Published online May 18, 2025. doi: 10.5312/wjo.v16.i5.106265
Revised: March 27, 2025
Accepted: April 15, 2025
Published online: May 18, 2025
Processing time: 85 Days and 1.9 Hours
Epiphyseal fracture is a significant etiology of limb deformity in children following fractures. However, unlike the rapid advancements in orthopedics, progress regarding the pathological changes, diagnosis, and treatment of epi
Core Tip: Epiphyseal fracture is a significant etiology of limb deformity in children following fractures. However, unlike the rapid advancements in orthopedics, progress regarding the pathological changes, diagnosis, and treatment of epiphyseal fractures has been slow. This review provides an overview of the epidemiology and classification of epiphyseal fractures, as well as the post-fracture pathological changes occurring within the epiphysis and its surrounding areas. Furthermore, it reviews recent advancements in the treatment of epiphyseal fractures. By summarizing laboratory-to-clinical progress related to this type of fracture, this article aims to assist pediatric orthopedists in accurately recognizing, diagnosing, and treating such injuries.
- Citation: Fan ZQ, Xie YY, Liu C, Chen YF, Yi YF, Tang ZW, Wen J, Xiao S, Li YF. Most vexing problem in pediatric fractures: Epiphyseal fractures. World J Orthop 2025; 16(5): 106265
- URL: https://www.wjgnet.com/2218-5836/full/v16/i5/106265.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i5.106265
In contrast to bone, which can undergo complete regeneration, cartilage exhibits a limited regenerative capacity due to its avascular nature. Epiphyses, a specialized type of cartilage found in children and adolescents, play a crucial role in the growth and development of long bones by facilitating both longitudinal and transverse growth. Fractures in pediatric patients frequently involve epiphyseal injuries. Understanding the epidemiological data of epiphyseal fractures is essential for effective management. Moreover, examining the pathological changes in the epiphysis following fractures can provide insights into disease progression. Investigating the specific pathological mechanisms can assist clinicians in selecting appropriate treatment options and guide the development of novel therapeutic strategies to improve the management of epiphyseal injury.
Mizuta et al[1] reported that 18% of nearly 2000 bone injuries were epiphyseal injuries, while another study found that 18.5% of all fractures involved epiphysis[2]. Mann and Rajmaira[3] noted that growth plate injuries comprised 30% of fractures in their cohort. A recent study from Japan indicated that epiphyseal injuries accounted for 17.9% of fractures in children[4]. In this Japanese study, the authors highlighted that the most commonly injured epiphysis was the phalanx of the hand, which contrasts with findings from other studies as detailed in the epidemiology summary section. Based on these data, it is evident that epiphyseal injuries are relatively common. Consequently, neglecting such injuries following fractures can have a significant adverse impact on the growth and development of children and adolescents. Due to the limited visibility of the epiphysis on X-ray, there is a high risk of missed diagnosis in cases of epiphyseal injury. Magnetic resonance imaging (MRI) has emerged as a crucial complementary tool to X-ray examination for such injuries, capable of revealing soft tissue damage, including ligamentous injuries around the fracture site. For instance, in the study by Iwinska-Zelder et al[5], among 10 children diagnosed with epiphyseal injury via plain radiography, MRI reclassified and altered the treatment plan for four patients. Similarly, Carey et al[6] conducted a comparable study. Moreover, MRI demonstrates excellent diagnostic capability for occult epiphyseal fractures[7]. Consequently, for epiphyseal fractures, MRI provides more comprehensive information to guide treatment planning and prognosis assessment[8]. Additionally, it serves as an effective tool for the early diagnosis of epiphyseal diseases, such as Kaschin-Beck disease[9].
The Salter-Harris classification is the predominant system utilized for categorizing epiphyseal fractures in pediatric patients[10]. This classification encompasses five distinct types: (1) Type I, involving fractures that traverse the physis; (2) Type II, involving fractures through the physis and metaphysis; (3) Type III, involving fractures through the physis and epiphysis; (4) Type IV, involving fractures through the physis, epiphysis, and metaphysis; and (5) Type V, characterized by physeal compression or crush injuries[11]. Among these, Type II is the most frequently observed[12-15], a finding corroborated by subsequent studies. For instance, Sananta et al[16] reported the prevalence of each type as follows: Type II (75%), Type III (10%), Type IV (10%), Type I (5%), and the relatively rare Type V.
Deng et al's study[17] encompassed 1124 children with epiphyseal injuries, involving 1147 fracture sites, of which 789 were boys and 335 were girls. The authors concluded that the highest incidence of epiphyseal fractures occurred in the adolescent group (ages between 4015 and 6570 days). In all age groups, boys exhibited a higher incidence of epiphyseal fractures compared to girls. Falls were identified as the most common cause of injury, affecting 494 boys and 226 girls. The distal radius was the most frequent site of epiphyseal fractures (460 cases), followed by the phalanx (233 cases). Among the 1147 epiphyseal fractures, Salter-Harris type II fractures were the most prevalent, accounting for 1002 cases[17].
In another study[18], which included 1020 children (282 girls and 738 boys) with a mean age of 8.3 years, the incidence of fractures in boys was approximately three times higher than that in girls. A total of 59 cases were identified as epiphyseal fractures. The leading cause of fractures was outdoor falls (705 cases), followed by indoor falls (239 cases). In this study, fractures predominantly occurred in the upper limb (76.6%) and on the left side (56.0%). This finding is consistent with other literature indicating that upper limb fractures are more common than lower limb fractures in children[19-21]. The most frequent fracture site was the radius (304 cases), particularly the distal radius, aligning with the aforementioned data. For lower extremity fractures, the femur was the most commonly affected bone (92 cases).
In the study by Kaewpornsawan et al[22], a total of 716 patients with 718 fractures were included. The gender distribution was 68% male and 32% female. The peak age for fractures was between 10 and 16 years, with an epiphyseal fracture incidence of 12.4%. Salter-Harris type II fractures were the most common, accounting for 11.3% of all cases. Fractures occurred more frequently on the right side (53.8%) compared to the left side (46.2%). The distal forearm was the most common fracture site, representing 18.87% of all fractures. The leading causes of fractures were falls (34.6%), traffic accidents (28.4%), and falls from height (24.1%).
In a review of hand fractures in children[23], the authors reported the following data: The gender distribution of fractures was 24.6% (57 cases) in females and 75.4% (175 cases) in males, with an average age of 11.1 ± 3.3 years. The peak incidence occurred at approximately 12 years of age, and physical activity was identified as the most common cause. The fifth metacarpal bone was the most frequently affected bone (21.1%). Epiphyseal fractures accounted for 39.8% of all cases, with Salter-Harris type II being the predominant fracture type.
In a study on the epidemiology of sports-related epiphyseal injuries of the lower limbs[24], the authors reported the following findings: Among the 85 cases of epiphyseal fractures, 60 were males and 25 were females, with ages ranging from 4 to 17 years (mean age: 12.6 years). Soccer accounted for 28% of the injuries, while alpine skiing accounted for 26%. The most common site of injury was the distal tibia epiphysis (31 cases), followed by the distal fibula (17 cases) and the proximal tibia epiphysis (15 cases). There were 30 cases classified as Salter-Harris type I and 25 cases as type II, which showed a slight variation compared to other studies.
In conclusion, the incidence of epiphyseal injury following fractures is approximately 20%, predominantly observed in adolescents, with a higher prevalence among males. The majority of these injuries result from falls, which are closely associated with increased physical activity and sports participation during adolescence. Epiphyseal fractures most commonly occur in the upper limbs, particularly at the distal radius. The predominant type of epiphyseal fracture is Salter-Harris Type II.
The epiphyseal plate (growth plate) is a cartilaginous structure located at the ends of long bones and consists of three distinct zones: The resting zone, the proliferative zone, and the hypertrophic zone[25]. Its primary function is to generate a mineralized cartilage scaffold that facilitates the formation of trabecular bone via endochondral ossification, which encompasses both chondrogenesis and osteogenesis[26]. The proliferative zone is the most frequently injured region among these three zones[27]. Chung et al[28] noted in their review of the injury response following epiphyseal fractures that the mechanisms governing growth plate injury response and bone repair appear to parallel those observed during fracture healing; however, the cellular and molecular processes underlying bone repair after growth plate injury remain incompletely understood. Previous animal studies have demonstrated that both intramembranous and endochondral osteogenesis mechanisms contribute to growth plate injury repair. Lee et al[29] reported no alterations in the expression of genes associated with endochondral ossification in a mouse model. In a rat model of burr-hole growth plate injury, Xian et al[30] observed the presence of Runx2 immunopositive osteoblasts during bone trabeculation at the site of growth plate injury, while the expression of genes related to chondrogenesis remained unchanged. However, a study using other rat growth plate injury models[31] have identified increased expression of cartilage-related genes (Sox9 and collagen 2). More significantly, a marked upregulation of collagen X, which is specifically expressed by hypertrophic chondrocytes during endochondral ossification, has been documented.
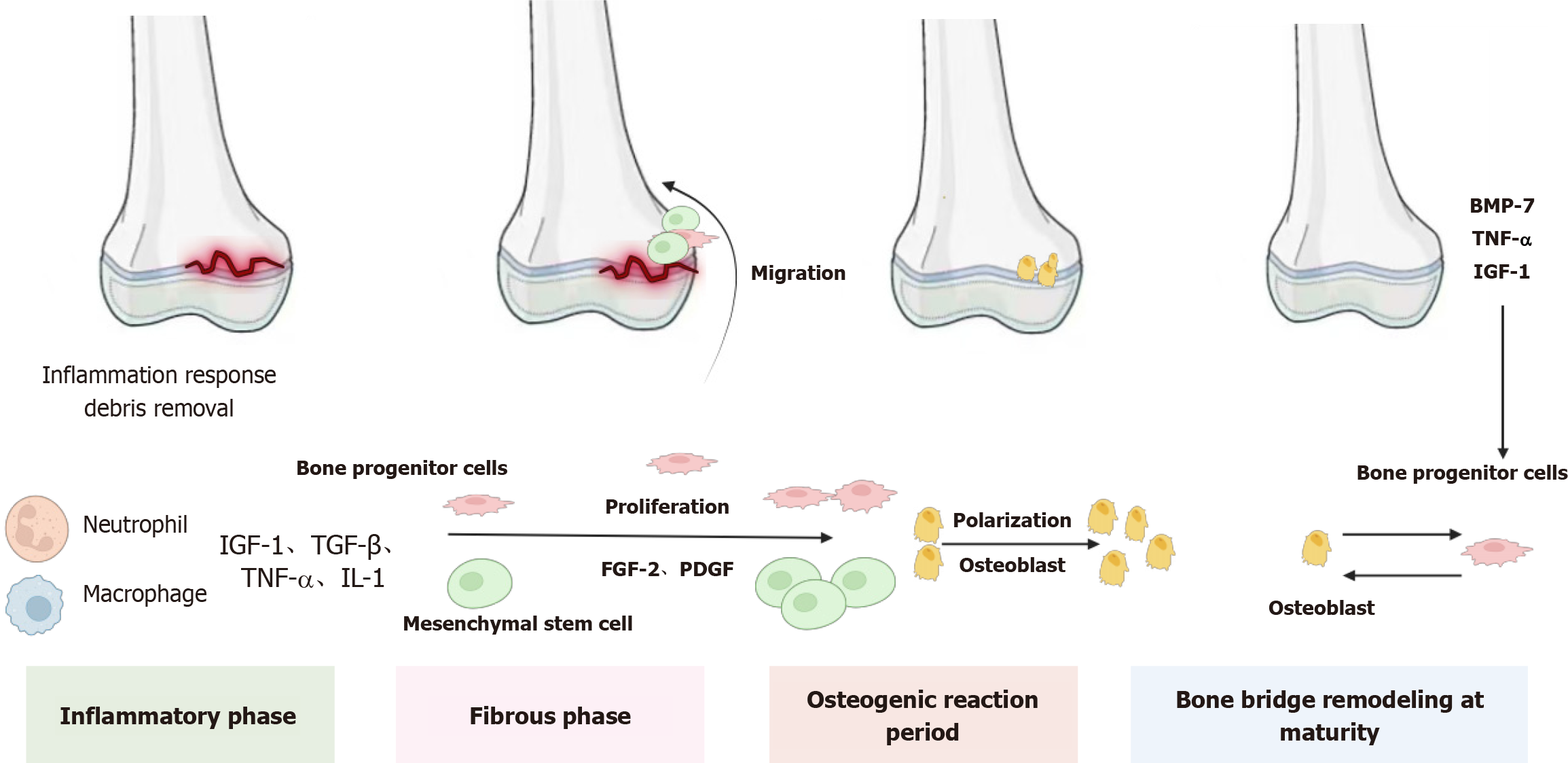
Previous studies have identified four distinct stages of injury response in the rat growth plate injury model: Inflammation (day 1-3), fibrosis (day 3-7), osteogenesis (day 7-14), and bone bridge maturation and remodeling (day 10-25). Comparable injury responses have been documented in growth plate injury models across various species, including mice, rabbits, pigs, and sheep[32-34].
As previously discussed, the initial response to growth plate injury is characterized by an inflammatory phase[35,36]. During this stage, a surge of inflammatory cells, primarily neutrophils along with macrophages/monocytes and lymphocytes, infiltrates the injured growth plate. Neutrophils actively remove bacteria and microdebris from the damaged area, facilitating soft tissue and bone healing[37,38]. Simultaneously, these cells secrete numerous growth factors and cytokines, which are crucial for regulating subsequent downstream reactions. The mRNA expression levels of these factors peak between 8 hours and 1 day post-injury. Notably, insulin-like growth factor-1 (IGF-I) and transforming growth factor (TGF)-beta exhibit upregulation in the early stages of injury repair. Bone morphogenetic protein (BMP), another key player during the inflammatory phase, promotes chondroblast and osteoblast differentiation while enhancing mesenchymal cell proliferation and migration[39,40]. Additionally, tumor necrosis factor-alpha (TNF-α) and interleukin-1alpha (IL-1α) show significant increases on day 1[41]. Birkl et al[42] have also emphasized TNF-α as a critical factor in healing and tissue repair. Collectively, the inflammatory phase is essential for repairing growth plate injuries as it orchestrates the downstream cascade of the healing response (Figure 1).
In the rat growth plate injury model, the inflammatory phase transitions into the fibrotic phase 3-7 days post-injury. The fibrotic response is characterized by the infiltration of vimentin-immunoreactive mesenchymal cells into the injured site. This phenomenon was also observed in mice, where undifferentiated spindle-shaped cells were present near the upper and lower regions of the growth plate injury site approximately 7 days after injury. During this period, the mRNA levels of the growth factors fibroblast growth factor 2 (FGF-2) and platelet-derived growth factor (PDGF)-BB were significantly upregulated, indicating their potential involvement in regulating the fibrotic response[43]. FGF-2 plays a critical role in various biological processes, including cell proliferation, differentiation, and migration[44]. Specifically, it promotes mesenchymal cell migration and proliferation[45,46], inhibits chondrocyte differentiation[47], enhances alkaline phosphatase activity[48,49], and stimulates bone resorption in vitro[50,51]. Additionally, FGF-2 stimulates the proliferation and migration of osteoprogenitor cells[52]. In fracture repair, PDGFs are essential for initiating events that lead to the migration and proliferation of fibroblasts and osteoblasts[53]. A study by Chung et al[54] demonstrated that inhibiting PDGF-R signaling during the fibrotic stage reduced mesenchymal cell proliferation and infiltration at day 4 post-injury and decreased the amount of bony or cartilaginous tissue at the injury site by day 14, highlighting the crucial role of PDGF in the fibrotic phase of growth plate repair.
After the fibrotic phase, the subsequent stage of the osteogenic reaction involves osteocytic differentiation among infiltrating mesenchymal cells, as evidenced by positive immunohistochemical staining for Runx-2 and alkaline phosphatase. These markers respectively indicate osteoblast differentiation and maturation[55].
In the final stage, the remodeling and maturation of the bone bridge involve the separation of bone trabeculae by numerous bone marrow cells and their surrounding by quiescent, flat, spindle-shaped osteoblasts. Osteoclasts, which are absorptive cells, occasionally appear in certain areas of the newly formed trabeculae at the injury site[56]. Additionally, growth factors such as TNF-α, IGF-1, and BMP-7 increase, promoting osteoclast differentiation and recruitment, thereby facilitating bone remodeling[57,58]. There is limited research on the pathological changes around epiphyseal injuries. An early study observed that growth plate injury affects the periphery, with cartilage tissue invading the metaphyseal region, leading to the disruption of continuous bone growth in the surrounding tissue[59]. Micro-computed tomography (CT) imaging revealed significant damage in the remaining undamaged growth plates when the bone bridge formed, including cellular disarray and a substantial reduction in overall growth plate thickness and volume[60]. Interestingly, Coleman et al[61] noted that tethering typically forms with age as the growth plate begins to close, appearing earlier in adjacent growth plates after injury. Macsai et al[62] reported that by day 60, bone bridge formation was detected in 60% of adjacent uninjured areas in injured animals. Immunohistochemical analysis showed reduced chondrocyte proliferation (PCNA marker) and increased apoptosis (TUNEL marker) in these adjacent uninjured regions.
The treatment of epiphyseal fractures encompasses both non-surgical methods, such as plaster immobilization, and surgical interventions. In addressing damaged growth cartilage, it is crucial to adhere to the principles of epiphyseal fracture management, which involve restoring and maintaining the continuity of the epiphysis[63]. Achieving good anatomical reduction, either through closed or open reduction with internal fixation, is a key factor in minimizing complications[64]. Cai et al[65] investigated the treatment strategies and surgical indications for distal tibial epiphyseal fractures in children. They recommended that treatment should be guided by the degree of fracture displacement. Specifically, for initial displacements less than 2 mm, long leg casts were applied following closed reduction. For displacements exceeding 2 mm, Kirschner wire or screw fixation was performed. Conservative management is appropriate for Salter-Harris type I and II fractures, while surgical intervention is preferred for types III and IV to reduce the risk of premature physeal closure[65]. Dahl et al[66] emphasized that the primary goal of epiphyseal fracture treatment is to minimize further damage to the epiphyseal plate. Experimental results demonstrated that the incidence of epiphyseal plate injury caused by smooth Kirschner wire cross-piercing is relatively low. In cases requiring open reduction and internal fixation, anatomical reduction should be achieved, with careful removal of entrapped periosteum and other soft tissues to prevent premature epiphyseal closure and malunion[67]. With the increasing participation of children and adolescents in group sports, anterior cruciate ligament (ACL) injuries have become a significant concern affecting the health of physically active young individuals. Treatment options for ACL injuries primarily consist of epiphyseal-sparing techniques, complete epiphyseal plate reconstruction, partial epiphyseal plate reconstruction, and trans-epiphyseal plate reconstruction. Based on prior studies, the first two approaches exhibit the least impact on the growth plate[68-70].
Due to the rapid growth characteristics of children, limb varus and valgus deformities may occur following epiphyseal injuries. For children with remaining growth potential and mild deformities, epiphyseal retardation is an appropriate choice due to its minimal invasiveness and high acceptability among patients and their families[71]. This technique is primarily suited for children who still possess growth potential and exhibit minor deformities. It is currently most frequently applied to coronal plane deformities around the knee joint; however, it is important to note that a rebound phenomenon of approximately 5° may occur after removal of the growth plate[72]. Nevertheless, there is limited research on the effects of this procedure on the growth plate, necessitating further fundamental studies to explore its implications. Conversely, for older children with severe deformities (involving multiple sites, limited growth potential, and deformities exceeding 20°), osteotomy has emerged as a corrective option[73].
For certain rare diseases, such as congenital spondyloepiphyseal dysplasia (SEDC), first described by Spranger and Wiedemann in 1966, it is an autosomal dominant genetic disorder characterized by asymmetric dwarfism, a short spine, a short neck, and varying degrees of coxa vara[74]. Orthopedic management in children should prioritize the cervical spine to prevent severe neurological deficits and/or mortality[74]. For coxa vara, proximal femoral valgus osteotomy has been demonstrated to be an effective treatment[75], while total hip and total knee arthroplasty can alleviate pain and enhance function[76,77]. However, due to the avascular nature of the epiphysis and limited cellular mobility, the regenerative capacity of articular cartilage after injury is severely constrained. Consequently, the effectiveness of these treatments diminishes significantly in patients with severe epiphyseal injuries, potentially impacting their growth and development. When the bone bridge occupies less than 50% of the growth plate area, surgical intervention is performed to remove the bone bridge and implant various interposition materials such as fat, bone wax, muscle, or polymer silicone. However, the clinical success rate of this procedure remains below 35%, primarily due to poor integration of the currently available interposition materials with host tissue, leading to subsequent complications. When the bone bridge exceeds 50% of the growth plate area, orthopedic surgery combined with limb lengthening becomes necessary; however, the outcomes in these cases are also unsatisfactory[78].
The application mechanism of skeletal stem cells (SSCs) in epiphyseal injury repair primarily relies on their self-renewal and multi-lineage differentiation capabilities. Following epiphyseal injury, SSCs can differentiate into osteoblasts via local or systemic signal transduction pathways, such as BMP and Wnt signaling, thereby facilitating bone damage repair[17,79]. Additionally, these stem cells can differentiate into chondrocytes, playing a critical role in the regeneration of epiphyseal cartilage[80]. During the repair process, SSCs also promote local angiogenesis by secreting vascular endothelial growth factor and other pro-angiogenic factors, enhancing blood supply to the injured area, providing essential nutrients and oxygen for tissue repair, and accelerating the regeneration process[81].
Currently, numerous researchers are dedicated to enhancing the regenerative potential of articular cartilage. Notably, significant efforts have been directed towards the research and development of mesenchymal stem cell (MSC) therapy, leveraging tissue engineering principles, as well as the introduction of cartilage growth factors. Cartilage tissue engineering employs a multifaceted approach that integrates various cell types and growth factors, such as bone marrow MSCs, chondrocytes, TGF-β, IGF-1, and FGF-2[82,83], along with diverse scaffolds fabricated from both natural and synthetic materials[84].
MSCs possess the capability to differentiate into various tissue cells, including cartilage, bone, and adipose tissue. Moreover, MSCs can be isolated from diverse sources such as periosteum, trabecular bone, adipose tissue, synovium, skeletal muscle, and bone marrow[85]. Notably, previous studies have demonstrated that synovial-derived MSCs exhibit enhanced chondrogenic differentiation potential compared to those derived from other mesenchymal tissues[86]. In a study by Yoshida et al[87], a 6-week-old rabbit growth arrest model was created by destroying the medial half of the proximal tibia. To evaluate the effect of synovial-derived MSCs, they were cultured in a scaffold-free structure, resulting in proliferation and differentiation into chondrocyte-like cells. This suggests that MSC-based therapy may offer a promising approach for treating growth plate injuries[87]. However, it has also been reported that bone marrow-derived MSCs (BM-MSCs) are particularly suitable for cartilage tissue engineering due to their superior proliferation rate and higher expression levels of cartilage-specific genes compared to MSCs from other tissues[88]. Additionally, BM-MSCs can be easily isolated and efficiently expanded[89]. Previous studies in rabbit models have indicated the feasibility and applicability of using MSCs to induce growth plate cartilage regeneration[90,91]. Nevertheless, the failure to achieve cartilage regeneration using autologous MSCs in a sheep growth plate injury model[92] underscores the need for further research to explore the potential of MSCs for growth plate regeneration in large animal models.
Given that chondrocytes are the predominant cell type in the growth plate, it is logical to implant chondrocytes within cartilage tissue engineering scaffolds for the treatment of growth plate injuries[93,94]. Both autologous chondrocyte studies[95] and allogeneic chondrocyte studies[96] have demonstrated that both types of chondrocytes can prevent bone bridge formation. However, the availability of autologous chondrocytes is limited and their harvest may cause additional damage[97]. Further experimental data are required to substantiate these findings.
Migration, proliferation, and differentiation of MSCs are significantly influenced by various signaling molecules, particularly growth factors. In the context of growth plate cartilage repair, an ideal approach involves the sequential application of multiple growth factors to first promote optimal MSC expansion and subsequently induce their differentiation into chondrocytes[98]. Previous studies have demonstrated that growth factors such as PDGF, IGF-1, FGF-2, and TGF alpha possess mitogenic properties for MSCs[99].
Additionally, growth factors with established chondrogenic effects include FGF-2, TGF-β1, TGF-β3, BMP-7, and IGF-1[100,101]. Wang et al[102] reviewed the critical roles of TGF-β, IGF-1, and FGF-2 in cartilage repair. According to reviews by Chen et al[103] and Dahlin et al[104], co-culture with chondrocytes and minimal exogenous TGF-β stimulation were found to be more effective for MSC differentiation or proliferation. IGF-1 not only stimulates the synthesis of matrix proteins such as type II collagen and aggrecan by chondrocytes but also inhibits chondrocyte degradation and apoptosis during cartilage injury by blocking the functions of IL-1 or TNF-α[105]. A clinical trial using IGF-1 to treat children with short stature for one year reported no adverse events, suggesting its potential for clinical applications[106].
Cartilage scaffolds must possess excellent biocompatibility, biodegradability, appropriate porosity, and suitable mechanical properties. Currently, both natural and synthetic materials are predominantly utilized for cartilage repair. Natural materials, such as extracellular matrix (ECM), alginate, agarose, and chitosan, exhibit superior biocompatibility and controlled biodegradability, making them ideal for initiating chondrocyte regeneration and promoting cartilage ECM secretion[107]. Synthetic materials, including poly (lactic-co-glycolic acid) (PLGA), polylactic acid, and polycaprolactone, are extensively employed in cartilage tissue engineering to fabricate scaffolds. These materials offer tunable mechanical properties and adjustable degradation rates by altering the degree of polymerization[108], providing greater mechanical strength and suitability for load-bearing applications and drug delivery compared to natural materials. Composite scaffolds have gained widespread use due to the limitations of individual materials. Combining materials with complementary advantages can address the shortcomings of single-material scaffolds[109,110]. For instance, Wang et al[111] developed a composite scaffold from PLGA, collagen, and silk fibroin for cartilage repair by optimizing the ratio of components. In vitro studies demonstrated that this composite scaffold promotes the proliferation and differentiation of MSCs without adverse effects. In vivo results indicated that this composite scaffold enhances articular cartilage regeneration and integration with surrounding cartilage. Consequently, this composite material holds significant promise for cartilage repair and regeneration.
In addition, several studies have demonstrated the potential of gene therapy in cartilage repair. For instance, chondrocytes cultured in vitro can sustain the expression of transgenic products such as TGF-β[112,113], BMP-7, and IGF-I[114] after modification with recombinant adenoviruses. However, Hu et al[115] noted that modifying MSCs may influence their differentiation into various tissue types. Palmer et al[116] found that only a specific subset of genes is necessary to induce chondrogenic differentiation of bone marrow-derived cells, and overexpression through gene-induced transduction could potentially hinder this process. Moreover, ex vivo gene therapy approaches present several challenges, including high costs, significant effort, and extended timeframes. Consequently, further investigation is required to determine the suitability of this type of cartilage engineering for growth plate cartilage regeneration[117].
Epiphyseal injuries are prevalent in adolescent fractures, significantly impacting patients' growth and development. Consequently, it is crucial to prioritize the identification of epiphyseal injuries during the treatment of adolescent fractures. Epidemiological studies on epiphyseal injuries in adolescents highlight the importance of fracture prevention and enhancing safety awareness among this population. Clinicians should focus on screening for epiphyseal injuries when treating fracture patients. Given the pathological changes associated with post-fracture epiphyseal injuries and current traditional treatment protocols, research into MSCs and cartilage growth factors should be intensified to improve therapeutic outcomes.
| 1. | Mizuta T, Benson WM, Foster BK, Paterson DC, Morris LL. Statistical analysis of the incidence of physeal injuries. J Pediatr Orthop. 1987;7:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 183] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Worlock P, Stower M. Fracture patterns in Nottingham children. J Pediatr Orthop. 1986;6:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 128] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Mann DC, Rajmaira S. Distribution of physeal and nonphyseal fractures in 2,650 long-bone fractures in children aged 0-16 years. J Pediatr Orthop. 1990;10:713-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 182] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Kawamoto K, Kim WC, Tsuchida Y, Tsuji Y, Fujioka M, Horii M, Mikami Y, Tokunaga D, Kubo T. Incidence of physeal injuries in Japanese children. J Pediatr Orthop B. 2006;15:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Iwinska-Zelder J, Schmidt S, Ishaque N, Hoppe M, Schmitt J, Klose KJ, Gotzen L. [Epiphyseal injuries of the distal tibia. Does MRI provide useful additional information?]. Radiologe. 1999;39:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Carey J, Spence L, Blickman H, Eustace S. MRI of pediatric growth plate injury: correlation with plain film radiographs and clinical outcome. Skeletal Radiol. 1998;27:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Gufler H, Schulze CG, Wagner S, Baumbach L. MRI for occult physeal fracture detection in children and adolescents. Acta Radiol. 2013;54:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Shi DP, Zhu SC, Li Y, Zheng J. Epiphyseal and physeal injury: comparison of conventional radiography and magnetic resonance imaging. Clin Imaging. 2009;33:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Li Y, Kang P, Zhou Z, Pei F, He Q, Ruan D. Magnetic resonance imaging at 7.0 T for evaluation of early lesions of epiphyseal plate and epiphyseal end in a rat model of Kashin-Beck disease. BMC Musculoskelet Disord. 2020;21:540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Salter RB. Injuries of the epiphyseal plate. Instr Course Lect. 1992;41:351-359. [PubMed] |
| 11. | Brown JH, DeLuca SA. Growth plate injuries: Salter-Harris classification. Am Fam Physician. 1992;46:1180-1184. [PubMed] |
| 12. | Della-Giustina K, Della-Giustina DA. Emergency department evaluation and treatment of pediatric orthopedic injuries. Emerg Med Clin North Am. 1999;17:895-922, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Peterson HA, Madhok R, Benson JT, Ilstrup DM, Melton LJ 3rd. Physeal fractures: Part 1. Epidemiology in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop. 1994;14:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Gnassingbe K, Walla A, Akakpo-Numado GK, Ketevi A, Tekou H. [Epiphyseal growth fractures: epidemiology and treatment. Retrospective report of 44 cases]. Mali Med. 2011;26:1-3. [PubMed] |
| 15. | Karlikowski M, Sułko J. Physeal fractures of the lower leg in children and adolescents: Therapeutic results, pitfalls and suggested management protocol - based on the experience of the authors and contemporary literature. Adv Med Sci. 2018;63:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Sananta P, Lesmana A, Alwy Sugiarto M. Growth plate injury in children: Review of literature on PubMed. J Public Health Res. 2022;11:22799036221104155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Deng H, Zhao Z, Xiong Z, Gao F, Tang S, Li Y, Li W, Huang J, Cui S, Chen X, Zeng S, Tang G, Sechi LA, Caggiari G, Doria C, Qiu X. Clinical characteristics of 1124 children with epiphyseal fractures. BMC Musculoskelet Disord. 2023;24:598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Aygun U. The feature assessment of the bone fractures in 1020 children and review of the literature. North Clin Istanb. 2020;7:460-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Landin LA. Fracture Patterns in Children. Acta Orthop Scand. 1983;54:3-109. [RCA] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Omori G. [Bone and joint diseases in children. Epidemiology of childhood fractures]. Clin Calcium. 2010;20:881-886. [PubMed] |
| 21. | Mathison DJ, Agrawal D. An update on the epidemiology of pediatric fractures. Pediatr Emerg Care. 2010;26:594-603; quiz 604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Kaewpornsawan K, Sukvanich P, Tujinda H, Eamsobhana P. Prevalence and patterns of fractures in children. J Med Assoc Thai. 2014;97 Suppl 9:S116-S120. [PubMed] |
| 23. | Mahabir RC, Kazemi AR, Cannon WG, Courtemanche DJ. Pediatric hand fractures: a review. Pediatr Emerg Care. 2001;17:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Krueger-Franke M, Siebert CH, Pfoerringer W. Sports-related epiphyseal injuries of the lower extremity. An epidemiologic study. J Sports Med Phys Fitness. 1992;32:106-111. [PubMed] |
| 25. | Iannotti JP. Growth plate physiology and pathology. Orthop Clin North Am. 1990;21:1-17. [PubMed] [DOI] [Full Text] |
| 26. | Yang X, Karsenty G. Transcription factors in bone: developmental and pathological aspects. Trends Mol Med. 2002;8:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Schurz M, Binder H, Platzer P, Schulz M, Hajdu S, Vécsei V. Physeal injuries of the distal tibia: long-term results in 376 patients. Int Orthop. 2010;34:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Chung R, Foster BK, Xian CJ. Injury responses and repair mechanisms of the injured growth plate. Front Biosci (Schol Ed). 2011;3:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Lee MA, Nissen TP, Otsuka NY. Utilization of a murine model to investigate the molecular process of transphyseal bone formation. J Pediatr Orthop. 2000;20:802-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Xian CJ, Zhou FH, McCarty RC, Foster BK. Intramembranous ossification mechanism for bone bridge formation at the growth plate cartilage injury site. J Orthop Res. 2004;22:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Chung R, Foster BK, Zannettino AC, Xian CJ. Potential roles of growth factor PDGF-BB in the bony repair of injured growth plate. Bone. 2009;44:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Jaramillo D, Shapiro F, Hoffer FA, Winalski CS, Koskinen MF, Frasso R, Johnson A. Posttraumatic growth-plate abnormalities: MR imaging of bony-bridge formation in rabbits. Radiology. 1990;175:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Mäkelä EA, Vainionpää S, Vihtonen K, Mero M, Rokkanen P. The effect of trauma to the lower femoral epiphyseal plate. An experimental study in rabbits. J Bone Joint Surg Br. 1988;70:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 122] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Wirth T, Byers S, Byard RW, Hopwood JJ, Foster BK. The implantation of cartilaginous and periosteal tissue into growth plate defects. Int Orthop. 1994;18:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Zhou FH, Foster BK, Sander G, Xian CJ. Expression of proinflammatory cytokines and growth factors at the injured growth plate cartilage in young rats. Bone. 2004;35:1307-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 593] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 37. | Franceschi RT. Biological approaches to bone regeneration by gene therapy. J Dent Res. 2005;84:1093-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 38. | Kim MH, Liu W, Borjesson DL, Curry FR, Miller LS, Cheung AL, Liu FT, Isseroff RR, Simon SI. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J Invest Dermatol. 2008;128:1812-1820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 39. | Zoricic S, Maric I, Bobinac D, Vukicevic S. Expression of bone morphogenetic proteins and cartilage-derived morphogenetic proteins during osteophyte formation in humans. J Anat. 2003;202:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1556] [Cited by in RCA: 1569] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 41. | Wang C, Xu CX, Alippe Y, Qu C, Xiao J, Schipani E, Civitelli R, Abu-Amer Y, Mbalaviele G. Chronic inflammation triggered by the NLRP3 inflammasome in myeloid cells promotes growth plate dysplasia by mesenchymal cells. Sci Rep. 2017;7:4880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Birkl D, Quiros M, García-Hernández V, Zhou DW, Brazil JC, Hilgarth R, Keeney J, Yulis M, Bruewer M, García AJ, O Leary MN, Parkos CA, Nusrat A. TNFα promotes mucosal wound repair through enhanced platelet activating factor receptor signaling in the epithelium. Mucosal Immunol. 2019;12:909-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Tatsuyama K, Maezawa Y, Baba H, Imamura Y, Fukuda M. Expression of various growth factors for cell proliferation and cytodifferentiation during fracture repair of bone. Eur J Histochem. 2000;44:269-278. [PubMed] |
| 44. | Farré J, Roura S, Prat-Vidal C, Soler-Botija C, Llach A, Molina CE, Hove-Madsen L, Cairó JJ, Gòdia F, Bragós R, Cinca J, Bayes-Genis A. FGF-4 increases in vitro expansion rate of human adult bone marrow-derived mesenchymal stem cells. Growth Factors. 2007;25:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Solchaga LA, Penick K, Goldberg VM, Caplan AI, Welter JF. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2010;16:1009-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 46. | Ito T, Sawada R, Fujiwara Y, Tsuchiya T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-beta signaling. Cytotechnology. 2008;56:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Wroblewski J, Edwall-Arvidsson C. Inhibitory effects of basic fibroblast growth factor on chondrocyte differentiation. J Bone Miner Res. 1995;10:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Rodan SB, Wesolowski G, Thomas KA, Yoon K, Rodan GA. Effects of acidic and basic fibroblast growth factors on osteoblastic cells. Connect Tissue Res. 1989;20:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Tanaka E, Ishino Y, Sasaki A, Hasegawa T, Watanabe M, Dalla-Bona DA, Yamano E, van Eijden TM, Tanne K. Fibroblast growth factor-2 augments recombinant human bone morphogenetic protein-2-induced osteoinductive activity. Ann Biomed Eng. 2006;34:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Kawaguchi H, Pilbeam CC, Harrison JR, Raisz LG. The role of prostaglandins in the regulation of bone metabolism. Clin Orthop Relat Res. 1995;36-46. [PubMed] |
| 51. | Kawaguchi H, Katagiri M, Chikazu D. Osteoclastic bone resorption through receptor tyrosine kinase and extracellular signal-regulated kinase signaling in mature osteoclasts. Mod Rheumatol. 2004;14:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Nasrabadi D, Rezaeiani S, Eslaminejad MB, Shabani A. Improved Protocol for Chondrogenic Differentiation of Bone Marrow Derived Mesenchymal Stem Cells -Effect of PTHrP and FGF-2 on TGFβ1/BMP2-Induced Chondrocytes Hypertrophy. Stem Cell Rev Rep. 2018;14:755-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Fujii H, Kitazawa R, Maeda S, Mizuno K, Kitazawa S. Expression of platelet-derived growth factor proteins and their receptor alpha and beta mRNAs during fracture healing in the normal mouse. Histochem Cell Biol. 1999;112:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Chung R, Cool JC, Scherer MA, Foster BK, Xian CJ. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J Leukoc Biol. 2006;80:1272-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Arasapam G, Scherer M, Cool JC, Foster BK, Xian CJ. Roles of COX-2 and iNOS in the bony repair of the injured growth plate cartilage. J Cell Biochem. 2006;99:450-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 56. | Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 57. | Fischer C, Reiner C, Schmidmaier G, Doll J, Child C, Grützner PA, Biglari B, Boxriker S, Moghaddam A. Safety study: is there a pathologic IGF-1, PDGF and TGF-β cytokine expression caused by adjunct BMP-7 in tibial and femoral non-union therapy? Ther Clin Risk Manag. 2018;14:691-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Kim Y, Kang BJ, Kim WH, Yun HS, Kweon OK. Evaluation of Mesenchymal Stem Cell Sheets Overexpressing BMP-7 in Canine Critical-Sized Bone Defects. Int J Mol Sci. 2018;19:2073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Craig JG, Cramer KE, Cody DD, Hearshen DO, Ceulemans RY, van Holsbeeck MT, Eyler WR. Premature partial closure and other deformities of the growth plate: MR imaging and three-dimensional modeling. Radiology. 1999;210:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Martin EA, Ritman EL, Turner RT. Time course of epiphyseal growth plate fusion in rat tibiae. Bone. 2003;32:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Coleman RM, Phillips JE, Lin A, Schwartz Z, Boyan BD, Guldberg RE. Characterization of a small animal growth plate injury model using microcomputed tomography. Bone. 2010;46:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Macsai CE, Hopwood B, Chung R, Foster BK, Xian CJ. Structural and molecular analyses of bone bridge formation within the growth plate injury site and cartilage degeneration at the adjacent uninjured area. Bone. 2011;49:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Havránek P, Hájková H. Treatment of children's epiphyseal injuries in the elbow region. Acta Univ Carol Med (Praha). 1985;31:243-268. [PubMed] |
| 64. | Seel EH, Noble S, Clarke NM, Uglow MG. Outcome of distal tibial physeal injuries. J Pediatr Orthop B. 2011;20:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Cai H, Wang Z, Cai H. Surgical indications for distal tibial epiphyseal fractures in children. Orthopedics. 2015;38:e189-e195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Dahl WJ, Silva S, Vanderhave KL. Distal femoral physeal fixation: are smooth pins really safe? J Pediatr Orthop. 2014;34:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Yuan Q, Zhen Y, Guo Z, Zhang F, Fang J, Zhu Z, Zhu L, Shen X, Yin C, Liu Y, Yao F, Wu L, Wang X. Open reduction and internal fixation for displaced Salter-Harris type II fractures of the distal tibia: a retrospective study of sixty-five cases in children. J Orthop Surg Res. 2021;16:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 68. | Wu J, Luo W, Zheng H, Ren F, Zhao Q, Huang J. [Management status of anterior cruciate ligament injury in children and adolescents]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022;36:495-499. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 69. | Gupta A, Tejpal T, Shanmugaraj A, Horner NS, Gohal C, Khan M. All-epiphyseal anterior cruciate ligament reconstruction produces good functional outcomes and low complication rates in pediatric patients: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2020;28:2444-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Ardern CL, Ekås GR, Grindem H, Moksnes H, Anderson AF, Chotel F, Cohen M, Forssblad M, Ganley TJ, Feller JA, Karlsson J, Kocher MS, LaPrade RF, McNamee M, Mandelbaum B, Micheli L, Mohtadi N, Reider B, Roe J, Seil R, Siebold R, Silvers-Granelli HJ, Soligard T, Witvrouw E, Engebretsen L. 2018 International Olympic Committee consensus statement on prevention, diagnosis and management of paediatric anterior cruciate ligament (ACL) injuries. Br J Sports Med. 2018;52:422-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 71. | Danino B, Rödl R, Herzenberg JE, Shabtai L, Grill F, Narayanan U, Segev E, Wientroub S. Guided growth: preliminary results of a multinational study of 967 physes in 537 patients. J Child Orthop. 2018;12:91-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 72. | Gupta P, Gupta V, Patil B, Verma V. Angular deformities of lower limb in children: Correction for whom, when and how? J Clin Orthop Trauma. 2020;11:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Pääkkönen M. Mini-invasive Osteotomy for Pediatric Distal Radius Malunion. Tech Hand Up Extrem Surg. 2022;26:89-92. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 74. | Al Kaissi A, Ryabykh S, Pavlova OM, Ochirova P, Kenis V, Chehida FB, Ganger R, Grill F, Kircher SG. The Managment of cervical spine abnormalities in children with spondyloepiphyseal dysplasia congenita: Observational study. Medicine (Baltimore). 2019;98:e13780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 75. | Bayhan IA, Abousamra O, Rogers KJ, Bober MB, Miller F, Mackenzie WG. Valgus Hip Osteotomy in Children With Spondyloepiphyseal Dysplasia Congenita: Midterm Results. J Pediatr Orthop. 2019;39:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Wyles CC, Panos JA, Houdek MT, Trousdale RT, Berry DJ, Taunton MJ. Total Hip Arthroplasty Reduces Pain and Improves Function in Patients With Spondyloepiphyseal Dysplasia: A Long-Term Outcome Study of 50 Cases. J Arthroplasty. 2019;34:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Sponer P, Korbel M, Kucera T. Total Knee Arthroplasty in Spondyloepiphyseal Dysplasia with Irreducible Congenital Dislocation of the Patella: Case Report and Literature Review. Ther Clin Risk Manag. 2021;17:275-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 78. | Ladenhauf HN, Jones KJ, Potter HG, Nguyen JT, Green DW. Understanding the undulating pattern of the distal femoral growth plate: Implications for surgical procedures involving the pediatric knee: A descriptive MRI study. Knee. 2020;27:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Lin Z, Willers C, Xu J, Zheng MH. The chondrocyte: biology and clinical application. Tissue Eng. 2006;12:1971-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 80. | Li Y, Li A, Junge J, Bronner M. Planar cell polarity signaling coordinates oriented cell division and cell rearrangement in clonally expanding growth plate cartilage. Elife. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 81. | Pascual-Leone N, Gross PW, Meza BC, Fabricant PD. Techniques in Pediatric Anterior Cruciate Ligament Reconstruction. Arthroscopy. 2022;38:2784-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 82. | Chen L, Liu J, Guan M, Zhou T, Duan X, Xiang Z. Growth Factor and Its Polymer Scaffold-Based Delivery System for Cartilage Tissue Engineering. Int J Nanomedicine. 2020;15:6097-6111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 83. | Wei P, Xu Y, Gu Y, Yao Q, Li J, Wang L. IGF-1-releasing PLGA nanoparticles modified 3D printed PCL scaffolds for cartilage tissue engineering. Drug Deliv. 2020;27:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 84. | Abdollahiyan P, Oroojalian F, Mokhtarzadeh A, de la Guardia M. Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Biotechnol J. 2020;15:e2000095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 85. | Xian CJ, Foster BK. Repair of injured articular and growth plate cartilage using mesenchymal stem cells and chondrogenic gene therapy. Curr Stem Cell Res Ther. 2006;1:213-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 86. | Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1089] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 87. | Yoshida K, Higuchi C, Nakura A, Nakamura N, Yoshikawa H. Treatment of partial growth arrest using an in vitro-generated scaffold-free tissue-engineered construct derived from rabbit synovial mesenchymal stem cells. J Pediatr Orthop. 2012;32:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Schmitt JF, See KH, Yang Z, Hui JH, Lee EH. Sequential differentiation of mesenchymal stem cells in an agarose scaffold promotes a physis-like zonal alignment of chondrocytes. J Orthop Res. 2012;30:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Yee CS, Manilay JO, Chang JC, Hum NR, Murugesh DK, Bajwa J, Mendez ME, Economides AE, Horan DJ, Robling AG, Loots GG. Conditional Deletion of Sost in MSC-Derived Lineages Identifies Specific Cell-Type Contributions to Bone Mass and B-Cell Development. J Bone Miner Res. 2018;33:1748-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 90. | Hui JH, Li L, Teo YH, Ouyang HW, Lee EH. Comparative study of the ability of mesenchymal stem cells derived from bone marrow, periosteum, and adipose tissue in treatment of partial growth arrest in rabbit. Tissue Eng. 2005;11:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Chen F, Hui JH, Chan WK, Lee EH. Cultured mesenchymal stem cell transfers in the treatment of partial growth arrest. J Pediatr Orthop. 2003;23:425-429. [PubMed] [DOI] [Full Text] |
| 92. | McCarty RC, Xian CJ, Gronthos S, Zannettino AC, Foster BK. Application of autologous bone marrow derived mesenchymal stem cells to an ovine model of growth plate cartilage injury. Open Orthop J. 2010;4:204-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Lee SU, Lee JY, Joo SY, Lee YS, Jeong C. Transplantation of a Scaffold-Free Cartilage Tissue Analogue for the Treatment of Physeal Cartilage Injury of the Proximal Tibia in Rabbits. Yonsei Med J. 2016;57:441-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Tomaszewski R, Wiktor Ł, Gap A. Orthotopic Autologous Chondrocyte Grafting as a Method of Treatment of Growth Plate Damage in Rabbits. Ortop Traumatol Rehabil. 2016;18:485-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | Tomaszewski R, Bohosiewicz J, Gap A, Bursig H, Wysocka A. Autogenous cultured growth plate chondrocyte transplantation in the treatment of physeal injury in rabbits. Bone Joint Res. 2014;3:310-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Li W, Xu R, Xue Y, Huang J, Gao Y. Treatment of growth plate injury with microencapsulated chondrocytes. Biotechnol Bioproc E. 2013;18:655-662. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Boopalan PRJVC, Varghese VD, Sathishkumar S, Arumugam S, Amarnath V. Similar regeneration of articular cartilage defects with autologous & allogenic chondrocytes in a rabbit model. Indian J Med Res. 2019;149:650-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Chung R, Xian CJ. Recent research on the growth plate: Mechanisms for growth plate injury repair and potential cell-based therapies for regeneration. J Mol Endocrinol. 2014;53:T45-T61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 99. | McCarty RC, Gronthos S, Zannettino AC, Foster BK, Xian CJ. Characterisation and developmental potential of ovine bone marrow derived mesenchymal stem cells. J Cell Physiol. 2009;219:324-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 100. | Yamaguchi A. Regulation of differentiation pathway of skeletal mesenchymal cells in cell lines by transforming growth factor-beta superfamily. Semin Cell Biol. 1995;6:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1828] [Cited by in RCA: 1734] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 102. | Wang X, Li Z, Wang C, Bai H, Wang Z, Liu Y, Bao Y, Ren M, Liu H, Wang J. Enlightenment of Growth Plate Regeneration Based on Cartilage Repair Theory: A Review. Front Bioeng Biotechnol. 2021;9:654087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Chen MJ, Whiteley JP, Please CP, Schwab A, Ehlicke F, Waters SL, Byrne HM. Inducing chondrogenesis in MSC/chondrocyte co-cultures using exogenous TGF-β: a mathematical model. J Theor Biol. 2018;439:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Dahlin RL, Ni M, Meretoja VV, Kasper FK, Mikos AG. TGF-β3-induced chondrogenesis in co-cultures of chondrocytes and mesenchymal stem cells on biodegradable scaffolds. Biomaterials. 2014;35:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 105. | Mahran YF, Badr AM, Aldosari A, Bin-Zaid R, Alotaibi HN. Carvacrol and Thymol Modulate the Cross-Talk between TNF-α and IGF-1 Signaling in Radiotherapy-Induced Ovarian Failure. Oxid Med Cell Longev. 2019;2019:3173745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Midyett LK, Rogol AD, Van Meter QL, Frane J, Bright GM; MS301 Study Group. Recombinant insulin-like growth factor (IGF)-I treatment in short children with low IGF-I levels: first-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2010;95:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 107. | Choi JH, Kim JS, Kim WK, Lee W, Kim N, Song CU, Jung JJ, Song JE, Khang G. Evaluation of Hyaluronic Acid/Agarose Hydrogel for Cartilage Tissue Engineering Biomaterial. Macromol Res. 2020;28:979-985. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Uz U, Gunhan K, Vatansever S, Kivanc M, Yuceturk AV. Novel Simple Strategy for Cartilage Tissue Engineering Using Stem Cells and Synthetic Polymer Scaffold. J Craniofac Surg. 2019;30:940-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 109. | Keplinger T, Wang X, Burgert I. Nanofibrillated cellulose composites and wood derived scaffolds for functional materials. J Mater Chem A. 2019;7:2981-2992. [DOI] [Full Text] |
| 110. | Trakoolwannachai V, Kheolamai P, Ummartyotin S. Characterization of hydroxyapatite from eggshell waste and polycaprolactone (PCL) composite for scaffold material. Compos B Eng. 2019;173:106974. [DOI] [Full Text] |
| 111. | Wang J, Yang Q, Cheng N, Tao X, Zhang Z, Sun X, Zhang Q. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater Sci Eng C Mater Biol Appl. 2016;61:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 112. | Shuler FD, Georgescu HI, Niyibizi C, Studer RK, Mi Z, Johnstone B, Robbins RD, Evans CH. Increased matrix synthesis following adenoviral transfer of a transforming growth factor beta1 gene into articular chondrocytes. J Orthop Res. 2000;18:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 113. | Smith P, Shuler FD, Georgescu HI, Ghivizzani SC, Johnstone B, Niyibizi C, Robbins PD, Evans CH. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43:1156-1164. [PubMed] [DOI] [Full Text] |
| 114. | Hidaka C, Quitoriano M, Warren RF, Crystal RG. Enhanced matrix synthesis and in vitro formation of cartilage-like tissue by genetically modified chondrocytes expressing BMP-7. J Orthop Res. 2001;19:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 115. | Hu C, Wu Y, Wan Y, Wang Q, Song J. Introduction of hIGF-1 gene into bone marrow stromal cells and its effects on the cell's biological behaviors. Cell Transplant. 2008;17:1067-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Palmer GD, Steinert A, Pascher A, Gouze E, Gouze JN, Betz O, Johnstone B, Evans CH, Ghivizzani SC. Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Mol Ther. 2005;12:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 117. | Chung R, Foster BK, Xian CJ. Preclinical studies on mesenchymal stem cell-based therapy for growth plate cartilage injury repair. Stem Cells Int. 2011;2011:570125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |