Published online Aug 18, 2025. doi: 10.5312/wjo.v16.i8.106982
Revised: May 14, 2025
Accepted: July 15, 2025
Published online: August 18, 2025
Processing time: 149 Days and 7.6 Hours
Intertrochanteric fractures, prevalent among older adults, pose significant clinical challenges due to high morbidity, mortality, and complication rates. Despite advancements in surgical methods and implant technology, one-year mortality remains between 20% and 30%, with up to 20% of survivors requiring revision surgery due to mechanical complications. Accurate fracture reduction and precise implant positioning are critical determinants of successful outcomes. This review synthesizes current literature on key radiographic parameters essential for evaluating fracture reduction quality and implant placement in intertrochanteric fracture fixation. Standardized intraoperative imaging techniques, such as correct anteroposterior and lateral fluoroscopic views, are fundamental for identifying malalignment. Important radiographic measures include the neck shaft angle, greater trochanter orthogonal line, anterior cortical line, and calcar displacement assessment. Reduction quality indices, notably the Baumgaertner and Chang Reduction Quality Criteria, provide reliable frameworks for predicting mechanical complications. Additionally, implant positioning parameters—including tip-apex distance, Calcar-referenced tip-apex distance, Cleveland zones, and Parker’s ratio index—are discussed as predictors of mechanical complications. Enhanced understanding and application of these radiographic criteria can improve surgical precision, reduce complications, and ultimately optimize patient outcomes in intertrochanteric fracture management.
Core Tip: Intertrochanteric fractures are common in the older adult population and are typically treated surgically with a dynamic hip screw or an intramedullary nail with one-fifth of patients requiring reoperation. Accurate fracture reduction and optimal implant positioning are crucial for successful fracture healing and reducing reoperation rates. In this review, we examine the radiographic parameters emphasized in the current literature for assessing fracture reduction quality and implant positioning following intertrochanteric fracture fixation. This review offers orthopaedic surgeons an overview of key radiographic parameters for assessing intertrochanteric fracture fixation and enhancing risk factor identification.
- Citation: Wittauer M, Henry J, Sánchez-Rosenberg G, Lambers AP, Jones CW, Yates PJ. Evaluation of reduction quality and implant positioning in intertrochanteric fracture fixation: A review of key radiographic parameters. World J Orthop 2025; 16(8): 106982
- URL: https://www.wjgnet.com/2218-5836/full/v16/i8/106982.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i8.106982
Hip fractures in the older adult population pose a significant challenge in orthopaedic practice, primarily due to their high associated morbidity and mortality rates[1-4]. With the global population aging, the incidence of hip fractures, including intertrochanteric fractures is projected to rise to 21.3 million cases annually by 2050[5]. This surge will inevitably place considerable strain on healthcare systems worldwide[6].
Intertrochanteric fractures, occurring in adults over 60 years of age, are the most frequent types of hip fracture. These fractures are typically managed surgically using dynamic hip screws or intramedullary nails, with a trend towards intramedullary implants[7]. Despite advances in surgical techniques and implant design, outcomes remain sobering. One-year mortality rates for patients with intertrochanteric fractures range from 20% to 30%[1,2], while 7% to 20% of survivors require reoperation due to mechanical complications such as secondary fracture displacement or implant failure[8,9].
Kaufer et al[10] identified the major risk factors for mechanical complications as bone quality, fracture pattern, implant selection, implant positioning, and reduction quality. Bone quality and fracture patterns are inherent patient factors, but meticulous surgical technique emphasising optimal fracture reduction and precise implant positioning is essential in order to minimize complications[11-16]. Poor reduction quality not only increases the risk of mechanical complications but also negatively impacts functional recovery and long-term quality of life[17,18].
This minireview aims to present a concise compilation of key radiographic parameters used to evaluate fracture reduction and implant positioning in intertrochanteric fracture fixation.
A literature review was conducted using PubMed to identify outcome studies on intertrochanteric fractures that assessed the quality of reduction and implant positioning. Various measurement and assessment techniques were extracted and independently evaluated by two experienced surgeons (Wittauer M and Henry J) for their clinical relevance and practicality. In collaboration with the senior author (Yates P), a set of key radiographic parameters was then selected. This minireview did not adhere to the formal methodology of a standardized systematic review.
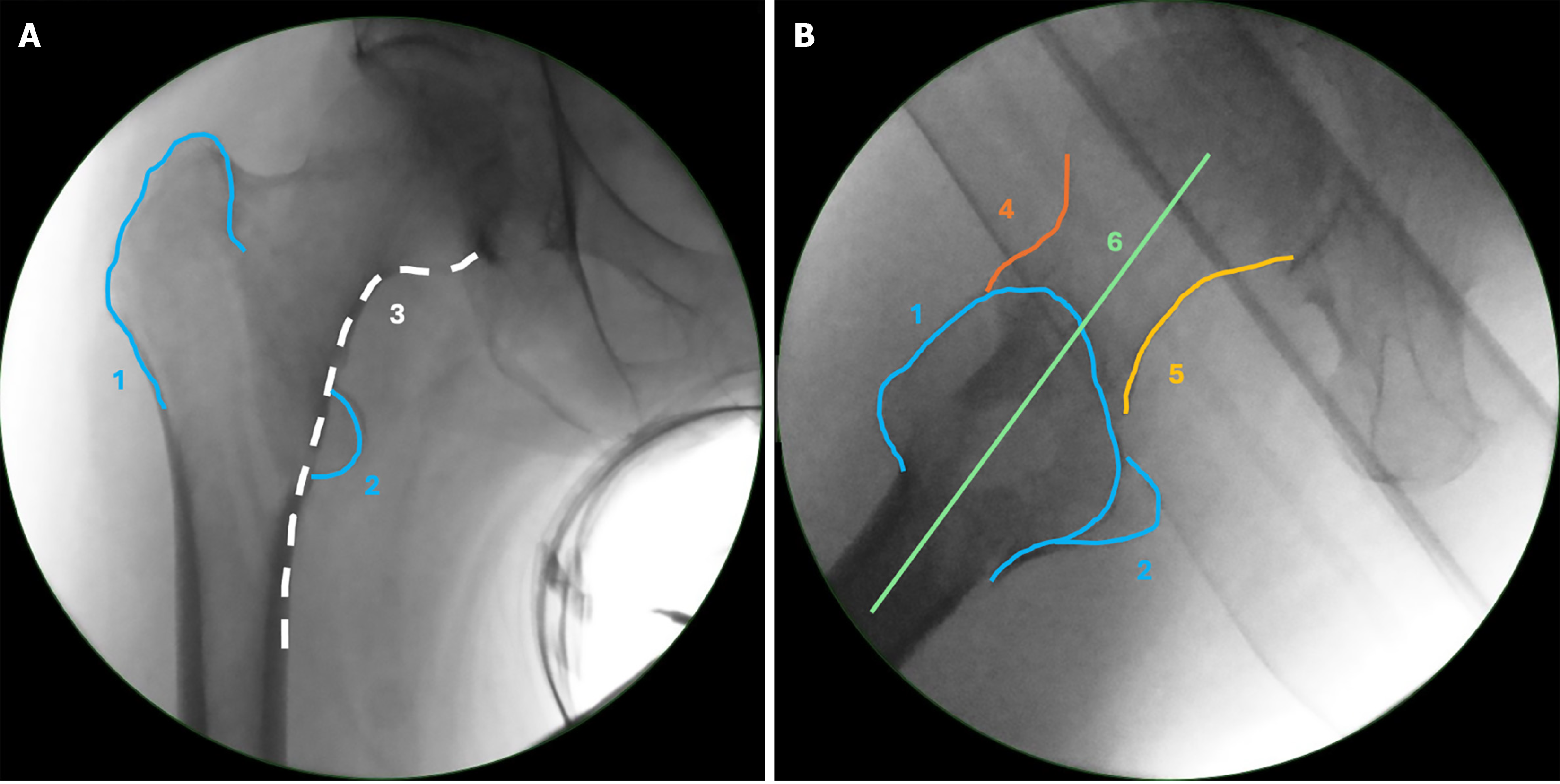
Optimal fracture reduction and accurate intraoperative imaging are closely interrelated[19]. Rikli et al[20,21] provided a detailed description of the anatomical structures required for correct imaging of the proximal femur. To obtain a correct anteroposterior (AP) image the fluoroscope is positioned perpendicular to the femoral shaft and coronal plane, with the leg internally rotated so that the patella faces upward. The entire femoral head with its joint space, the femoral neck, both trochanters, and the proximal portion of the shaft should be visible (Figure 1). This imaging modality allows observation of varus or valgus malalignment, rotational malalignment and coronal translational displacement.
To obtain a correct lateral image, the beam track should avoid the contralateral hip, aided by contralateral leg abduction, angled 30°-45° to the longitudinal axis of the injured leg. The fluoroscope must typically be positioned between 0° and 25° to the horizontal plane, depending on the patient’s femoral anteversion angle and limb rotation. A true lateral view is achieved when a straight diagonal line can be drawn from the middle of the femoral head, through the neck axis, and into the shaft (Figure 1). Correct fluoroscope positioning and proper fracture reduction are prerequisites for obtaining an accurate lateral view. In a well-reduced fracture on a true lateral view, the anterior line and posterior line appear continuous, without gaps or step-offs. The anterior line is traced along the anterior aspect of the femoral head, neck, and shaft in the lateral view, while the posterior line follows the corresponding posterior contour (Figure 1). Any displacement (ad latus deformity) between the head/neck fragment and the shaft results in step-offs along these lines and indicates sagittal plane translational displacement. Similarly, angulation, gaps, or openings in the anterior or posterior lines indicate rotational or angular deformities, such as external rotation/extension or internal rotation/flexion, respectively[20].
Achieving precise fluoroscopic AP and lateral views, as well as accurately assessing fracture displacement, is critical for correcting malalignment and achieving optimal reduction prior to implant positioning[19,22]. A good reduction may facilitate precise implant placement, as implants are designed to align with the anatomy of a reduced bone. Imaging, reduction, and implant placement exert reciprocal influence: Improving imaging may therefore enhance reduction accuracy[20].
The importance of varus and valgus malreduction: Varus malreduction has been considered more detrimental than valgus due to the biomechanical disadvantage of increased stress at the fracture site. This occurs in varus malreduction because the centre of rotation at the hip joint moves further from the intramedullary axis. As the reduction is moved from valgus to varus, a compressive force close to the axis of the intramedullary nail transitions to one of high bending stress[23]. Varus angulation has been associated with higher complication rates[16,23,24] and the theory is further supported by high union rates despite a high prevalence of valgus malreduction[25].
Measuring the neck shaft angle: Accurately assessing coronal plane angular reduction on radiographs is challenging, primarily due to the influence of femoral rotation. Variations in femoral positioning can alter the measured neck shaft angle (NSA) by up to 10 degrees on plain radiographs[26]. Excessive traction during surgery can also increase the NSA through valgus malreduction.
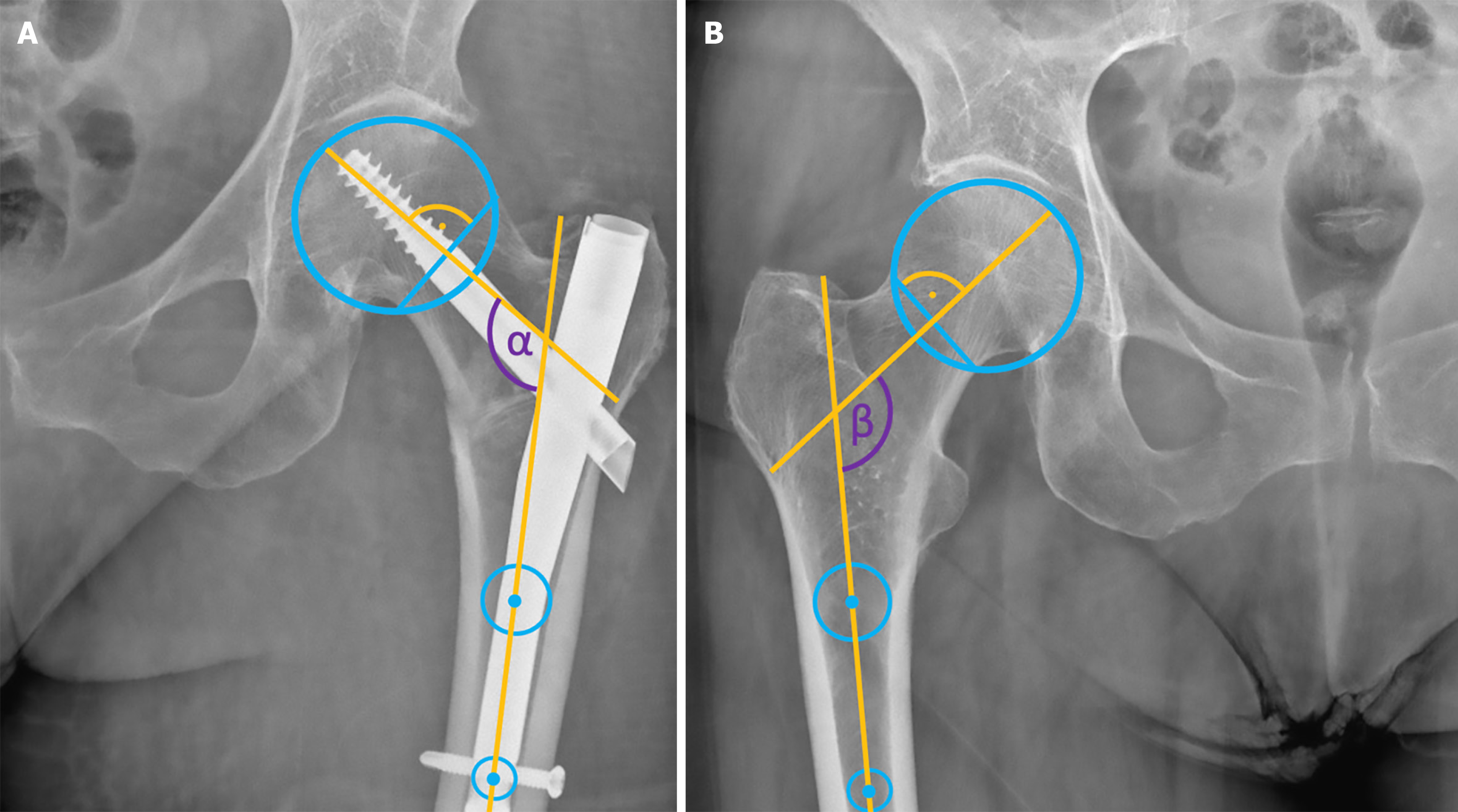
Many studies assess NSA by comparing side-to-side differences on plain radiographs, provided the contralateral hip is native and uninjured[24]. In our view, the NSA during and after cephalomedullary nailing should be compared to that of the uninjured hip, as it serves as a prognostic factor in the operative treatment of intertrochanteric fractures[27]. The method for measuring the NSA, originally described by Müller[28], remains valid today. For accurate measurement, the femoral head centre is determined using a circular template. The points where the template intersects the superior and inferior aspects of the femoral neck are connected to form a straight line. The femoral neck axis is then defined as the perpendicular line to this straight line that passes through the femoral head centre. The shaft axis is determined by two central points in the proximal femoral shaft, and the angle between these two axes represents the NSA (Figure 2).
However, surgeons must recognize the limitations of this approach, as it often involves comparing preoperative radiographs with intraoperative post-fixation images, where leg positioning is even less reliable. Additionally, individual patients may naturally exhibit small side-to-side differences in NSA due to congenital variations or acquired factors, with an average discrepancy of approximately 1.3 degrees[26].
Parry et al[29] described a method to calculate the true ‘corrected’ postoperative NSA using the known NSA of the implant. While valuable for research purposes, this method is time-consuming and impractical for routine clinical assessment. Another study classified any NSA below 160 degrees as malreduced[16]. However, this approach fails to account for individual variations in pre-fracture NSA, leading to both false positives and false negatives in coronal reduction assessment. Moreover, 160 degrees is an unusual threshold for defining a ‘normal’ NSA. Postoperatively, the NSA can continue to change, with Pajarinen et al[30] reporting a mean decrease of 5 degrees within the first six weeks after fixation.
Strictly speaking, while side-to-side comparisons of NSA are possible, quantifying and comparing NSA remains fundamentally flawed and inaccurate. The only truly reliable method for assessing NSA reduction quality would require access to recent pre-injury imaging of the fractured side, along with postoperative imaging where femoral rotation has been controlled or computed tomography has been used.
The greater trochanter orthogonal line & anterior cortical line: To assist with varus/valgus reduction Yoon et al[31] introduced a simple intraoperative technique in a CT-based radiographic cadaver study. The Greater Trochanter Orthogonal Line (GTOL) is an imaginary line drawn perpendicular to the anatomical axis of the femur, intersecting the tip of the greater trochanter. They proposed using the population mean of the GTOL passing 2 mm proximal to the centre of the femoral head as an intraoperative guide for varus/valgus reduction, demonstrating a consistent correlation with NSA. Using the GTOL for varus/valgus reduction assessment is easy and reliable, especially when compared to the healthy, uninjured side (Figure 3).
On the lateral image, another intraoperative reference line, the Anterior Cortical Line (ACL), has been described in the same article by Yoon et al[31]. This line, visible on a lateral projection of the proximal femur, follows the anterior cortex of the femur and extends proximally to intersect the femoral head. On average, the ACL passes 10 mm posterior to the centre of the femoral head (as seen on a lateral X-ray) and demonstrates a consistent correlation with femoral anteversion.
A concern with this methodology is that the reported figures represent mean values, whereas the range of results relative to the femoral head centre were broad (GTOL; -10 to 11 mm, ACL; -8 to 25 mm). Additionally, both lines will be influenced by limb positioning —GTOL by abduction/adduction and ACL by limb rotation. In our view, these measurements lack the precision needed to serve as definitive targets for individual patients in the absence of pre-injury imaging. However, they may still be useful in challenging cases where other landmarks are limited, providing a rough intraoperative guide based on the mean values or using the healthy, uninjured side as reference.
The wedge effect – iatrogenic varus: The “Wedge Effect” first described by O'Malley et al[32] is the distraction of an intertrochanteric fracture site on nail insertion causing lateralization of the femoral shaft and varus malreduction of the femoral neck. It likely occurs when either the bony path for the proximal femur is under-prepared and the reamer ‘falls in’ to the fracture rather than reaming a passage, or the nail is accidentally inserted into a new location in the fracture that wasn’t prepared at all. Mingo-Robinet et al[33] expand on this concept by describing two intraoperative fluoroscopic signs that help identify the occurrence of the Wedge Effect: The Medialized Greater Trochanter (GT) Sign and the Cross Wire Sign. Comparing fluoroscopy images saved prior to nail insertion of a reduced fracture with the final post-fixation radiographs quantified the incidence of the Wedge Effect, which was observed in 9% of their patients. The Medialized GT sign is when the GT is moved medially by the nail’s insertion such that it overlies the intramedullary canal or medial cortex of the femur instead of sitting more laterally as would be expected. The Cross Wire Sign is a crossing of the axis of the guidewire with an imaginary line that passes through the centre of the femoral neck to the centre of the femoral head (the ideal location of the guidewire). The “Wedge Effect” causes the two lines to cross because the separation of the fracture site by the intramedullary nail proximally tends to hinge the neck medially causing a varus deformity.
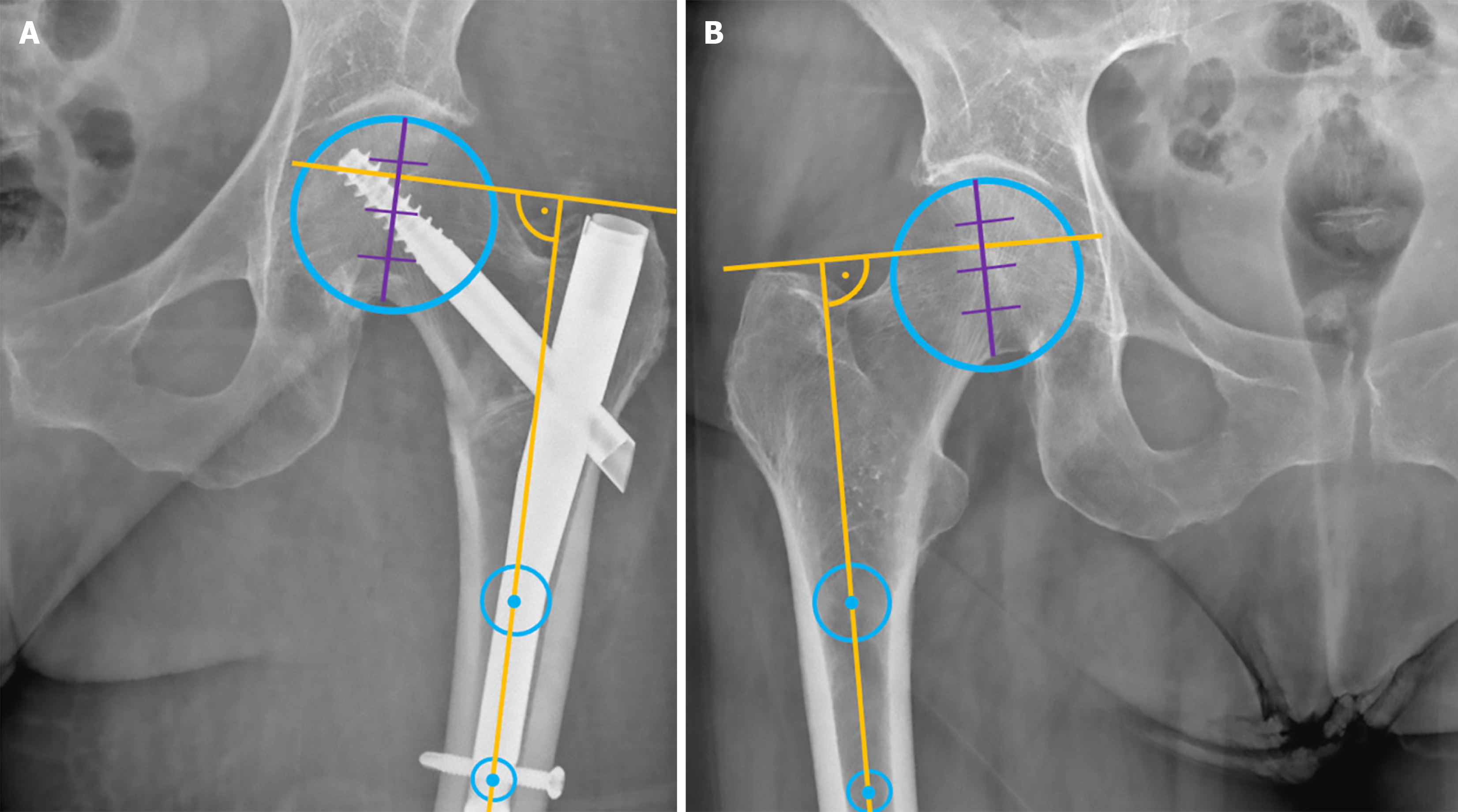
The importance of calcar displacement and its influence on anteromedial cortical support in intertrochanteric fractures has been shown to be essential for achieving secondary stability. Chang et al[34] established a classification system distinguishing between positive, neutral, and negative cortical support in AP radiographs. Positive cortical support was defined as the medial cortex of the head–neck fragment being positioned medially relative to the medial cortex of the shaft. If the neck cortex is located laterally to the shaft, it is considered negative, indicating no cortical buttress. A neutral position is defined when the two cortices align smoothly in contact. The same concept was adapted by Tsukada et al[35] for postoperative stability on lateral radiographs. Calcar displacement was again classified into three types based on the relative position of the proximal head–neck fragment and the distal anterior femoral cortex: Reduced, anteriorly displaced proximal fragment, and posteriorly displaced proximal fragment. Their findings indicate that positive or neutral cortical support in the AP view, combined with a reduced or anteriorly displaced fragment in the lateral view, is associated with improved secondary stability. In contrast, a negative and posteriorly displaced head–neck fragment relative to the shaft leads to excessive sliding, femoral neck shortening, loss of NSA, and poor outcomes, and should therefore be avoided[36]. Figure 4 illustrates an example of a negative and posteriorly displaced head–neck fragment in both the AP and lateral views.
Baumgaertner Reduction Quality Criteria: Based on a retrospective analysis of hip fractures treated with sliding hip screws Baumgaertner et al[37] introduced their reduction quality criteria in 1995. Fracture reduction quality, and its correlation with cut-out and failure, was also assessed using immediate postoperative radiographs and categorized as “good”, “acceptable”, or “poor” (Table 1). They observed a statistically significant increase in cut-out rates in the “poor” reduction group compared to the “good” reduction group. This suggests that achieving “good” reduction is associated with a lower risk of mechanical complications.
| I Alignment |
| a: AP view: Normal or slight valgus NSA |
| b: Lateral view: Less than 20° of angulation |
| II Displacement |
| a: AP view: Less than 4 mm of displacement of any fragments |
| b: Lateral view: Less than 4 mm of displacement of any fragments |
| Reduction quality: |
| Good: Both main criteria met |
| Acceptable: Only one main criterion met |
| Poor: Neither main criterion met |
Chang Reduction Quality Criteria: Chang et al[36] in 2015 introduced the concept of positive medial cortical support and used this to develop a quality of reduction index based on both alignment and fracture displacement. For the NSA slight valgus or anatomical alignment is desired on AP view, and a range from 160-180 degrees on the lateral view. Fragment displacement focused on their anteromedial cortical support theory. These criteria are used to classify the quality of fracture reduction as “excellent”, “acceptable”, or “poor” (Table 2).
| I Alignment |
| a: AP view: Normal or slight valgus NSA |
| b: Lateral view: Less than 20° of angulation |
| II Displacement |
| a: AP view: Neutral or positive medial cortical support |
| b: Lateral view: Smooth anterior cortical contact |
| Reduction quality: |
| Excellent: All four sub criteria met |
| Acceptable: Two or three sub criteria met |
| Poor: One or no sub criterion met |
The authors emphasise the importance of achieving positive medial cortical support, as it contributes significantly to fracture stability and healing. While valgus alignment is important, the authors stress that it's not synonymous with positive medial cortical support. The two should be assessed independently. The results of their study showed that patients with “excellent” or “acceptable” reductions had significantly better functional outcomes than those with “poor” reductions. Additionally, the tip-apex distance (TAD) was significantly smaller in the “excellent” and “acceptable” reduction groups compared to the “poor” reduction group at all follow-up points. This indicates the relationship between the quality of reduction and optimal implant placement.
Comparison of Baumgaertner Reduction Quality Criteria and Chang Reduction Quality Criteria: Mao et al[38] directly compared these two indices for assessing the quality of fracture reduction in a retrospective review of 127 proximal femur fractures treated with short intramedullary nailing. The authors applied the Baumgaertner Reduction Quality Criteria (BRQC) and Chang Reduction Quality Criteria (CRQC) to their cohort and looked for correlation with mechanical complications. They also analysed the TAD and the Cleveland zones for femoral neck component position. Mechanical complications were observed in 20.5% of patients. The most common complications were varus displacement, excessive lateral migration, as well as implant failure.
While the initial univariate analysis showed significant associations with both BRQC and CRQC, as well as TAD and femoral neck component position, further multivariate analysis to account for confounding variables showed that only CRQC and TAD were independent predictors for mechanical complications. They also found much greater interobserver reliability with the CRQC compared to BRQC. The authors acknowledged the retrospective nature and small sample size but concluded that the CRQC is a reliable tool for both clinical assessments and research applications, urging its adoption over the BRQC in the evaluation of trochanteric fracture reduction quality.
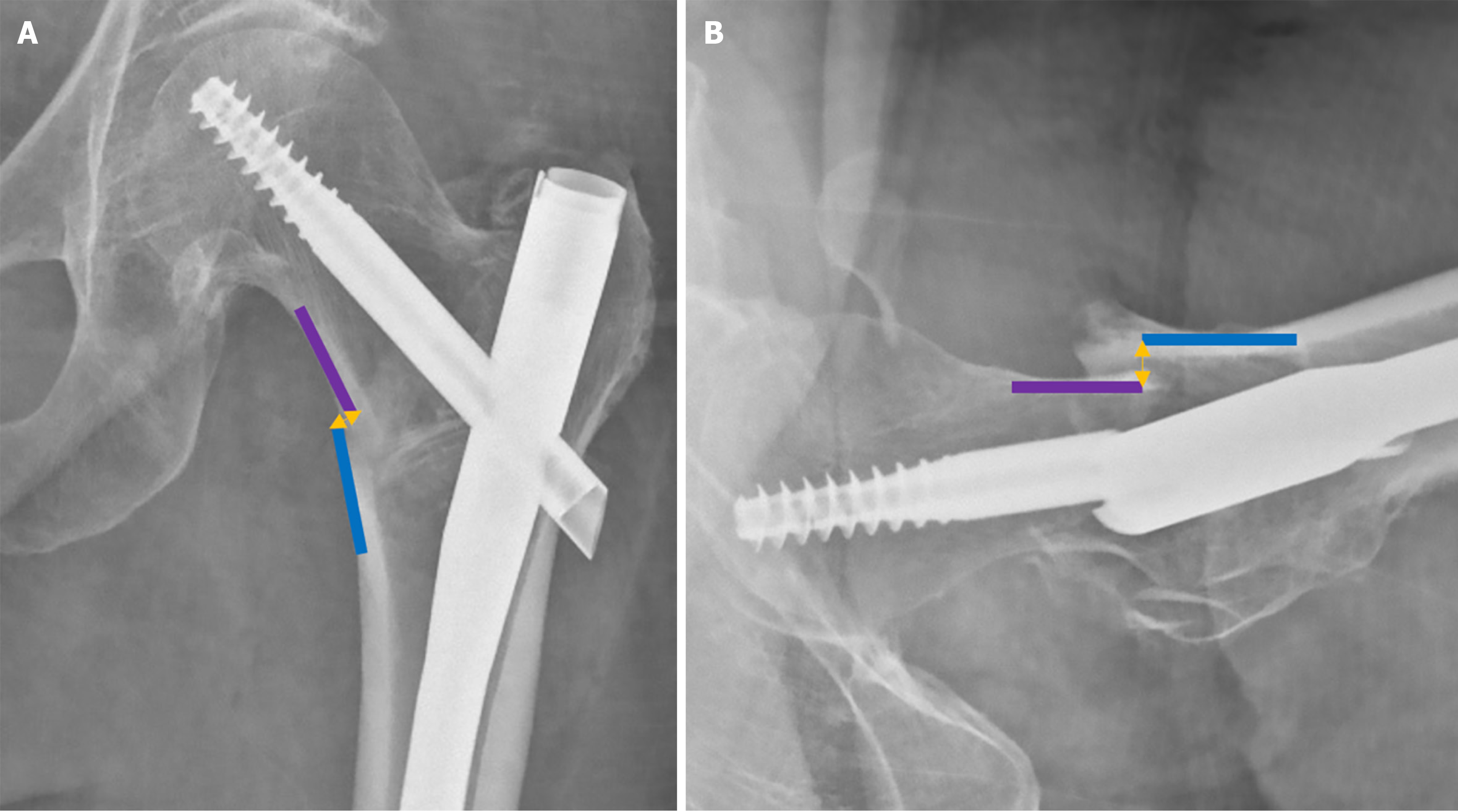
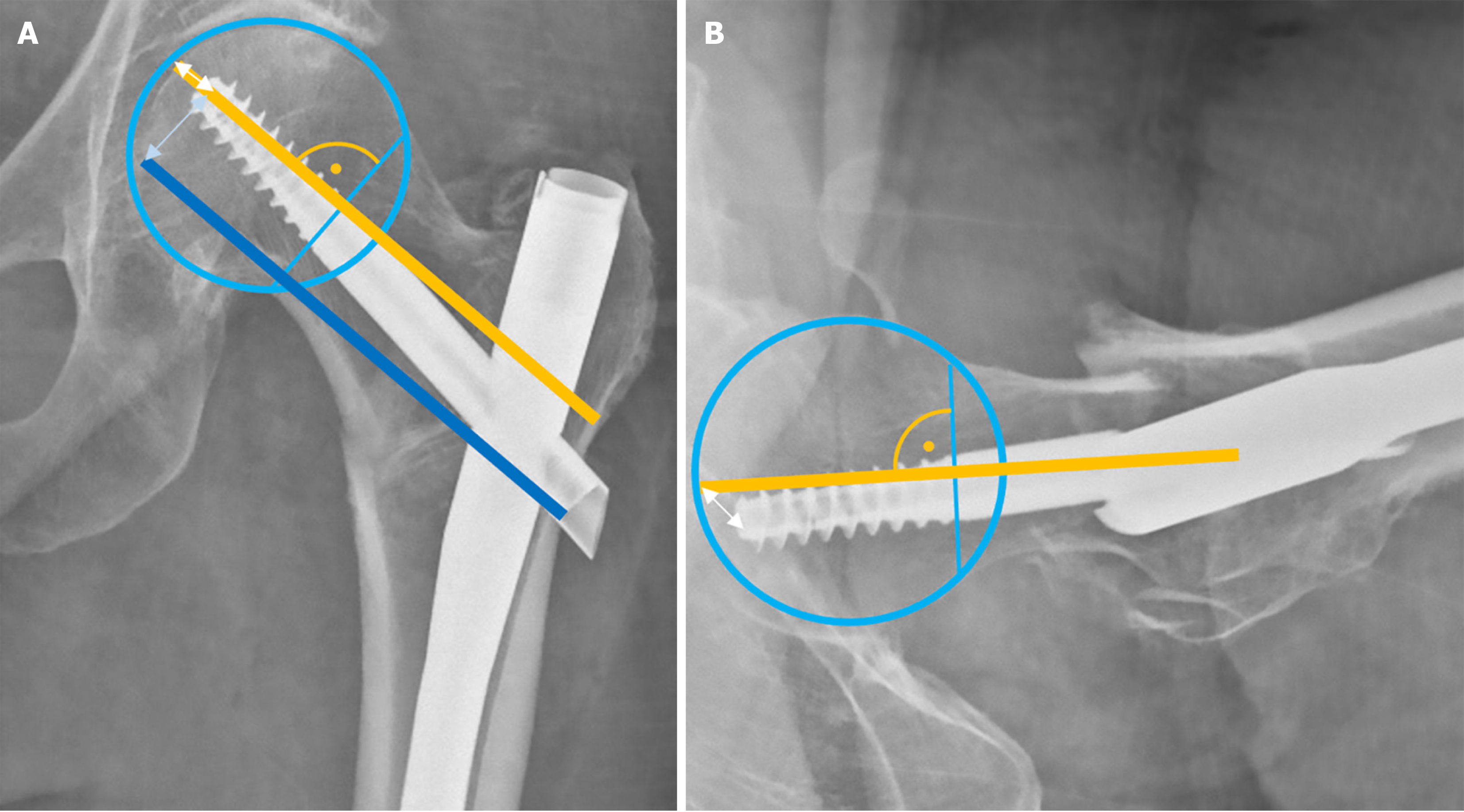
The concept of TAD was introduced by Baumgaertner et al[37] in 1995 to help to prevent nail cut-out, defined as the collapse of the neck-shaft angle into varus, resulting in the protrusion of the femoral neck component (screw or blade) from the femoral head[39]. Reported cut-out rates generally range from 2% to 15%[40-42]. TAD establishes a “no-go zone” for the placement of the intertrochanteric femoral neck component tip. It is calculated as the sum of the distances from the tip of the femoral neck component to the apex of the femoral head on both AP and lateral radiographs, measured along the femoral neck component’s central axis (Figure 5). A TAD of > 25 mm is associated with a significantly higher risk of femoral neck component cut-out[37,43].
Although a recent review suggests that computer-assisted navigation systems may help reduce TAD and improve lag screw positioning[44], several prospective studies have not demonstrated a consistent benefit in optimizing TAD or reducing outliers[45,46]. Similarly, evidence supporting robot-assisted orthopaedic trauma surgery remains limited[47]. While future advances in artificial intelligence may enhance these technologies and help with real-time reduction assessment, structured education and training continue to provide the most reliable improvements, particularly for less experienced surgeons[48].
Kuzyk et al[49] expanded on the TAD concept, recognizing that the calcar region provides greater resistance to screw breakage and varus collapse due to its higher bone density. The main difference lies in the measurement of the distance in the AP radiograph, where Calcar-referenced tip-apex distance (CalTAD) is measured from the apex of the screw or blade to a line adjacent the calcar (Figure 5), thus favouring a more inferior placement of the femoral neck component[50]. The TAD measurement of the lateral radiograph is then added. Although definitive cut-off values have not been set, cut-out has been more commonly observed in cases with a CalTAD exceeding 25 mm[40,51,52].
In a direct comparison, Kashigar et al[50] found a higher predictive value of CalTAD over TAD. Garabano et al[52] found CalTAD to be the only significant predictor of cut-out when greater than 25 mm in a multivariate analysis. Conversely, Lopes-Coutinho et al[53] found no superiority of CalTAD over TAD. Murena et al[54] showed that both TAD and CalTAD were significant predictors of cut out, but only TAD was found significant in multivariate analysis.
Although TAD and CalTAD are useful parameters for determining optimal femoral neck component positioning, high-quality evidence remains limited. Current studies report varying optimal cutoff values, making it difficult to establish definitive recommendations. However, most studies suggest that a TAD or CalTAD exceeding 25 millimetres should be avoided.
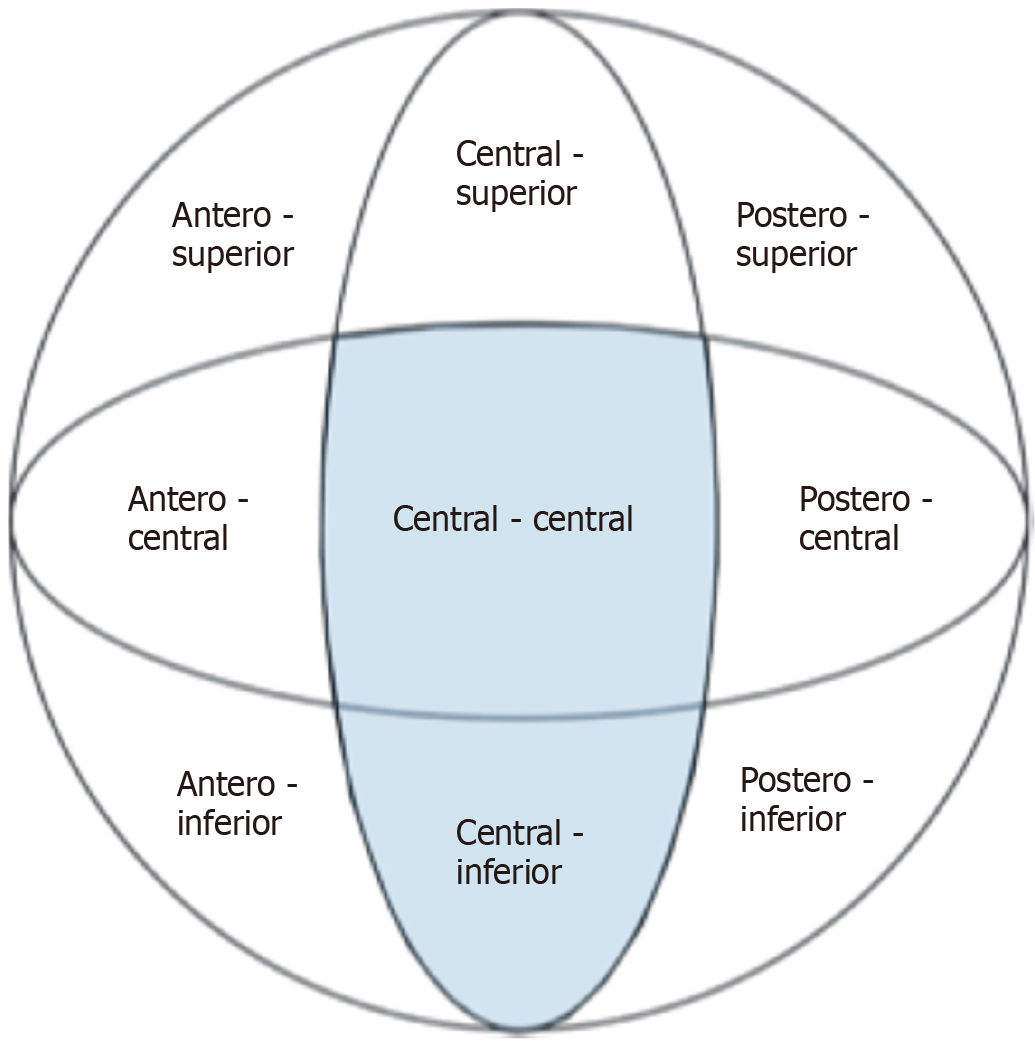
The Cleveland zones system, first introduced by Cleveland et al[55] in 1959, provides a standardized framework for categorizing implant placement. This system divides the femoral head into nine zones: Three in the superior–inferior direction, assessed on an AP radiograph, and three in the anterior–posterior direction, assessed on a lateral radiograph (Figure 6). Generally, a centre-centre or central-inferior position of the femoral neck component within the femoral head is associated with a lower risk of cut-out[37,49,56-58].
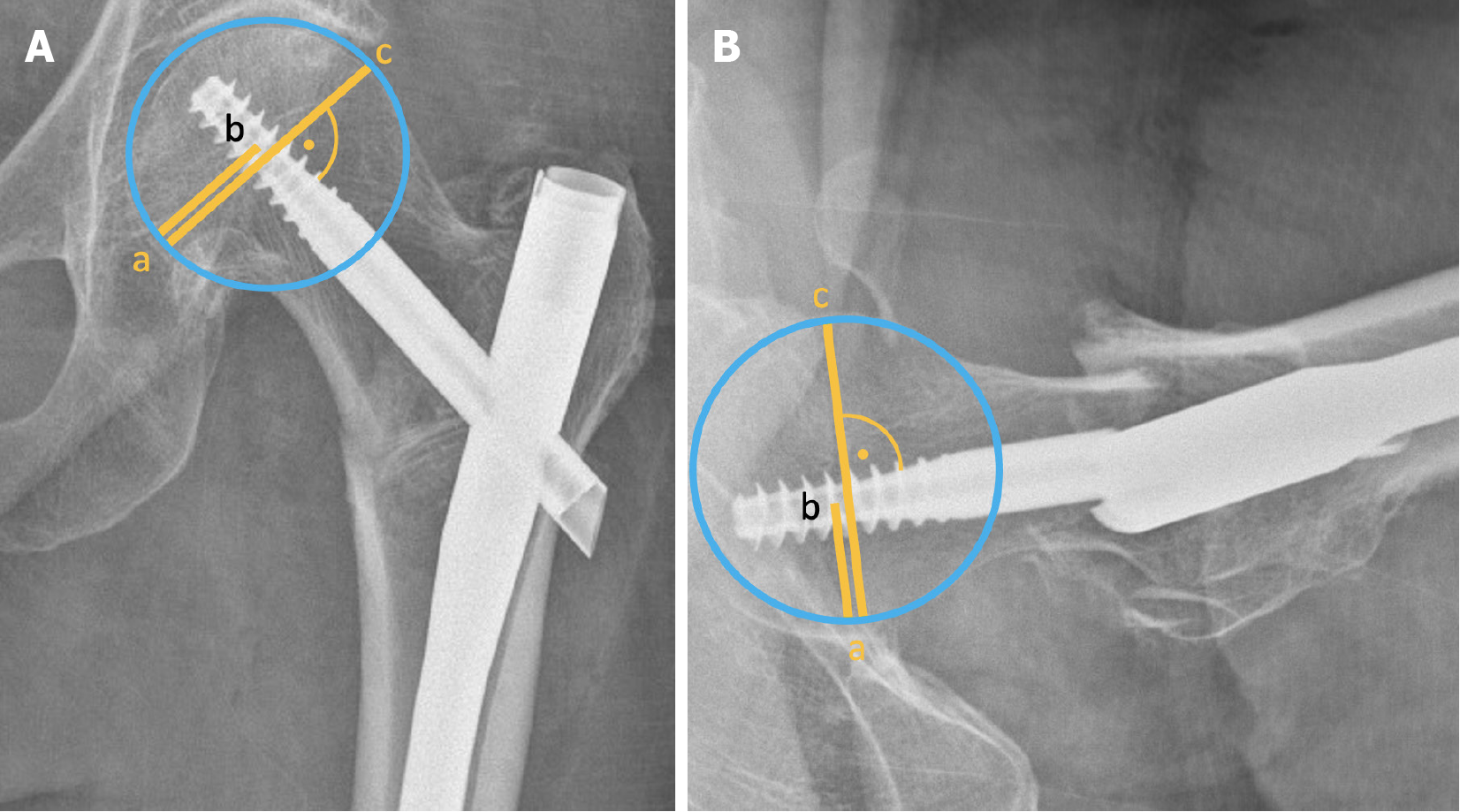
First published in 1992, the Parker's ratio index assesses the position of the femoral neck component (screw or blade) within the femoral head[59]. It calculates the ratio of the distance from the centre of the femoral neck component to the inferior (or posterior) cortex, divided by the femoral head diameter, multiplied by 100 (Figure 7). This results in a value between 0 and 100, indicating the component's position from inferior/posterior (0) to superior/anterior (100) in both the AP and lateral views[60]. The reported cutoff values for the Parker's ratio index for femoral component cut-out range from 58 to 65[60,61]. Several studies have shown that superiorly or anteriorly placed femoral neck components, analogous to the Cleveland zones, are associated with a higher risk of mechanical complications[50,52].
Table 3 presents an overview of the key radiographic parameters used to assess fracture reduction and implant positioning.
| Parameter | Imaging modality | Strengths | Limitations | Recommendation |
| Parameters for quality of reduction | ||||
| Neck shaft angle | AP X-ray | Simple, familiar measure; side-to-side comparison possible | Affected by femoral rotation and traction; intraoperative leg positioning alters measurement; side-to-side variation exists naturally | NSA best compared to uninjured side; not reliably accurate without pre-injury imaging; avoid varus malreduction |
| Greater trochanter orthogonal line | AP X-ray | Easy intraoperative estimation; correlates with NSA; uses anatomical landmarks | Influenced by abduction/adduction; population-based average has wide range | Use as a rough intraoperative guide; more useful with contralateral comparison |
| Anterior cortical line | Lateral X-ray | Consistent mean correlation with femoral anteversion; helps identify rotational issues | Affected by limb rotation; broad range around mean; limited individual specificity | Can assist intraoperatively when other landmarks are limited |
| Calcar Displacement | AP and lateral X-ray | Highlights medial cortical support; distinguishes positive/neutral/negative support | Requires high-quality views; subjective classification | Positive or neutral support (AP) + reduced/anterior displacement (lateral) associated with better outcomes |
| Wedge Effect signs (Medialized GT, Cross Wire Sign) | Fluoroscopy (Intraoperative) | Identifies iatrogenic varus malreduction during nail insertion | Requires saved pre- and post-insertion images for comparison | Avoid medialization and improper entry; contributes to varus malalignment |
| Baumgaertner Reduction Quality Criteria | AP and lateral X-ray | Simple alignment/displacement criteria | Less interobserver reliability; not predictive after multivariate analysis | Achieving 'good' BRQC predicts fewer mechanical complications, but CRQC is preferred |
| Chang Reduction Quality Criteria | AP and lateral X-ray | Includes medial cortical support; better interobserver reliability; predictive of outcomes | Slightly more complex; requires careful assessment of cortical contact | Recommended over BRQC; better predictor of complications and reduction quality |
| Parameters for implant positioning | ||||
| TAD | AP and lateral X-ray | Easy to measure; well-established cut-off; widely used | Influenced by positioning; variability in measurement | TAD > 25 mm associated with increased cut-out risk; aim for < 25 mm |
| Calcar-Referenced TAD | AP and lateral X-ray | Accounts for stronger calcar bone; inferior placement favoured | Cut-off values vary; some studies show limited superiority over TAD | CalTAD > 25 mm linked to cut-out; may be more predictive than TAD, but not conclusively superior |
| Cleveland zones | AP and lateral X-ray | Standardized 9-zone grid; easy to visualize component position | No direct distance measurement; qualitative zone allocation | Centre-centre and central-inferior positions have lowest cut-out risk |
| Parker’s ratio index | AP and lateral X-ray | Quantitative position assessment; applicable in both planes | Calculation required; multiple cut-off values proposed (58–65) | Higher index (superior/anterior) linked to increased complications; lower index < 60 (posterior/inferior) preferred |
This review of radiographic parameters for evaluating fracture reduction and implant positioning in intertrochanteric fracture fixation highlights several key points: Standardized imaging techniques are crucial for accurate assessment of fracture reduction and implant positioning. Proper fluoroscopic views in both AP and lateral planes are essential for identifying and correcting malalignment. NSA restoration is critical, with varus malreduction being particularly detrimental. However, accurately measuring NSA remains challenging due to the influence of femoral rotation and individual anatomical variations. Novel radiographic aids such as the GTOL and ACL offer potential simple intraoperative guides for reduction assessment. The "Wedge Effect" is an important iatrogenic complication to recognize and avoid during nail insertion, as it can lead to varus malreduction. Calcar displacement and anteromedial cortical support play crucial roles in achieving secondary stability and predicting outcomes. Quality of fracture reduction indices, particularly the CRQC, show promise in standardizing reduction assessment and predicting mechanical complications. Implant positioning evaluation tools such as TAD, CalTAD, Cleveland zones, and Parker's ratio index provide valuable guidance for optimal femoral neck component placement within the femoral head. Ultimately, the successful management of intertrochanteric fractures requires a nuanced understanding of these radiographic parameters, combined with careful preoperative planning, meticulous surgical technique, and individualized patient care. As the incidence of these fractures continues to rise, ongoing refinement of assessment tools and surgical techniques will be crucial in improving outcomes and reducing the significant morbidity and mortality associated with these challenging injuries.
| 1. | Klop C, Welsing PM, Cooper C, Harvey NC, Elders PJ, Bijlsma JW, Leufkens HG, de Vries F. Mortality in British hip fracture patients, 2000-2010: a population-based retrospective cohort study. Bone. 2014;66:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1215] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 3. | Walter N, Szymski D, Kurtz S, Alt V, Lowenberg DW, Lau E, Rupp M. Factors associated with mortality after proximal femoral fracture. J Orthop Traumatol. 2023;24:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Nazrun AS, Tzar MN, Mokhtar SA, Mohamed IN. A systematic review of the outcomes of osteoporotic fracture patients after hospital discharge: morbidity, subsequent fractures, and mortality. Ther Clin Risk Manag. 2014;10:937-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1375] [Cited by in RCA: 1485] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 6. | Harris E, Clement N, MacLullich A, Farrow L. The impact of an ageing population on future increases in hip fracture burden. Bone Joint J. 2024;106-B:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 7. | T J, Kwek EBK. Are Intertrochanteric Fractures Evolving? Trends in the Elderly Population over a 10-Year Period. Clin Orthop Surg. 2022;14:13-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Tucker A, Donnelly KJ, Rowan C, McDonald S, Foster AP. Is the Best Plate a Nail? A Review of 3230 Unstable Intertrochanteric Fractures of the Proximal Femur. J Orthop Trauma. 2018;32:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Palm H, Jacobsen S, Sonne-Holm S, Gebuhr P; Hip Fracture Study Group. Integrity of the lateral femoral wall in intertrochanteric hip fractures: an important predictor of a reoperation. J Bone Joint Surg Am. 2007;89:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Kaufer H. Mechanics of the Treatment of Hip Injuries. Clin Orthop Relat R. 1980;146:53-61. [DOI] [Full Text] |
| 11. | Khanna V, Tiwari M. Significance of Tip Apex Distance in Intertrochanteric Fracture femur managed with Proximal femoral nailing. Orthop Traumatol Surg Res. 2021;107:103009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Yoon YC, Oh CW, Sim JA, Oh JK. Intraoperative assessment of reduction quality during nail fixation of intertrochanteric fractures. Injury. 2020;51:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Gordon M, Berntsson PO, Sjölund E, Demir Y, Hedbeck CJ, Stark A, Sköldenberg O. Loss of offset after pertrochanteric hip fractures affects hip function one year after surgery with a short intramedullary nail. A prospective cohort study. Int Orthop. 2016;40:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Paul O, Barker JU, Lane JM, Helfet DL, Lorich DG. Functional and radiographic outcomes of intertrochanteric hip fractures treated with calcar reduction, compression, and trochanteric entry nailing. J Orthop Trauma. 2012;26:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Lobo-Escolar A, Joven E, Iglesias D, Herrera A. Predictive factors for cutting-out in femoral intramedullary nailing. Injury. 2010;41:1312-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Bojan AJ, Beimel C, Taglang G, Collin D, Ekholm C, Jönsson A. Critical factors in cut-out complication after Gamma Nail treatment of proximal femoral fractures. BMC Musculoskelet Disord. 2013;14:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 17. | Li J, Zhang L, Zhang H, Yin P, Lei M, Wang G, Wang S, Tang P. Effect of reduction quality on post-operative outcomes in 31-A2 intertrochanteric fractures following intramedullary fixation: a retrospective study based on computerised tomography findings. Int Orthop. 2019;43:1951-1959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Studer P, Suhm N, Wang Q, Rosenthal R, Saleh HA, Jakob M. Displaced trochanteric fragments lead to poor functional outcome in pertrochanteric fractures treated by cephalomedullary nails. Injury. 2015;46:2384-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Devitt BM, O'Byrne JM. I can C clearly now the rail has gone! Injury. 2007;38:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Rikli D, Goldhahn S, Blauth M, Mehta S, Cunningham M, Joeris A; PIP Study group. Optimizing intraoperative imaging during proximal femoral fracture fixation - a performance improvement program for surgeons. Injury. 2018;49:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Rikli D, Blauth M, Mehta S, Seibert F. Intraoperative imaging of the proximal femur. In: Baumgaertner M, Nousiainen MT, editors. AO Surgery Reference, 2022. [cited 23 June 2025]. Available from: https://surgeryreference.aofoundation.org/orthopedic-trauma/adult-trauma/proximal-femur/further-reading/intraoperative-imaging-of-the-proximal-femur. |
| 22. | Herman A, Landau Y, Gutman G, Ougortsin V, Chechick A, Shazar N. Radiological evaluation of intertrochanteric fracture fixation by the proximal femoral nail. Injury. 2012;43:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Marmor M, Liddle K, Buckley J, Matityahu A. Effect of varus and valgus alignment on implant loading after proximal femur fracture fixation. Eur J Orthop Surg Traumatol. 2016;26:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Ciufo DJ, Zaruta DA, Lipof JS, Judd KT, Gorczyca JT, Ketz JP. Risk Factors Associated With Cephalomedullary Nail Cutout in the Treatment of Trochanteric Hip Fractures. J Orthop Trauma. 2017;31:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Kusnezov N, Prabhakar G, Vanden Berge D, Dabash S, Thabet AM, Abdelgawad A. Incidence, predictors, and impact of valgus reduction of traumatic intertrochanteric femoral fractures (OTA 31A1-3) treated with the helical blade system: Is anatomic reduction necessary? A retrospective case series. Curr Orthop Pract. 2020;31:41-47. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Kay RM, Jaki KA, Skaggs DL. The effect of femoral rotation on the projected femoral neck-shaft angle. J Pediatr Orthop. 2000;20:736-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Jiamton C, Boernert K, Babst R, Beeres FJP, Link BC. The nail-shaft-axis of the of proximal femoral nail antirotation (PFNA) is an important prognostic factor in the operative treatment of intertrochanteric fractures. Arch Orthop Trauma Surg. 2018;138:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Müller ME. Die Hüftnahen Femurosteotomien unter Berücksichtigung der Form, Funktion und Beanspruchung des Hüftgelenkes. Stuttgart: Thieme, 1957. |
| 29. | Parry JA, Barrett I, Schoch B, Cross W, Yuan B. Validation of Neck-Shaft Angle Correction After Cephalomedullary Nail Fixation. J Orthop Trauma. 2018;32:505-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Pajarinen J, Lindahl J, Savolainen V, Michelsson O, Hirvensalo E. Femoral shaft medialisation and neck-shaft angle in unstable pertrochanteric femoral fractures. Int Orthop. 2004;28:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Yoon YC, Kim J, Cho JW, Cho WT, Kim HJ, Oh JK. Simple guidelines for evaluating intraoperative alignment after the reduction of intertrochanteric fractures. Asian J Surg. 2021;44:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | O'Malley MJ, Kang KK, Azer E, Siska PA, Farrell DJ, Tarkin IS. Wedge effect following intramedullary hip screw fixation of intertrochanteric proximal femur fracture. Arch Orthop Trauma Surg. 2015;135:1343-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Mingo-Robinet J, Gonzalez-Alonso C, Alonso Del Olmo JA. Fluoroscopic landmarks to recognize iatrogenic varus displacement (wedge effect) during cephalomedullary nailing of intertrochanteric fractures. Injury. 2021;52 Suppl 4:S47-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Chang SM, Zhang YQ, Du SC, Ma Z, Hu SJ, Yao XZ, Xiong WF. Anteromedial cortical support reduction in unstable pertrochanteric fractures: a comparison of intra-operative fluoroscopy and post-operative three dimensional computerised tomography reconstruction. Int Orthop. 2018;42:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Tsukada S, Okumura G, Matsueda M. Postoperative stability on lateral radiographs in the surgical treatment of pertrochanteric hip fractures. Arch Orthop Trauma Surg. 2012;132:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Chang SM, Zhang YQ, Ma Z, Li Q, Dargel J, Eysel P. Fracture reduction with positive medial cortical support: a key element in stability reconstruction for the unstable pertrochanteric hip fractures. Arch Orthop Trauma Surg. 2015;135:811-818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 37. | Baumgaertner MR, Curtin SL, Lindskog DM, Keggi JM. The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. J Bone Joint Surg Am. 1995;77:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 881] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 38. | Mao W, Ni H, Li L, He Y, Chen X, Tang H, Dong Y. Comparison of Baumgaertner and Chang reduction quality criteria for the assessment of trochanteric fractures. Bone Joint Res. 2019;8:502-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Mavrogenis AF, Panagopoulos GN, Megaloikonomos PD, Igoumenou VG, Galanopoulos I, Vottis CT, Karabinas P, Koulouvaris P, Kontogeorgakos VA, Vlamis J, Papagelopoulos PJ. Complications After Hip Nailing for Fractures. Orthopedics. 2016;39:e108-e116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Caruso G, Corradi N, Caldaria A, Bottin D, Lo Re D, Lorusso V, Morotti C, Valpiani G, Massari L. New tip-apex distance and calcar-referenced tip-apex distance cut-offs may be the best predictors for cut-out risk after intramedullary fixation of proximal femur fractures. Sci Rep. 2022;12:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Swift B, Stewart A, Grammatopoulos G, Papp S, Wilkin G, Liew A. Comparing the rates and modes of failure of two third generation cephalomedullary nail systems in the treatment of intertrochanteric hip fractures. Injury. 2022;53:2846-2852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 42. | Andruszkow H, Frink M, Frömke C, Matityahu A, Zeckey C, Mommsen P, Suntardjo S, Krettek C, Hildebrand F. Tip apex distance, hip screw placement, and neck shaft angle as potential risk factors for cut-out failure of hip screws after surgical treatment of intertrochanteric fractures. Int Orthop. 2012;36:2347-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 43. | Rubio-Avila J, Madden K, Simunovic N, Bhandari M. Tip to apex distance in femoral intertrochanteric fractures: a systematic review. J Orthop Sci. 2013;18:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Li H, Wang D, Zhang W, Xu G, Xu C, Zhang H, Zhang L, Li J, Tang P. Does computer-assisted orthopaedics system (ADAPT system) improve outcomes of intertrochanteric hip fractures? Injury. 2023;54:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Hansen RH, Rölfing JD, Nielsen CL, Brink O, Gundtoft PH. Computer-Assisted Intramedullary Nailing of Intertrochanteric Fractures Did Not Prevent Tip-Apex Distance Outliers. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Lilly RJ, Koueiter DM, Graner KC, Nowinski GP, Sadowski J, Grant KD. Computer-assisted navigation for intramedullary nail fixation of intertrochanteric femur fractures: A randomized, controlled trial. Injury. 2018;49:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Schuijt HJ, Hundersmarck D, Smeeing DPJ, van der Velde D, Weaver MJ. Robot-assisted fracture fixation in orthopaedic trauma surgery: a systematic review. OTA Int. 2021;4:e153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 48. | Wittauer M, Sklorz P, Przybilla P, Vach W, Eckardt H. Optimising reduction and implant positioning in intertrochanteric fracture treatment: An evaluation of the effects of a structured educational program. Injury. 2025;56:112146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 49. | Kuzyk PR, Zdero R, Shah S, Olsen M, Waddell JP, Schemitsch EH. Femoral head lag screw position for cephalomedullary nails: a biomechanical analysis. J Orthop Trauma. 2012;26:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 50. | Kashigar A, Vincent A, Gunton MJ, Backstein D, Safir O, Kuzyk PR. Predictors of failure for cephalomedullary nailing of proximal femoral fractures. Bone Joint J. 2014;96-B:1029-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 51. | Barra AE, Barrios C. Predictive value of tip-apex distance and calcar-referenced tip-apex distance for cut-out in 398 femoral intertrochanteric fractures treated in a private practice with dynamic intramedullary nailing. Front Surg. 2024;11:1438858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 52. | Garabano G, Juri A, Perez Alamino L, Rodriguez JA, Pesciallo CA. Predicting cut-out in intertrochanteric fractures fixed with cephalomedullary nails: the role of tip-to-apex distance referenced to calcar (calTAD)--A retrospective analysis of 158 cases. Eur J Orthop Surg Traumatol. 2024;35:24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Lopes-Coutinho L, Dias-Carvalho A, Esteves N, Sousa R. Traditional distance "tip-apex" vs. new calcar referenced "tip-apex" - which one is the best peritrochanteric osteosynthesis failure predictor? Injury. 2020;51:674-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 54. | Murena L, Moretti A, Meo F, Saggioro E, Barbati G, Ratti C, Canton G. Predictors of cut-out after cephalomedullary nail fixation of pertrochanteric fractures: a retrospective study of 813 patients. Arch Orthop Trauma Surg. 2018;138:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 55. | Cleveland M, Bosworth DM, Thompson FR, Wilson HJ, Ishizuka T. A Ten-Year Analysis of Intertrochanteric Fractures of the Femur. J Bone Jt Surg. 1959;41:1399-1408. [DOI] [Full Text] |
| 56. | Caruso G, Bonomo M, Valpiani G, Salvatori G, Gildone A, Lorusso V, Massari L. A six-year retrospective analysis of cut-out risk predictors in cephalomedullary nailing for pertrochanteric fractures: Can the tip-apex distance (TAD) still be considered the best parameter? Bone Joint Res. 2017;6:481-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | Fang Q, Han J, Liu W, Wang D, Ge Z, Wang G. Predictors of and predictive nomogram for cut-out of proximal femur nail anti-rotation device in intertrochanteric fractures. Arch Orthop Trauma Surg. 2023;143:3985-3995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | De Bruijn K, den Hartog D, Tuinebreijer W, Roukema G. Reliability of predictors for screw cutout in intertrochanteric hip fractures. J Bone Joint Surg Am. 2012;94:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 59. | Parker MJ. Cutting-out of the dynamic hip screw related to its position. J Bone Joint Surg Br. 1992;74:625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Parmar V, Kumar S, Aster A, Harper WH. Review of methods to quantify lag screw placement in hip fracture fixation. Acta Orthop Belg. 2005;71:260-263. [PubMed] |
| 61. | Selim A, Al-Hadithy N, Diab NM, Ahmed AM, Kader KFA, Hegazy M, Azeem HA, Barakat AS. Proposal of a modified tip apex distance for prediction of lag screw cut-out in trochanteric hip fractures. SICOT J. 2023;9:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |