD 18 mos. | 12 | 1 | 12 |
| Dürr et al[56], 2019 | 16 | Female | Talus | R | SURG | 12 | SD | 1 | REC as Ganglioma | 24 | 0 | 24 |
| Sydlik et al[57], 2019 | 6 | Male | Femur | P | 24 | CR IR | 1 | SD | 12 | 0 | 12 | |
| Kotaka et al[58], 2023 | 38 | Male | Spine L3 | P | 3 | CR IR | 1 | REC DRCH ongoing SD 37 | 17 | 1 | 37 | |
| Vanderniet et al[59], 2023 | 13 | Male | Spine | P | 12 | IR | 1 | SD | 36 | 0 | 36 | |
| Vanderniet et al[59], 2023 | 12 | Female | Spine | P | SURG SAE | 8 | IR | 1 | SD | 6 | 0 | 6 |
| Evangelisti et al[54], 2024 | 20 | Male | Spine C6-C7 | P | SURG SAE 1 | 12 | CR IR | 1 | SD | 43 | 0 | 43 |
| Kulkarni et al[65], 2019 | 14 | Female | Spine | R | SURG | 6 | CR IR | 1 | SD | 24 | 0 | 24 |
| Evangelisti et al[54], 2024 | 26 | Female | Spine C4 | P | MSC 1 | 17 | CR IR | 1 | REC DRCH ongoing 20 mos. | 10 | 1 | 43 |
| Kurucu et al[51], 2017 | 12 | Male | Pelvis | P | 14 | CR IR | 2 | SD | 10 | 0 | 10 | |
| Kurucu et al[51], 2017 | 16 | Male | Sacrum | R | SURG | 14 | CR PIR | 2 | SD | 12 | 0 | 12 |
| Upfill-Brown et al[60], 2019 | 10 | Female | Pelvis | R | SURG | 11 | CR IR | 2 | SD | 13 | 0 | 13 |
| Raux et al[55], 2019 | 8 | Male | Spine | R | SURG SAE | 17 | CR IR | 2 | SD | 6 | 0 | 6 |
| Sydlik et al[57], 2019 | 11 | Male | Sacrum | P | 16 | CR IR | 2 | SD | 12 | 0 | 12 | |
| Harcus 2020[61], | 13 | Male | Proximal tibia | R | SURG n 3 | 27 | CR | 2 | SD | 16 | 0 | 16 |
| Del Sindaco et al[62], 2021 | 8 | Male | Spine | P | 12 | CR IR | 2 | REC 9 mos. DRCH DTS SD | 15 | 0 | 15 | |
| Deodati et al[63], 2022 | 10 | Male | Pelvis | P | 10 | ND | 2 | SD | 19 | 0 | 19 | |
| Vanderniet et al[59], 2023 | 12 | Male | Spine | P | SURG SAE ST | 18 | IR | 2 | SD | 42 | 0 | 42 |
| Vanderniet et al[59], 2023 | 10 | Female | Spine | P | 18 | IR | 2 | SD | 24 | 0 | 24 | |
| Vanderniet et al[59], 2023 | 13 | Male | Spine | P | SURG SAE ST | 12 | IR | 2 | SD | 12 | 0 | 12 |
| Gandolfi et al[64], 2023 | 10 | Female | Sacrum | R | SURG SAE | 25 | CR | 2 | SD | 6 | 0 | 6 |
Clinical response was defined as pain relief with or without reduction in tumor volume. Imaging response was defined as an increase in peripheral or internal bone mineralization in a radiograph or computed tomography (CT) compared with baseline values. Recurrence of the disease was usually defined as an increase in the size of osteolytic areas on radiography, CT, or magnetic resonance imaging (MRI) with or without the reappearance of clinical symptoms.
In the context of our meta-analysis, after discontinuation of DT, patients were divided into two groups. The first cohort consisted of patients who had no indications for BP use (Group 1). The second cohort included cases with RH who received BPs as a part of therapy (Group 2).
The significantly lower age in Group 2 (Table 2) can be explained by the occurrence of RH mainly in children.
| Feature | Group 1 (n = 31) | Group 2 (n = 12) | P value |
| Gende | 0.3 | ||
| Male | 18 (58) | 9 (75) | |
| Female | 13 (42) | 3 (25) | |
| Age (years) | 16 (5-42) | 10.5 (8-16) | 0.03 |
| Site | 0.2 | ||
| Axial | 23 (74) | 11 (92) | |
| Extremities | 8 (26) | 1 (8) | |
| Primary/failed initial treatment/poor response | 22 (71) | 7 (58) | 0.4 |
| Recurrent | 9 (29) | 5 (42) | |
| DT duration (months) | 12 (4-41) | 15 (10-27) | 0.05 |
| Clinical and/or imaging response to DT | 0.9 | ||
| Yes | 27 (90) | 10 (91) | |
| No | 3 (10) | 1 (9) | |
| Post-DT withdrawal follow-up (months) | 16 (3-43) | 12 (6-42) | 0.4 |
| Clinical and/or imaging and/or histological relapse | 0.02 | ||
| Yes | 10 (32) | 0 (0) | |
| No | 21 (68) | 12 (100) |
Local control was assessed using the reported clinical, imaging, and in some cases pathological examinations. In cases of tumor recurrences following DT, additional data were extracted regarding the clinical management approaches used. To illustrate the role of ART in difficult-to-treat patients, we present two cases from our own clinical experience regarding the treatment of locally advanced ABCs, that failed to show clinical or radiological response after multiple cyst decompression procedures.
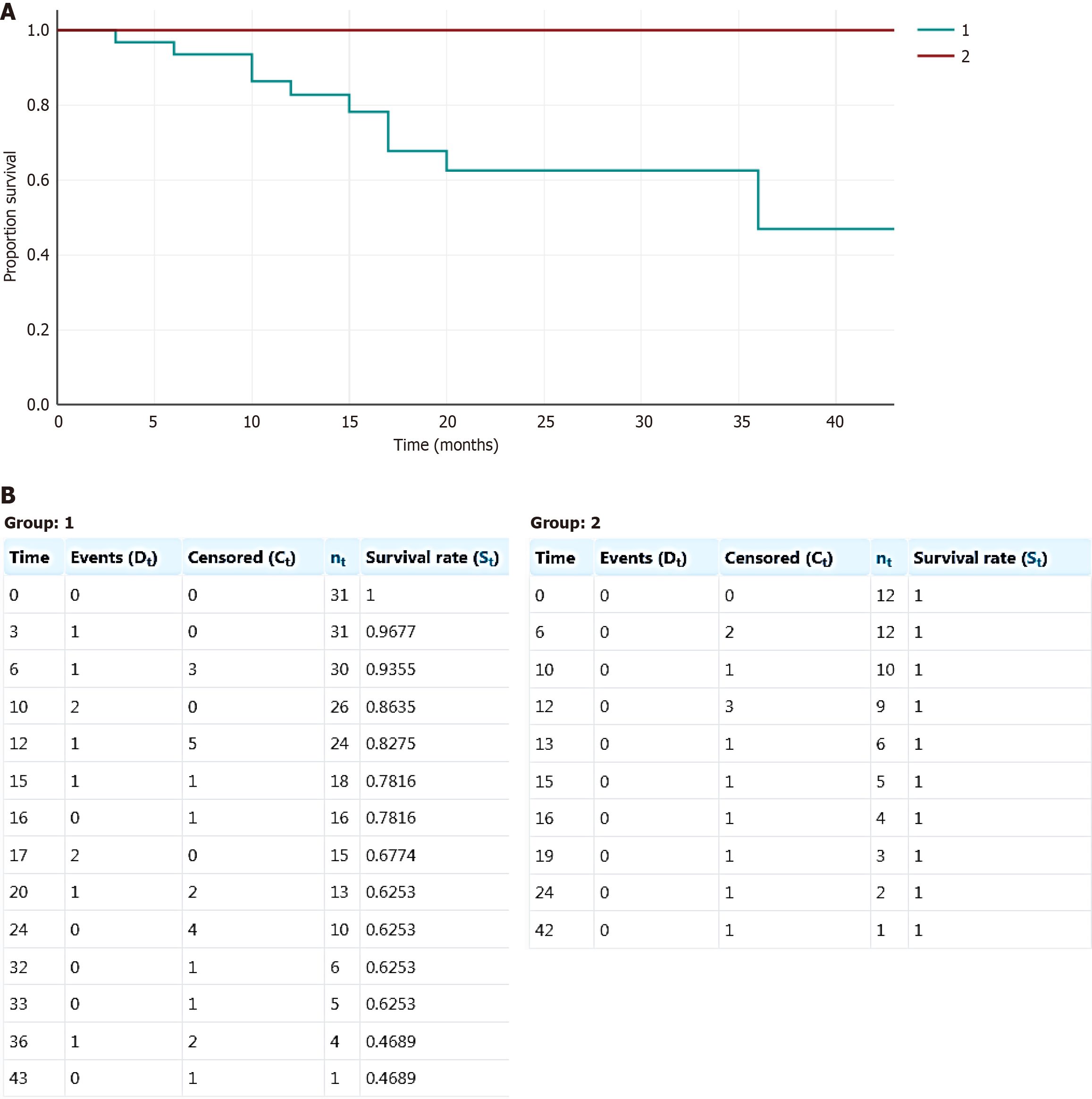
The medians and range of demographic information and outcomes were calculated and reported. The χ2 test was used to compare recurrence rates between the two patient cohorts, with significance set at P < 0.05. The Kaplan-Meier method was used to estimate the survival function from lifetime data in both groups.
There were 27 males and 16 females with a mean age of 15.8 years. (range 5-42), Tables 1 and 2. In 35 cases (81%), the tumor involved the axial skeleton, and in eight cases (19%), the tumor localization was in the extremities. Among axial lesions, the most common was the spine (n = 27), followed by the pelvis (n = 7) and mandible (n = 1). Twenty-nine patients had untreated tumors (17 cases) or were refractory to ongoing therapy (debulking surgery, SAE, and ST) (12 cases). The remaining 14 patients had recurrent lesions after initial intralesional surgery alone (7) or combined with SAE, ST, or DT (7). Thirty-one patients were included in Group 1, while Group 2 included 12 patients (Figure 1). Details of patient demographics, clinical features, treatment, and outcomes of both groups are presented in Table 2.
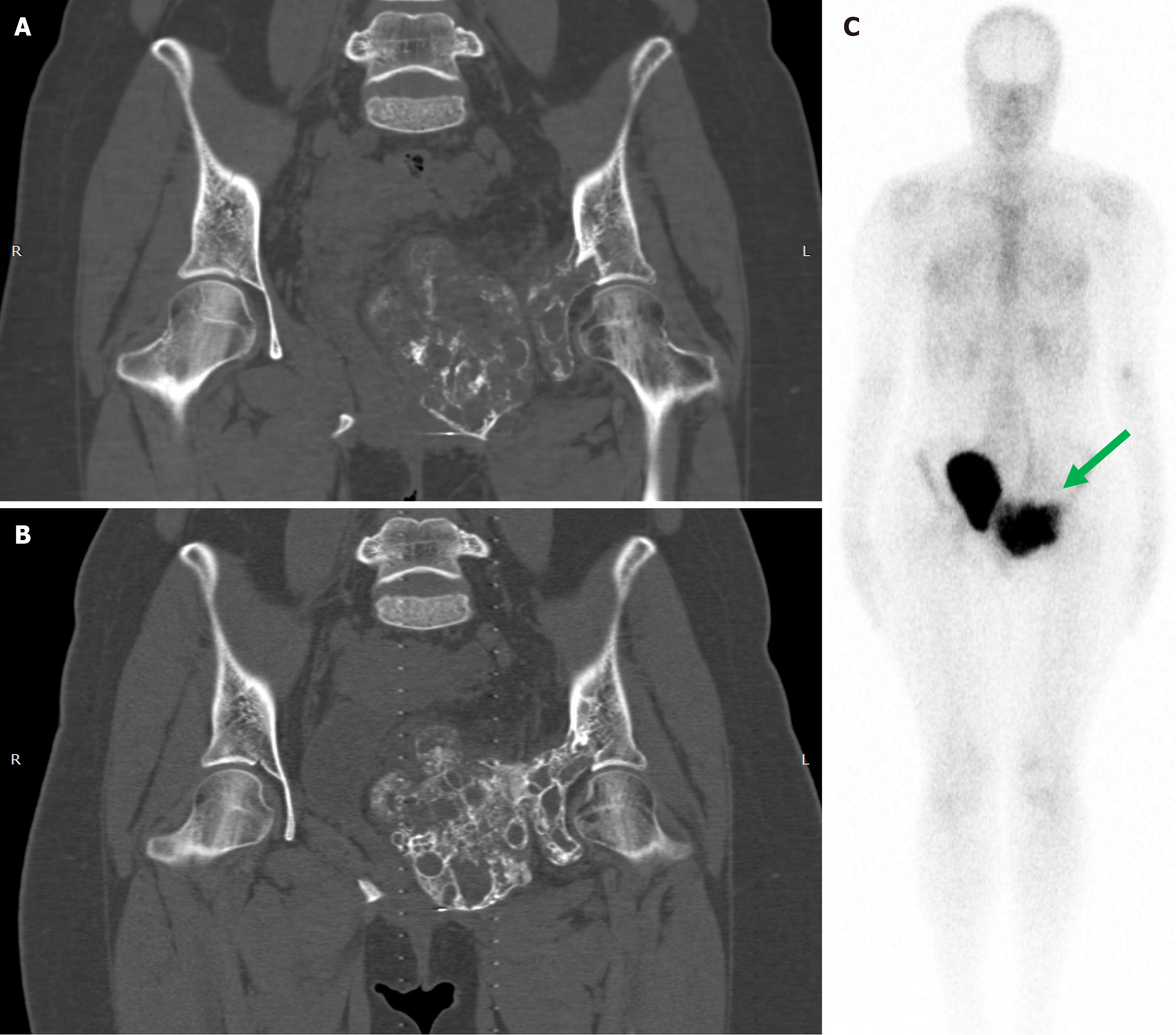
DT was administered according to the GCTB protocol (denosumab 120 mg SC every 4 weeks with loading doses on study days 8 and 15) (57%) or monthly without loading doses (43%)[47]. The duration of the DT varied from 3 to 41 months (median 14). A good clinical and/or imaging response was documented in 37 of 41 evaluated patients (90%), while four patients (10%) had a partial response or stabilization of the disease (Table 1). The difference between the response rates in both groups was statistically insignificant (P = 0.9). In one case from our clinical experience, a 19-year-old girl with locally advanced pelvic ABC failed to respond to multiple decompressions and was treated with ART. Monthly DT for one year resulted in pain relief, pronounced tumor ossification, no pelvic dysfunction, and a good quality of life, Figure 2.
In the context of our analysis, we focused on the incidence of RH during or after DT. RH alone or in combination with other side effects was observed exclusively in 15 (35.7%) skeletally immature children (80% male) with a median age of 10 years (range 6-16), Table 3. All episodes of RH occurred after discontinuation of denosumab or during DT over the extended treatment time.
| Side effects | Frequency |
| Hypercalcemia | 12 (29) |
| Hypercalcemia + Growth plate ossification | 1 (2) |
| Hypocalcemia + Hypercalcemia | 1 (2) |
| Hypocalcemia + Hypophosphatemia + Hypercalcemia | 1 (2) |
| Hypocalcemia + Hypophosphatemia | 1 (2) |
| Vomiting | 1 (2) |
| Not reported | 26 (60) |
| Total | 43 |
BPs therapy was a part of intensive care for RH, which also included diuretics, corticosteroids, calcitonin, intravenous infusions of 0.9% saline, 5% dextrose, and other solutions. As shown in Table 4, pamidronate (PAMI) was used alone in one patient and combination with zoledronic acid (ZA) in two others. ZA alone was administered in one patient and combined with risedronate in three patients. One patient was treated with neridronate and another four with unspecified BP regimens. The duration of BP exposure ranged from one to six months (median 2.5). RH was always resolved and the duration of BP therapy was relatively short (Table 4) with no side effects described as seen with long-term BP use in cancer or osteoporosis.
| Ref. | Age/gender | Site | BPs therapy | BPs therapy in months |
| Kurucu et al[51], 2017 | 12/Male | Pelvis | BPs 1 dose | 1 |
| Kuruku et al[51], 2017 | 16/Male | Sacrum | BPs 1 dose | 1 |
| Raux et al[55], 2019 | 8/Male | Spine | BPs 3 doses | 6 |
| Sydlik et al[57], 2019 | 11/Male | Sacrum | Neridronate 2 doses (2 mg/kg) | 1 |
| Upfill-Brown et al[60], 2019 | 10/Female | Pelvis | BPs 1 dose | 1 |
| Harcus et al[61], 2020 | 13/Male | PT | PAMI 2 IV doses (0.25 mg/kg and then 0.5 mg/kg, 24 hours apart). PAMI one IV dose (0.5 mg/kg). ZA one IV dose (0.05 mg/kg) | 2 |
| Del Sindaco et al[62], 2021 | 8/Male | Spine | ZA six IV doses (0.05 mg/kg) | 6 |
| Deodati et al[63], 2022 | PAMI one IV dose (1 mg/kg) in 1 week. PAMI five IV doses every 15 days (0.5 mg/kg) | 3 | ||
| Vanderniet et al[59], 2023 | 12/Male | Spine | ZA two IV doses (0.025 mg/kg), oral risedronate up to 6 months | Up to 6 |
| Vanderniet et al[59], 2023 | 10/Female | Spine | ZA two IV doses (0.025 mg/kg), oral risedronate up to 6 months | Up to 6 |
| Vanderniet et al[59], 2023 | 13/Male | Spine | ZA one IV dose (0.025 mg/kg), oral risedronate up to 6 months | Up to 6 |
| Gandolfi et al[64], 2023 | 10/Female | Sacrum | PAMI two IV doses (1 mg/kg). ZA one IV dose (0.03 mg/kg) | 1 |
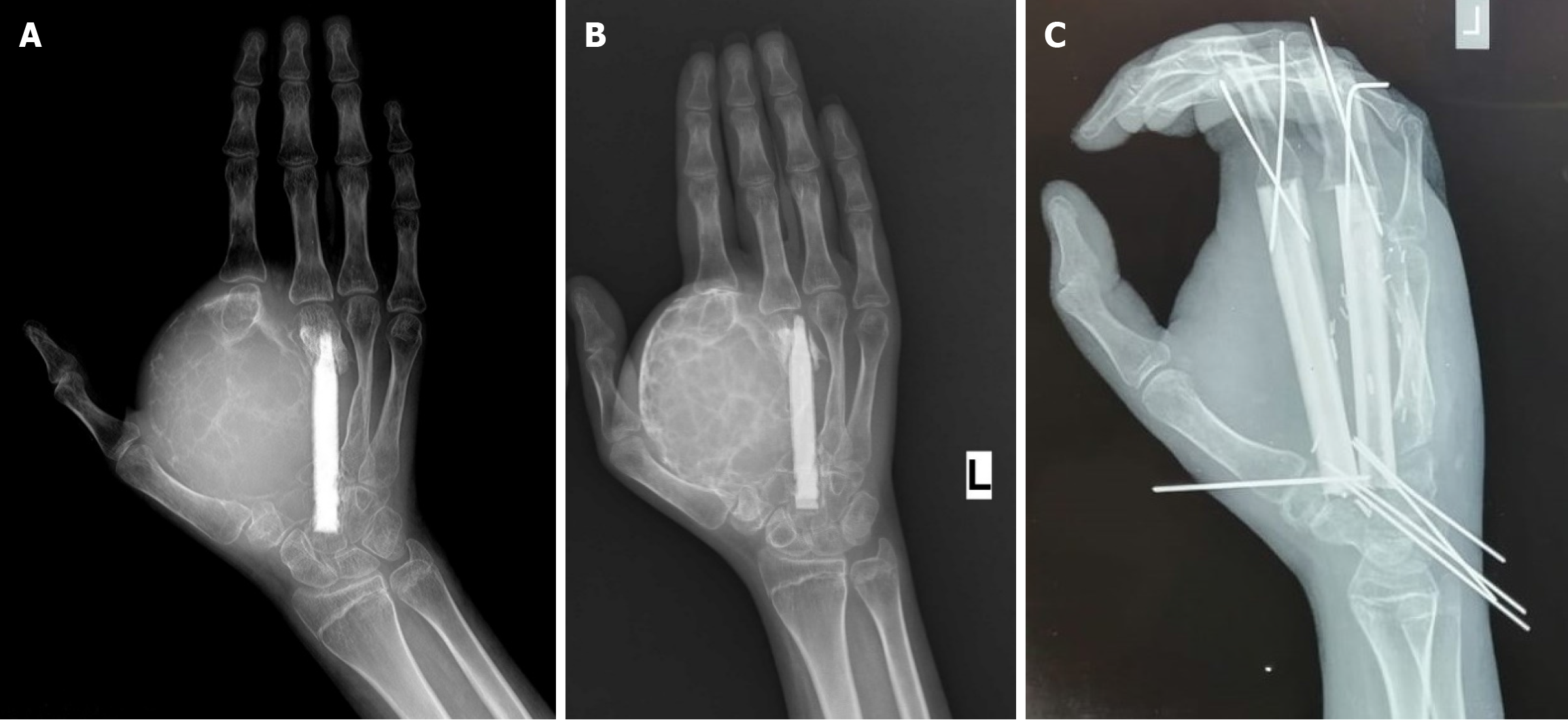
Another of our patients, a 16-year-old girl with recurrent and unresectable ABC of the metacarpal bones, also failed to respond to decompressions, Figure 3A. In order to convert the tumor into a resectable form, neoadjuvant therapy with BP was initiated. After six monthly PAMI infusions, peripheral ossification of the tumor allowed us to perform a function-preserving surgery without intraoperative complications and to achieve a long-term relapse-free interval, Figure 3B and C.
As is shown in Table 2, the groups were unbalanced in terms of age and duration of DT, with a predominance of children and longer DT in the BPs group. At a median follow-up time of 15.5 months (range of 3-43), there were 10 confirmed and two pathologically unconfirmed clinical and/or imaging relapses among the 43 patients who discontinued DT, Tables 1 and 2. All local relapses occurred in patients who did not receive BPs after DT withdrawal. Thus, the rate of local relapses in Group 1 and Group 2 was 33% (10/31) and 0% (0/12), respectively (P = 0.02). The median time to relapse was 13.5 months (range 3-36). Seven relapsed patients (70%) received denosumab re-challenge with stable tumors during ongoing DT. One patient was treated with mesenchymal stem cells and was progression-free during 56 months of follow-up. Two additional patients underwent surgery and ST for local recurrence. Among the 31 patients in Group 1, 21 patients were relapse-free at a median follow-up time of 20 months (range 6-43). Thus, all patients in Group 1 achieved disease control, seven of whom continued long-term DT at the time of publication of the articles. Interestingly, no local recurrences were observed among children treated with BPs for post-denosumab RH. Disease control in this group was maintained at a median follow-up time of 12.5 months (range 6-42). The difference in local control rates between the two groups was statistically significant when the χ2 test was used. Kaplan-Meier estimates show the same trend, but due to the small sample size and short follow-up in Group 2, this difference did not reach statistical significance (Figure 1).
Locally advanced and rapidly growing ABCs are difficult to treat surgically and require new approaches to facilitate surgery or achieve definitive control in inoperable cases. Percutaneous procedures can fail in non-stabilized ABCs that are highly cellular, contain numerous MNGCs, and have minimal blood-filled cystic components. Pathogenetic treatment targeting the RANKL-RANK axis in ABC has recently been reviewed[47,65-67]. DT is used in difficult situations such as axial tumors often associated with high surgical morbidity and in cases refractory to standard treatments. However, several issues are associated with DT, including limited, if any, direct antitumor effect on mutated cells, as documented in GCTB[68,69], and frequent disease reactivation after treatment discontinuation. Moreover, there are no established guidelines regarding the optimal duration of DT and potential toxicity with long time use is a concern. In addition, RANKL inhibition in children is associated with a high risk of RH and metaphyseal sclerosis, making prolonged DT highly problematic in skeletally immature patients. This may imply shorter treatment duration and careful considerations of the risk-benefit ratio as well as modalities to prevent RH[47,59].
Vanderniet et al[59] reported that the majority of ABC patients (65%) discontinued DT for various reasons, mainly due to complete clinical and imaging response. In the current meta-analysis, we observed that among patients receiving DT alone, the relapse rate after drug withdrawal was 32%. In cases of ABCs relapse, re-challenge with denosumab is reported to provide additional tumor control[54] and disease stabilization rates reached 90%; however, this comes at the cost of long-term DT and the risk of late side effects[70,71]. To avoid long-term toxicity, various strategies have been proposed for DT discontinuation, such as drug holidays until relapse, extended dosing intervals, second-look surgery or mesenchymal stem cells therapy[54].
Thus, in a significant proportion (68%), of ABC patients (Table 2), long-term local control was achieved exclusively after RANKL inhibition, but the exact mechanism of this effect remains unclear. These positive results should be interpreted with caution since the post-denosumab follow-up period was relatively short. We cannot exclude that DT may delay relapse rather than prevent it, and with longer follow-up, relapse rates may increase. Residual USP6+ cells[72] are likely responsible for tumor reactivation after DT discontinuation in ABC. This scenario was also described in GCTB, which demonstrates significant but reversible responses to denosumab[70,71,73], and a longer follow-up is needed to draw a definitive conclusion in ABC.
BPs have been used as first-line ART in patients with locally advanced/inoperable ABCs of the axial skeleton. To date, several case reports and case series have described this approach as definitive treatment with positive results[46,74-76]. Simm et al[75] treated an 8-year-old boy with locally advanced lumbosacral ABC with ZA infusions at 0.04 mg/kg. The patient received seven doses at 4-month intervals over 24 months. Dramatic improvement in symptoms was noted after the first infusion. Although residual cysts were visible on MRI after two years of treatment, the patient remained asymptomatic at 12-month follow-up after stopping the infusions. Seven patients with axial ABC were reported in two overlapping papers[46,74]. After BP therapy, which lasted from 3 to 16 months (median 8 months), significant symptomatic and radiological improvement was achieved. Notably, at a median follow-up of 14 months (range 0-42), no local relapses were reported. Kumar et al[76] reported a 16-year-old male with recurrent ABC of the Th9 vertebra. He underwent SAE alone, following which his radicular pain markedly improved. Subsequently, he was also treated with BPs therapy, intravenous ZA at a dose of 0.04 mg/ kg every 4-month interval, for 1 year (3 doses). At 2-year follow-up with MRI and CT studies, there was complete bone formation within the lytic areas and pain relief.
The sustained tumor control during and after BPs treatment suggests that these drugs have a broader spectrum of action than just osteoclastic inhibition, potentially through direct antitumor action on neoplastic cells. In vitro and in vivo studies have shown that BPs, and ZA in particular, are the only class of drugs directly affecting the biology of the neoplastic stromal cell population in GCTB[77-81]. In addition to its apoptotic effect, it is speculated that BPs may also induce, directly or indirectly, differentiation of GCTB stromal cells[82,84] and stimulate osteogenesis through the formation of apoptotic bodies, which have anabolic effects on bone in vivo[84,85], possibly via reverse signaling through the vesicular RANK-mRANKL axis and/or MNGC-derived apoptotic bodies[86]. Supporting these experimental data, a recent meta-analysis showed that ZA reduced the recurrence rate after surgical treatment of GCTB and was therefore recommended after aggressive extended curettage[87].
The currently available experimental and still limited clinical experience with the use of BPs for the treatment of ABC has demonstrated the ability of these drugs to improve the clinical condition and induce significant and sustained tumor ossification, likely, by promoting the apoptosis of USP6+ cells. In light of the substrate-dependent effectiveness of BPs, the significant imaging responses even in these purely osteolytic lesions warrant further explanation. While this aspect plays a role in GCTB, it is unclear whether the same applies to ABC.
Although DT is well tolerated in most cases, lifelong treatment is not considered an optimal and safe option in benign bone tumors. Some concerns are the possible side effects associated with the prolonged treatment such as arthralgia, chronic muscle pain, peripheral neuropathy, fatigue, skin rash, electrolyte disturbances, osteonecrosis of the jaw, atypical bone fractures, and malignant transformation[70,71,88]. Various approaches have been used to reduce the cumulative toxicity of long-term DT in ABC and GCTB, including treatment discontinuation[71], “drug holidays”[89], and increased dose intervals[90-94]. Another approach in this context consists of using BPs alongside denosumab. This was described in our previous case report of a patient with GCTB, who achieved long-term disease control after denosumab induction and ZA maintenance[48]. In that study, we hypothesized that after denosumab-induced tumor ossification, higher local concentrations of an amino-BP may play a protective role by accumulating substrate to target its biological effect on mutated residual cells.
Given the limited data regarding the use of RANKL inhibitors in combination with BPs in ABC, we sought additional evidence in the current literature to support the viability of this concept. We identified a specific subgroup of ABC patients who discontinued DT and subsequently received BPs for treatment or prevention of RH. Interestingly, we found no reported relapses among patients who received BPs after DT discontinuation. Notably, the majority of patients in this group (75%) were followed for at least one year. Del Sindaco et al[62] reported a case of an 8-year-old male who had a relapsed, non-resectable ABC of C4-C7. Denosumab 70 mg/m2 was administered for one year. After complete ossification of the tumor, DT was discontinued. Six months later, the patient developed severe RH that required the administration of one dose of ZA. Tumor evaluation performed 9 months after the end of denosumab showed an asymptomatic recurrence and DT was resumed for 2.5 years. Two episodes of hypercalcemia that occurred during the re-challenge were again treated with ZA. In order to prevent RH, six-monthly ZA were administered. At the last follow-up, 15 months after the last denosumab injection, the patient remained relapse-free. Vanderniet et al[59] reported another three post-DT cases with BP therapy aimed to prevent RH. The authors proposed to cease denosumab after 6 months if there was normalization of tumor metabolism on positron emission tomography/CT and spinal stability. They recommended that after DT discontinuation, BPs should be administered for at least 6 months. Progression-free intervals ranging from 12 to 42 months were achieved. A recent review published by Maximen et al[67] noted a statistically significant difference in relapse rates between adults and children. We speculate that the use of BPs in the pediatric population with RH may also have influenced these results. In the context of our analysis, this strategy can be rethought as a strategy to achieve the goal of definitive non-surgical control of inoperable ABC.
In support of this, we present a clinical observation from our own practice, which demonstrates that ossified post-DT ABCs accumulate considerable amounts of 99mTc-BP as seen in diagnostic bone scintigraphy, Figure 2C. Such a case could conceptually be regarded as a good candidate for BPs maintenance. It is conceivable that high concentrations of an amino-BP in the ossified lesion may provide an anti-tumor effect on residual USP6+ cells, leading to sustained or definitive local control.
We view these results with caution and acknowledge that additional cases with longer follow-ups are needed to strengthen this promising trend and draw definitive conclusions.
Regarding the duration of BP therapy or its discontinuation, we believe that at least two scenarios can be considered today:
Long-term therapy with BPs at subsequently extended intervals (every 6 or 12 months with daily calcium/vit. D3 supplementation).
Discontinuation of BP treatment in case of complete clinical and imaging response with mandatory follow-up examinations every 3 months during the first two years of observation.
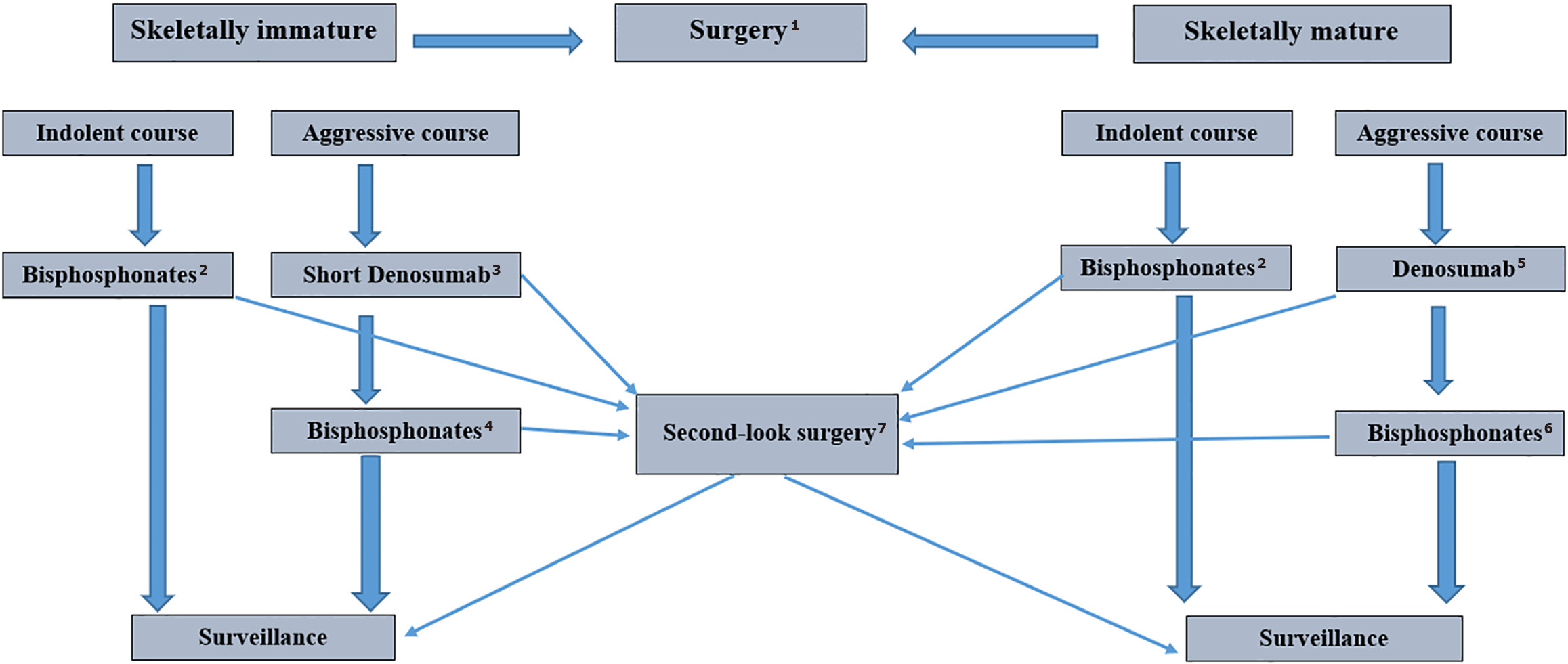
Given the therapeutic benefit of both groups of ART drugs, it is necessary to explore individual treatment strategies for inoperable ABC, taking the patient’s age, tumor location, skeletal stability, developmental phase, and clinical and imaging response into consideration. Based on the currently available clinical experience, we propose a potential treatment algorithm for locally advanced, progressive, and inoperable ABCs tailored to the age of the patient and clinical aggressiveness (Figure 4). Given their favorable safety profile when used in children, BPs can be regarded as a definitive treatment, except in cases when emergency surgery is required due to instability. In actively growing tumors with a minimal cystic component on MRI and when a rapid clinical response is required, treatment can be initiated with denosumab for a limited time, followed by BP maintenance. Continuing DT with prolonged intervals or stopping treatment with re-challenge in case of relapse seems less preferable due to the risk of complications. In skeletally mature patients with aggressive ABC, induction DT can initiate rapid tumor ossification. After approximately one year of treatment and/or when complete response is achieved based on clinical, neurological, imaging, metabolic, and biochemical data, maintenance therapy with BPs can be initiated.
The optimal duration of BP therapy remains to be determined by further studies. In our opinion, maintenance therapy could be administered for approximately 6 months, which would avoid the side effects of long-term antiresorptive treatment.
During continuous ART, periodic reassessment of tumor resectability and surgical risks is recommended. To further stimulate ossification of residual ABC elements and increase the hardness of newly formed bone, ART can be used after minimally invasive procedures such as cyst decompression, “curopsy”, ST, SAE, etc., Figure 3.
At the end of our analysis, we formulate and put forward for discussion some unanswered questions: How does the self-limiting nature of ABC interact with the pathogenetic effects of ART and how can these two elements be optimally combined to achieve the best effect? What are the optimal time intervals for RANKL inhibition and BPs administration? What is the best post-DT treatment option? BPs maintenance, continuation of DT at extended intervals, or DT drug holidays with re-challenge in case of relapse.
Our review and meta-analysis have several limitations. First, the eligible studies comprising our literature review mainly consisted of case reports and small case series with considerable heterogeneity in terms of patient population and treatment regimens. The majority of patients in the BPs group were children who received longer-term DT. At the same time, it should be emphasized that both of these factors did not have a significant effect on the clinical and imaging response to DT, which makes it possible to exclude their influence on the results of BP maintenance. Secondly, there are currently no standardized protocols for the use of BPs in RH. In addition, there are differences in the affinity of BPs for bone tissue or in the dosing regimens of BPs, which may affect the outcome. Third, most local recurrences occur during the first two years of follow-up after DT withdrawal. Given the median follow-up time in the BPs group of 12 months, the difference in local control rates requires cautious interpretation.
The strengths of our review include the identification of all published cases of ABC treated with denosumab to date, with careful analysis of complications, in particular RH, and their treatment with BPs. In addition, all patients with a short follow-up period were excluded from the analysis. To the best of our knowledge, this is the first meta-analysis to examine relapse rates after discontinuation of DT and the role of BPs for the treatment of RH in the context of their impact on local control in ABC.
The novelty of our study lies in having a “critical look” into previously published papers/clinical series to scrutinize the support for our combination strategy previously published for aggressive GCBT[48] as a novel option for severe ABC cases. The trend toward achieving local control in denosumab-treated ABC using BPs suggests that these drugs might play a protective role, probably due to better accumulation of last-generation (nitrogenous) BPs in the mineralizing newly formed bone matrix. The internalization by cells of TME may be sufficiently high to provide a direct and irreversible effect on residual USP6+ cells.
This review and meta-analysis highlight the outcomes after DT cessation in all patients with ABCs not eligible for surgery reported in the scientific literature to date. A significant proportion of patients benefit from DT alone when other treatments have failed. The mechanisms of disease control by RANKL inhibitors in ABC remain unclear. However, approximately one-third of patients experience tumor reactivation and require either denosumab re-treatment or second-attempt surgery. BPs used in post-denosumab ossifying lesions appear to play a protective role in ABC, presumably by acting directly on tumor elements containing residual USP6+ cells. ART should be adapted to the patient’s age, clinical aggressiveness, and the degree of stability of the involved skeletal compartment. The optimal approach for withdrawal from standard doses of denosumab remains to be determined. Our hypothesis-generating analysis provides a rationale for further experimental and clinical studies aimed at incorporating this promising two-stage combined ART strategy into the clinical management of difficult-to-treat ABCs.
| 1. | World Health Organization. Soft tissue and bone tumours. [cited 8 July 2025]. Available from: https://publications.iarc.fr/588. |
| 2. | Leithner A, Windhager R, Lang S, Haas OA, Kainberger F, Kotz R. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin Orthop Relat Res. 1999;176-179. [PubMed] |
| 3. | Althof PA, Ohmori K, Zhou M, Bailey JM, Bridge RS, Nelson M, Neff JR, Bridge JA. Cytogenetic and molecular cytogenetic findings in 43 aneurysmal bone cysts: aberrations of 17p mapped to 17p13.2 by fluorescence in situ hybridization. Mod Pathol. 2004;17:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Oliveira AM, Hsi BL, Weremowicz S, Rosenberg AE, Dal Cin P, Joseph N, Bridge JA, Perez-Atayde AR, Fletcher JA. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004;64:1920-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26:265-266. [PubMed] [DOI] [Full Text] |
| 6. | Balko J, Golas W, Kaspar L, Krskova L, Strnadova M, Kotis J, Zamecnik J. Novel and unusual USP6 fusion partners in aneurysmal bone cyst and their role in pathogenesis and histopathological evaluation of this disease. J Clin Pathol. 2025;78:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Blackburn PR, Davila JI, Jackson RA, Fadra N, Atiq MA, Pitel BA, Nair AA, VanDeWalker TJ, Hessler MG, Hovel SK, Wehrs RN, Fritchie KJ, Jenkins RB, Halling KC, Geiersbach KB. RNA sequencing identifies a novel USP9X-USP6 promoter swap gene fusion in a primary aneurysmal bone cyst. Genes Chromosomes Cancer. 2019;58:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Guseva NV, Jaber O, Tanas MR, Stence AA, Sompallae R, Schade J, Fillman AN, Miller BJ, Bossler AD, Ma D. Anchored multiplex PCR for targeted next-generation sequencing reveals recurrent and novel USP6 fusions and upregulation of USP6 expression in aneurysmal bone cyst. Genes Chromosomes Cancer. 2017;56:266-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Panagopoulos I, Gorunova L, Andersen K, Lobmaier I, Lund-Iversen M, Micci F, Heim S. Fusion of the Lumican (LUM) Gene With the Ubiquitin Specific Peptidase 6 (USP6) Gene in an Aneurysmal Bone Cyst Carrying a t(12;17)(q21;p13) Chromosome Translocation. Cancer Genomics Proteomics. 2020;17:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Šekoranja D, Zupan A, Mavčič B, Martinčič D, Salapura V, Snoj Ž, Limpel Novak AK, Pižem J. Novel ASAP1-USP6, FAT1-USP6, SAR1A-USP6, and TNC-USP6 fusions in primary aneurysmal bone cyst. Genes Chromosomes Cancer. 2020;59:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Warren M, Xu D, Li X. Gene fusions PAFAH1B1-USP6 and RUNX2-USP6 in aneurysmal bone cysts identified by next generation sequencing. Cancer Genet. 2017;212-213:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Oliveira AM, Perez-Atayde AR, Inwards CY, Medeiros F, Derr V, Hsi BL, Gebhardt MC, Rosenberg AE, Fletcher JA. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so-called secondary aneurysmal bone cysts. Am J Pathol. 2004;165:1773-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Lau AW, Pringle LM, Quick L, Riquelme DN, Ye Y, Oliveira AM, Chou MM. TRE17/ubiquitin-specific protease 6 (USP6) oncogene translocated in aneurysmal bone cyst blocks osteoblastic maturation via an autocrine mechanism involving bone morphogenetic protein dysregulation. J Biol Chem. 2010;285:37111-37120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Pringle LM, Young R, Quick L, Riquelme DN, Oliveira AM, May MJ, Chou MM. Atypical mechanism of NF-κB activation by TRE17/ubiquitin-specific protease 6 (USP6) oncogene and its requirement in tumorigenesis. Oncogene. 2012;31:3525-3535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Dabska M, Buraczewski J. Aneurysmal bone cyst. Pathology, clinical course and radiologic appearances. Cancer. 1969;23:371-389. [PubMed] [DOI] [Full Text] |
| 16. | Quick L, Young R, Henrich IC, Wang X, Asmann YW, Oliveira AM, Chou MM. Jak1-STAT3 Signals Are Essential Effectors of the USP6/TRE17 Oncogene in Tumorigenesis. Cancer Res. 2016;76:5337-5347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Madan B, Walker MP, Young R, Quick L, Orgel KA, Ryan M, Gupta P, Henrich IC, Ferrer M, Marine S, Roberts BS, Arthur WT, Berndt JD, Oliveira AM, Moon RT, Virshup DM, Chou MM, Major MB. USP6 oncogene promotes Wnt signaling by deubiquitylating Frizzleds. Proc Natl Acad Sci U S A. 2016;113:E2945-E2954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Oliveira AM, Chou MM. The TRE17/USP6 oncogene: a riddle wrapped in a mystery inside an enigma. Front Biosci (Schol Ed). 2012;4:321-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Kumta SM, Huang L, Cheng YY, Chow LT, Lee KM, Zheng MH. Expression of VEGF and MMP-9 in giant cell tumor of bone and other osteolytic lesions. Life Sci. 2003;73:1427-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Li L, Yang H, He Y, Li T, Feng J, Chen W, Ao L, Shi X, Lin Y, Liu H, Zheng E, Lin Q, Bu J, Zeng Y, Zheng M, Xu Y, Liao Z, Lin J, Lin D. Ubiquitin-Specific Protease USP6 Regulates the Stability of the c-Jun Protein. Mol Cell Biol. 2018;38:e00320-e00317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Stevens KJ, Stevens JA. Aneurysmal Bone Cysts. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 22. | Ye Y, Pringle LM, Lau AW, Riquelme DN, Wang H, Jiang T, Lev D, Welman A, Blobel GA, Oliveira AM, Chou MM. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-kappaB. Oncogene. 2010;29:3619-3629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Shinde A, Mehlman CT, Collins MH. Aneurysmal bone cysts express vascular markers. Pediatr Dev Pathol. 2006;9:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Pelle DW, Ringler JW, Peacock JD, Kampfschulte K, Scholten DJ 2nd, Davis MM, Mitchell DS, Steensma MR. Targeting receptor-activator of nuclear kappaB ligand in aneurysmal bone cysts: verification of target and therapeutic response. Transl Res. 2014;164:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Suster DI, Kurzawa P, Neyaz A, Jarzembowski JA, Lozano-Calderon S, Raskin K, Schwab J, Choy E, Chebib I, Deshpande V. Chondroblastoma Expresses RANKL by RNA In Situ Hybridization and May Respond to Denosumab Therapy. Am J Surg Pathol. 2020;44:1581-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Taylor RM, Kashima TG, Hemingway FK, Dongre A, Knowles HJ, Athanasou NA. CD14- mononuclear stromal cells support (CD14+) monocyte-osteoclast differentiation in aneurysmal bone cyst. Lab Invest. 2012;92:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Won KY, Kalil RK, Kim YW, Park YK. RANK signalling in bone lesions with osteoclast-like giant cells. Pathology. 2011;43:318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Yamagishi T, Kawashima H, Ogose A, Ariizumi T, Sasaki T, Hatano H, Hotta T, Endo N. Receptor-Activator of Nuclear KappaB Ligand Expression as a New Therapeutic Target in Primary Bone Tumors. PLoS One. 2016;11:e0154680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26:615-625. [PubMed] [DOI] [Full Text] |
| 30. | Lichtenstein L. Aneurysmal bone cyst.A pathological entity commonly mistaken for giant-cell tumor and occasionally for hemangioma and osteogenic sarcoma. Cancer. 1950;3:279-289. |
| 31. | Restrepo R, Zahrah D, Pelaez L, Temple HT, Murakami JW. Update on aneurysmal bone cyst: pathophysiology, histology, imaging and treatment. Pediatr Radiol. 2022;52:1601-1614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 32. | Hiemcke-Jiwa LS, van Gorp JM, Fisher C, Creytens D, van Diest PJ, Flucke U. USP6-Associated Neoplasms: A Rapidly Expanding Family of Lesions. Int J Surg Pathol. 2020;28:816-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Ibrahim T, Howard AW, Murnaghan ML, Hopyan S. Percutaneous curettage and suction for pediatric extremity aneurysmal bone cysts: is it adequate? J Pediatr Orthop. 2012;32:842-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Louahem D, Kouyoumdjian P, Ghanem I, Mazeau P, Perrochia H, L’kaissi M, Cottalorda J. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Malghem J, Maldague B, Esselinckx W, Noel H, De Nayer P, Vincent A. Spontaneous healing of aneurysmal bone cysts. A report of three cases. J Bone Joint Surg Br. 1989;71:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | McQueen MM, Chalmers J, Smith GD. Spontaneous healing of aneurysmal bone cysts. A report of two cases. J Bone Joint Surg Br. 1985;67:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Reddy KI, Sinnaeve F, Gaston CL, Grimer RJ, Carter SR. Aneurysmal bone cysts: do simple treatments work? Clin Orthop Relat Res. 2014;472:1901-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Park HY, Yang SK, Sheppard WL, Hegde V, Zoller SD, Nelson SD, Federman N, Bernthal NM. Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med. 2016;9:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 39. | Boriani S, Lo SF, Puvanesarajah V, Fisher CG, Varga PP, Rhines LD, Germscheid NM, Luzzati A, Chou D, Reynolds JJ, Williams RP, Zadnik P, Groves M, Sciubba DM, Bettegowda C, Gokaslan ZL; AOSpine Knowledge Forum Tumor. Aneurysmal bone cysts of the spine: treatment options and considerations. J Neurooncol. 2014;120:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Cottalorda J, Chotel F, Kohler R, de Gauzy JS, Louahem D, Lefort G, Dimeglio A, Bourelle S. Aneurysmal bone cysts of the pelvis in children: a multicenter study and literature review. J Pediatr Orthop. 2005;25:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Rossi G, Mavrogenis AF, Papagelopoulos PJ, Rimondi E, Ruggieri P. Successful treatment of aggressive aneurysmal bone cyst of the pelvis with serial embolization. Orthopedics. 2012;35:e963-e968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Varshney MK, Rastogi S, Khan SA, Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin Orthop Relat Res. 2010;468:1649-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Terzi S, Gasbarrini A, Fuiano M, Barbanti Brodano G, Ghermandi R, Bandiera S, Boriani S. Efficacy and Safety of Selective Arterial Embolization in the Treatment of Aneurysmal Bone Cyst of the Mobile Spine: A Retrospective Observational Study. Spine (Phila Pa 1976). 2017;42:1130-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Fritz J, Sonnow L, Morris CD. Adjuvant MRI-guided percutaneous cryoablation treatment for aneurysmal bone cyst. Skeletal Radiol. 2019;48:1149-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Docquier PL, Delloye C. Treatment of aneurysmal bone cysts by introduction of demineralized bone and autogenous bone marrow. J Bone Joint Surg Am. 2005;87:2253-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Kieser DC, Mazas S, Cawley DT, Fujishiro T, Tavolaro C, Boissiere L, Obeid I, Pointillart V, Vital JM, Gille O. Bisphosphonate therapy for spinal aneurysmal bone cysts. Eur Spine J. 2018;27:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Sandhu V, Ajit Singh V, Puri A. Exploring Denosumab’s potential in aneurysmal bone cyst treatment: A scoping review. J Orthop Surg (Hong Kong). 2024;32:10225536241297105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Machak GN, Bruland ØS, Romanova TN, Kovalev AV. Denosumab induction and Zoledronic acid maintenance therapy for recurrent unresectable giant cell tumour of the distal tibia: A case report with sustained tumour control after drug withdrawal. J Bone Oncol. 2024;45:100596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Ghermandi R, Terzi S, Gasbarrini A, Boriani S. Denosumab: non-surgical treatment option for selective arterial embolization resistant aneurysmal bone cyst of the spine and sacrum. Case report. Eur Rev Med Pharmacol Sci. 2016;20:3692-3695. [PubMed] |
| 50. | Ntalos D, Priemel M, Schlickewei C, Thiesen DM, Rueger JM, Spiro AS. Therapeutic Management of a Substantial Pelvic Aneurysmatic Bone Cyst Including the Off-Label Use of Denosumab in a 35-Year-Old Female Patient. Case Rep Orthop. 2017;2017:9125493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Kurucu N, Akyuz C, Ergen FB, Yalcin B, Kosemehmetoglu K, Ayvaz M, Varan A, Aydin B, Kutluk T. Denosumab treatment in aneurysmal bone cyst: Evaluation of nine cases. Pediatr Blood Cancer. 2018;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 52. | Patel RS, Dhamne CA, Gopinathan A, Kumar N, Kumar N. Denosumab: a potential treatment option for aneurysmal bone cyst of the atlas. Eur Spine J. 2018;27:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Palmerini E, Ruggieri P, Angelini A, Boriani S, Campanacci D, Milano GM, Cesari M, Paioli A, Longhi A, Abate ME, Scoccianti G, Terzi S, Trovarelli G, Franchi A, Picci P, Ferrari S, Leopardi MP, Pierini M. Denosumab in patients with aneurysmal bone cysts: A case series with preliminary results. Tumori. 2018;104:344-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 54. | Evangelisti G, Altorfer FCS, Falzetti L, Palmerini E, Griffoni C, Ghermandi R, Boriani S, Monetta A, Cesari M, Ibrahim T, Gasbarrini A. Denosumab Re-Challenge and Long-Term Efficacy for Aneurysmal Bone Cyst of the Spine: Enhanced Treatment Algorithm. J Clin Med. 2024;13:4522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Raux S, Bouhamama A, Gaspar N, Brugières L, Entz-Werlé N, Mallet C, Dijoud F, Gouin F, Marec-Bérard P. Denosumab for treating aneurysmal bone cysts in children. Orthop Traumatol Surg Res. 2019;105:1181-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Dürr HR, Grahneis F, Baur-Melnyk A, Knösel T, Birkenmaier C, Jansson V, Klein A. Aneurysmal bone cyst: results of an off label treatment with Denosumab. BMC Musculoskelet Disord. 2019;20:456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 57. | Sydlik C, Dürr HR, Pozza SB, Weißenbacher C, Roeb J, Schmidt H. Hypercalcaemia after treatment with denosumab in children: bisphosphonates as an option for therapy and prevention? World J Pediatr. 2020;16:520-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Kotaka S, Fujiwara Y, Ota R, Manabe H, Adachi N. The Efficacy of Denosumab in Treating Spinal Aneurysmal Bone Cyst: A Case Report. Cureus. 2023;15:e39954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 59. | Vanderniet JA, Wall CL, Mullins A, London K, Lim L, Hibbert S, Briody J, Padhye B, Poon M, Biggin A, Dalla-Pozza L, Munns CF. Denosumab for central giant cell granuloma in an Australian tertiary paediatric centre. Bone. 2022;159:116395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Upfill-Brown A, Bukata S, Bernthal NM, Felsenfeld AL, Nelson SD, Singh A, Wesseling-Perry K, Eilber FC, Federman NC. Use of Denosumab in Children With Osteoclast Bone Dysplasias: Report of Three Cases. JBMR Plus. 2019;3:e10210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 61. | Harcus M, Aldridge S, Abudu A, Jeys L, Senniappan S, Morgan H, Pizer B. The Efficacy of Denosumab in the Management of a Tibial Paediatric Aneurysmal Bone Cyst Compromised by Rebound Hypercalcaemia. Case Rep Pediatr. 2020;2020:8854441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Del Sindaco G, Berlanga P, Brugières L, Thebault E, Mantovani G, Wicart P, Linglart A. Mineral and Bone Consequences of High Dose Denosumab Therapy to Treat an Aneurysmal Bone Cyst, a Child Case Report. Front Endocrinol (Lausanne). 2021;12:698963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Deodati A, Fintini D, Levtchenko E, Rossi M, Ubertini G, Segers H, Battafarano G, Cappa M, Del Fattore A. Mechanisms of acute hypercalcemia in pediatric patients following the interruption of Denosumab. J Endocrinol Invest. 2022;45:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 64. | Gandolfi A, Shaaban S. Denosumab-Induced Rebound Hypercalcemia Treated With Bisphosphonates in a Pediatric Patient. JCEM Case Rep. 2023;1:luad133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 65. | Kulkarni AG, Patel A. Denosumab: A potential new treatment option for recurrent Aneurysmal Bone Cyst of the spine. SICOT J. 2019;5:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Giantini-Larsen AM, Chakravarthy VB, Barzilai O, Newman WC, Wexler L, Bilsky MH. The role of neoadjuvant denosumab in the treatment of aneurysmal bone cysts: a case series and review of the literature. J Neurosurg Pediatr. 2022;30:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 67. | Maximen J, Robin F, Tronchot A, Rossetti A, Ropars M, Guggenbuhl P. Denosumab in the management of Aneurysmal bone cyst. Joint Bone Spine. 2022;89:105260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Hayashida K, Kawabata Y, Kato I, Kamiishi T, Matsuo K, Takeyama M, Inaba Y. Clinical and pathological analysis of giant cell tumor of bone with denosumab treatment and local recurrence. J Orthop Sci. 2022;27:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 69. | Yang L, Zhang H, Zhang X, Tang Y, Wu Z, Wang Y, Huang H, Fu X, Liu J, Hogendoorn PCW, Cheng H. Clinicopathologic and molecular features of denosumab-treated giant cell tumour of bone (GCTB): Analysis of 21 cases. Ann Diagn Pathol. 2022;57:151882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 70. | Chawla S, Blay JY, Rutkowski P, Le Cesne A, Reichardt P, Gelderblom H, Grimer RJ, Choy E, Skubitz K, Seeger L, Schuetze SM, Henshaw R, Dai T, Jandial D, Palmerini E. Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20:1719-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 71. | Palmerini E, Chawla NS, Ferrari S, Sudan M, Picci P, Marchesi E, Leopardi MP, Syed I, Sankhala KK, Parthasarathy P, Mendanha WE, Pierini M, Paioli A, Chawla SP. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): For how long? Eur J Cancer. 2017;76:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 72. | Hung YP, Bredella MA, Lobmaier IVK, Lozano-Calderón SA, Rosenberg AE, Nielsen GP. Aneurysmal bone cyst and osteoblastoma after neoadjuvant denosumab: histologic spectrum and potential diagnostic pitfalls. APMIS. 2022;130:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 73. | Agarwal MG, Gundavda MK, Gupta R, Reddy R. Does Denosumab Change the Giant Cell Tumor Treatment Strategy? Lessons Learned From Early Experience. Clin Orthop Relat Res. 2018;476:1773-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 74. | Cornelis F, Truchetet ME, Amoretti N, Verdier D, Fournier C, Pillet O, Gille O, Hauger O. Bisphosphonate therapy for unresectable symptomatic benign bone tumors: a long-term prospective study of tolerance and efficacy. Bone. 2014;58:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Simm PJ, O’Sullivan M, Zacharin MR. Successful treatment of a sacral aneurysmal bone cyst with zoledronic acid. J Pediatr Orthop. 2013;33:e61-e64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Kumar BY, Thirumal R, Chander SG. Aneurysmal bone cyst of thoracic spine with neurological deficit and its recurrence treated with multimodal intervention - A case report. Surg Neurol Int. 2020;11:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Cheng YY, Huang L, Lee KM, Xu JK, Zheng MH, Kumta SM. Bisphosphonates induce apoptosis of stromal tumor cells in giant cell tumor of bone. Calcif Tissue Int. 2004;75:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Lau CP, Huang L, Tsui SK, Ng PK, Leung PY, Kumta SM. Pamidronate, farnesyl transferase, and geranylgeranyl transferase-I inhibitors affects cell proliferation, apoptosis, and OPG/RANKL mRNA expression in stromal cells of giant cell tumor of bone. J Orthop Res. 2011;29:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Lau CP, Huang L, Wong KC, Kumta SM. Comparison of the anti-tumor effects of denosumab and zoledronic acid on the neoplastic stromal cells of giant cell tumor of bone. Connect Tissue Res. 2013;54:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 80. | Shibuya I, Takami M, Miyamoto A, Karakawa A, Dezawa A, Nakamura S, Kamijo R. In Vitro Study of the Effects of Denosumab on Giant Cell Tumor of Bone: Comparison with Zoledronic Acid. Pathol Oncol Res. 2019;25:409-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 81. | Zwolak P, Manivel JC, Jasinski P, Kirstein MN, Dudek AZ, Fisher J, Cheng EY. Cytotoxic effect of zoledronic acid-loaded bone cement on giant cell tumor, multiple myeloma, and renal cell carcinoma cell lines. J Bone Joint Surg Am. 2010;92:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Wang X, Su P, Kang Y, Xu C, Qiu J, Wu J, Sheng P, Huang D, Zhang Z. Combination of Melatonin and Zoledronic Acid Suppressed the Giant Cell Tumor of Bone in vitro and in vivo. Front Cell Dev Biol. 2021;9:690502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 83. | Yang T, Zheng XF, Li M, Lin X, Yin QS. Stimulation of osteogenic differentiation in stromal cells of giant cell tumour of bone by zoledronic acid. Asian Pac J Cancer Prev. 2013;14:5379-5383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Ma Q, Liang M, Wu Y, Ding N, Duan L, Yu T, Bai Y, Kang F, Dong S, Xu J, Dou C. Mature osteoclast-derived apoptotic bodies promote osteogenic differentiation via RANKL-mediated reverse signaling. J Biol Chem. 2019;294:11240-11247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 85. | Stepan JJ, Alenfeld F, Boivin G, Feyen JH, Lakatos P. Mechanisms of action of antiresorptive therapies of postmenopausal osteoporosis. Endocr Regul. 2003;37:225-238. [PubMed] |
| 86. | Takegahara N, Kim H, Choi Y. RANKL biology. Bone. 2022;159:116353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 87. | Kumar A, Sinha S, Haider Y, Jameel J, Kumar S. Role of Zoledronic Acid Supplementation in Reducing Post-Surgical Recurrence of Giant Cell Tumor of Bone: A Meta-Analysis of Comparative Studies. Cureus. 2021;13:e16742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Luengo-Alonso G, Mellado-Romero M, Shemesh S, Ramos-Pascua L, Pretell-Mazzini J. Denosumab treatment for giant-cell tumor of bone: a systematic review of the literature. Arch Orthop Trauma Surg. 2019;139:1339-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 89. | Gaston CL, Grimer RJ, Parry M, Stacchiotti S, Dei Tos AP, Gelderblom H, Ferrari S, Baldi GG, Jones RL, Chawla S, Casali P, LeCesne A, Blay JY, Dijkstra SP, Thomas DM, Rutkowski P. Current status and unanswered questions on the use of Denosumab in giant cell tumor of bone. Clin Sarcoma Res. 2016;6:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 90. | Anusitviwat C, Ruangchainikom M, Korwutthikulrangsri E, Sutipornpalangkul W. Total neurological recovery after surgical decompression and treatment with denosumab of large unresectable spinal giant cell tumour expanding to mediastinum. BMJ Case Rep. 2022;15:e248837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 91. | De Vita A, Vanni S, Miserocchi G, Fausti V, Pieri F, Spadazzi C, Cocchi C, Liverani C, Calabrese C, Casadei R, Recine F, Gurrieri L, Bongiovanni A, Ibrahim T, Mercatali L. A Rationale for the Activity of Bone Target Therapy and Tyrosine Kinase Inhibitor Combination in Giant Cell Tumor of Bone and Desmoplastic Fibroma: Translational Evidences. Biomedicines. 2022;10:372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 92. | Jiang CY, Zhao L, Schuetze SM, Chugh R. Giant Cell Tumor of Bone: Effect of Longer Dosing Intervals of Denosumab on Tumor Control and Bone-related Complications. Oncologist. 2022;27:595-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Sambri A, Medellin MR, Errani C, Campanacci L, Fujiwara T, Donati D, Parry M, Grimer R. Denosumab in giant cell tumour of bone in the pelvis and sacrum: Long-term therapy or bone resection? J Orthop Sci. 2020;25:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 94. | Tsukamoto S, Tanaka Y, Mavrogenis AF, Kido A, Kawaguchi M, Errani C. Is Treatment with Denosumab Associated with Local Recurrence in Patients with Giant Cell Tumor of Bone Treated with Curettage? A Systematic Review. Clin Orthop Relat Res. 2020;478:1076-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |