Published online May 18, 2025. doi: 10.5312/wjo.v16.i5.106183
Revised: March 18, 2025
Accepted: April 17, 2025
Published online: May 18, 2025
Processing time: 87 Days and 5.3 Hours
Spinal cord injury (SCI) is a severe and permanent trauma that often leads to significant motor, sensory, and autonomic dysfunction. Neuronal apoptosis is a major pathomechanism underlying secondary injury in SCI. Long non-coding RNAs (lncRNAs) have emerged as key regulators of gene expression and cellular processes, including apoptosis. However, the role of lncRNA growth arrest-specific transcript 5 (GAS5) in SCI-induced neuronal apoptosis remains unclear.
To investigate the role of lncRNA GAS5 in SCI-induced neuronal apoptosis via its interaction with microRNA (miR)-21 and the phosphatase and tensin homolog (PTEN)/AKT pathway.
SCI rat models and hypoxic neuronal cell models were established. Motor function was assessed using the Basso-Beattie-Bresnahan score. Expression levels of GAS5, miR-21, PTEN, caspase 3, B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), and AKT were measured using quantitative PCR or Western blot analysis. Neuronal apoptosis was determined by TUNEL staining. Dual-luciferase reporter assays validated GAS5-miR-21 binding. Knockdown and overexpression experiments explored the functional effects of the GAS5/miR-21 axis.
GAS5 was significantly upregulated in the spinal cord following SCI, coinciding with increased neuronal apoptosis and decreased AKT activation. In vitro experiments demonstrated that GAS5 acted as a molecular sponge for miR-21, leading to increased PTEN expression and inhibition of the AKT signaling pathway, thereby promoting apoptosis. In vivo, GAS5 knockdown attenuated neuronal apoptosis, enhanced AKT activation, and improved motor function recovery in SCI rats.
GAS5 promotes neuronal apoptosis in SCI by binding to miR-21 and upregulating PTEN expression, inhibiting the AKT pathway. Targeting GAS5 may represent a novel therapeutic strategy for SCI.
Core Tip: This study investigated the role of long non-coding RNA growth arrest-specific transcript 5 (GAS5) in spinal cord injury (SCI)-induced neuronal apoptosis. We demonstrate that GAS5 acts as a molecular sponge to bind miR-21, thereby upregulating phosphatase and tensin homolog (PTEN) expression and inhibiting the AKT signaling pathway, ultimately promoting neuronal apoptosis. Inhibiting GAS5 expression alleviates neuronal apoptosis and improves motor function recovery in SCI rat models, suggesting that targeting GAS5 may be a promising therapeutic strategy for SCI.
- Citation: Wang YJ, Zhi ZZ, Liu T, Kang J, Xu GH. Long non-coding RNA GAS5 promotes neuronal apoptosis in spinal cord injury via the miR-21/PTEN axis. World J Orthop 2025; 16(5): 106183
- URL: https://www.wjgnet.com/2218-5836/full/v16/i5/106183.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i5.106183
Spinal cord injury (SCI) is a severe and permanent trauma frequently resulting from traffic accidents or falls, leading to loss of motor, sensory, and autonomic functions[1]. SCI affects approximately 250000 to 1000000 people globally each year, posing a substantial global health concern[2]. SCI is characterized by a high incidence rate, challenging medical care, unfavorable prognosis, and substantial treatment costs. The process of SCI can be divided into primary and secondary injuries[3,4]. Primary injuries are caused by uncontrollable mechanical factors such as fractures and compressions. Secondary injuries, involving inflammatory reactions, tissue hypoxia, neuronal apoptosis, and necrosis, exacerbate the condition[5]. Therefore, it is crucial to study the mechanisms behind these pathological changes to minimize secondary injuries and prevent further damage.
Long non-coding RNAs (lncRNAs) are RNA molecules with transcript lengths exceeding 200 nucleotides that do not encode proteins[6]. These molecules play a crucial role in regulating gene expression through various mechanisms, including epigenetic, transcriptional, and post-transcriptional regulation. Numerous studies have shown that lncRNAs are involved in regulating various pathological processes following SCI. For example, Zhou et al[7] demonstrated that lncGBP9 regulates macrophage phenotypes via the signal transducer and activator of transcription 1 (STAT1)/STAT6 signaling pathway. Yang et al[8] found that inhibiting lncRNA X chromosome inactivation exacerbates SCI by promoting microglia M2 polarization through the miR-124-3p/interferon regulatory factor 1 axis. Bai et al[9] discovered that the lncRNA nuclear enriched abundant transcript 1 facilitates spinal cord regeneration after injury by targeting microRNA (miR)-29b. Growth arrest-specific transcript 5 (GAS5), an lncRNA, has been extensively studied and is implicated in the progression of various diseases including cancer, cardiovascular diseases, rheumatoid arthritis, diabetes, and neurolo
LncRNAs can function as competitive endogenous RNAs (ceRNAs) by binding to miRs and releasing their inhibitory effects on target mRNAs[13]. GAS5 interacts with various miRs, including miR-429, miR-26, and miR-128-3p, affecting cellular functions[14-16]. However, the effects of GAS5 on neuronal apoptosis following SCI have not been extensively studied. Therefore, this study investigated the role of GAS5 in neuronal apoptosis in SCI and elucidated its potential mechanisms through in vitro and in vivo experiments.
All animal procedures were conducted in strict compliance with the guidelines set by the Animal Care and Use Commi
Adult Sprague Dawley rats (weighing 200 ± 20 g) were obtained from Vital River Laboratories (Beijing, China) and housed in a specific pathogen-free environment at 23 ± 2 °C with a 12-hour light-dark cycle. The rats were allowed free access to food and water. Rats were acclimated to the environment for at least 1 week before the experiment. Anesthesia was induced using 1% pentobarbital sodium (50 mg/kg). After sterile preparation, the rats’ backs were shaved, and the skin was sterilized. A midline incision was made to expose the spinal cord, followed by laminectomy at the T9/10 level. The SCI model was induced using Allen’s method, with a 10-g weight dropped from a height of 5 cm onto the dura mater at the T10 level[17]. Successful SCI was confirmed by paralysis of the hind limbs, spasmodic tail movement, and spinal cord hematoma. The muscle and skin were sutured layer by layer, and the rats’ bladders were manually expressed twice daily until voluntary urination resumed. The sham group underwent laminectomy without SCI.
The sample size for this study was determined based on prior research and statistical power analysis. Previous studies on SCI models have shown that a minimum of six animals per group is required to detect significant differences in motor function and histological outcomes with a power of 80% and a significance level of 0.05[18]. Therefore, we used six rats per group in our study to ensure sufficient statistical power to detect significant differences between the SCI and sham groups. This sample size was also validated through preliminary experiments to ensure the reliability and reproducibility of our results.
PC-12 cells, derived from rat pheochromocytoma, were used to mimic neuronal injury in SCI models[19]. The cells were purchased from ATCC (Manassas, VA, United States) and maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C. The OGD/R method was used to create the injury model. Briefly, once the cells reached the logarithmic growth phase, glucose- and serum-free medium replaced the culture medium, and the cells were placed in a low-oxygen chamber (1% oxygen) for 2 hours. Subsequently, the cells were returned to normal conditions for reoxygenation at various time points (0, 0.5, 1, 2, 4, 8, 16, and 24 hours).
The locomotor function of rats was assessed using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale[20]. Rats were placed on a circular platform, and their hindlimb walking and limb activity were scored. The BBB score ranges from 0 to 21 points, with higher scores indicating better motor function. Each group was scored one day before surgery and at 1, 3, 7, and 14 days post-injury.
Total RNA was isolated from spinal cord tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer's instructions. Reverse transcription was performed using the EasyScript RT Reagent Kit (TransGen Biotech, Beijing, China). Quantitative PCR (qPCR) analysis was conducted using SYBR Premix Ex Taq II (Takara, Kyoto, Japan) on the Roche Light Cycler 480 system (Roche, Welwyn Garden City, Hertfordshirem, United Kingdom). Target gene mRNA levels were normalized to β-actin as the reference gene. Primer sequences are listed in Supplementary Table 1.
Total protein was extracted from rat spinal cord tissue or cells using RIPA lysis buffer (Solarbio, Beijing, China). Protein concentration was determined using the BCA Protein Assay Kit (Beyotime, Wuhan, China). Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked in 5% bovine serum albumin at room temperature for 1 hour, followed by incubation with primary antibodies (1:1000 to 2000) overnight at 4 °C. After washing, horseradish peroxidase-conjugated secondary antibodies (1:5000 to 8000) were applied for 1 hour at room temperature. Bands were visualized using an ECL detection kit (Beyotime, Wuhan, China), and grayscale values were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, United States).
Binding sites between GAS5 and miR-21 were predicted using TargetScan (www.targetscan.org). Wild-type and mutant GAS5 3’-UTR fragments containing miR-21 binding sites were amplified by PCR and cloned into pcDNA vectors. PC-12 cells were co-transfected with miR-21 mimic and wild-type/mutant GAS5 vectors using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, United States). The control group was co-transfected with empty vectors and miR-21 mimic. Luciferase activity was measured 48 hours post-transfection using the Dual-Luciferase Reporter Assay System (Promega Corporation, Madison, WI, United States).
Cells were fixed in 4% formaldehyde, permeabilized, and incubated with primary antibodies (phosphorylated AKT [p-AKT], 1:100; B-cell lymphoma-2 [Bcl-2]-associated X protein [Bax], 1:100) and secondary antibodies for 1 hour each. Samples were counterstained with DAPI (0.5 µg/mL) and examined using an Imager A2 Axio upright microscope (Zeiss, Jena, Germany) to analyze specific gene expression.
Cellular apoptosis was analyzed using the TUNEL assay kit (Beyotime, Wuhan, China) according to the manufacturer's instructions. Images were captured using a fluorescence microscope for further analysis.
Sprague Dawley rats received intrathecal injection of 10 μL lentivirus (5 × 108 TU/mL) 30 minutes before SCI surgery. Rats were then fed normally for 1 week, after which relevant tissues were collected.
Data are presented as the mean ± SD (n ≥ 3). Statistical analyses were performed using GraphPad Prism 9.0 software. Comparisons between two groups were made using non-parametric t-tests. For multiple group compari
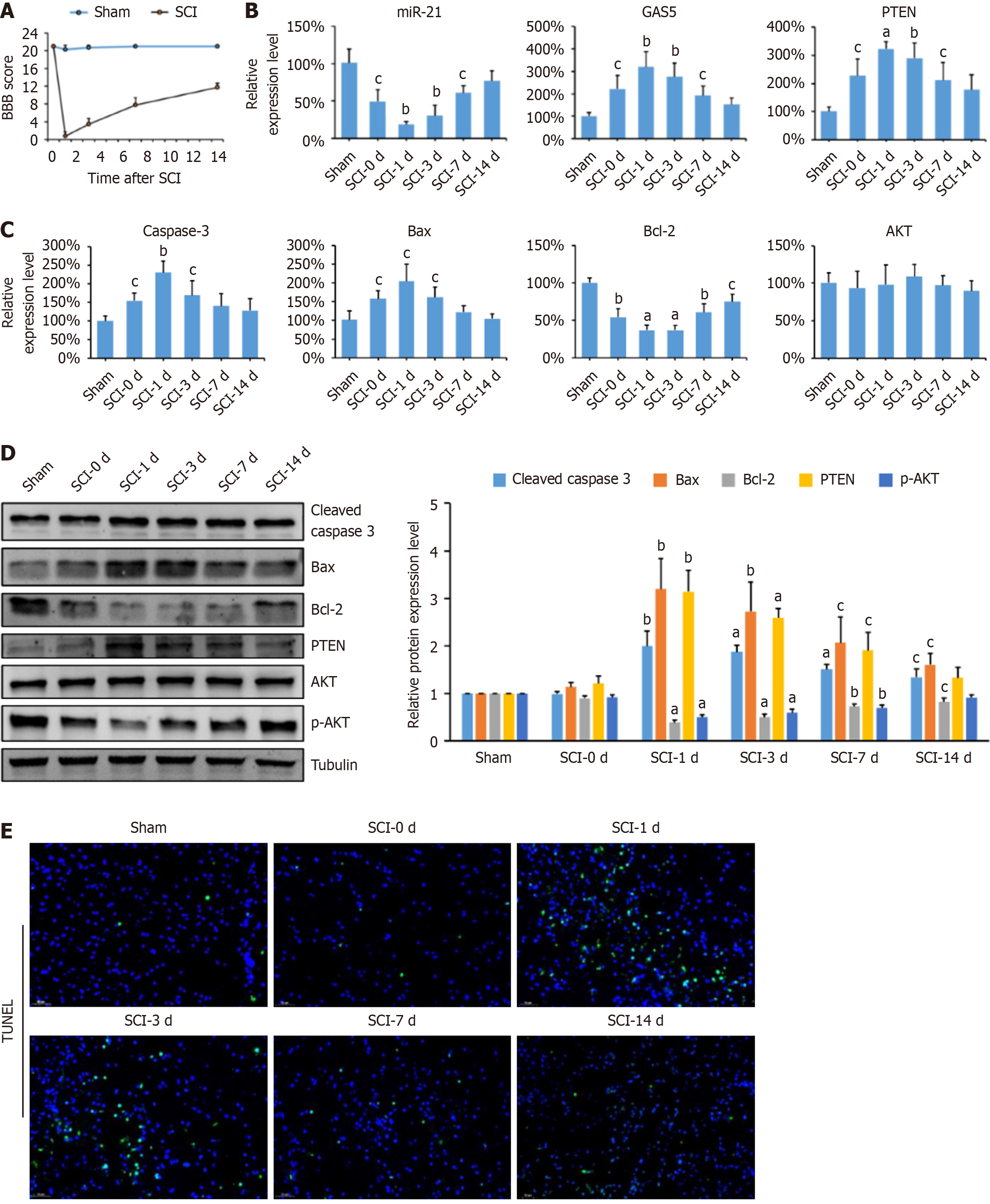
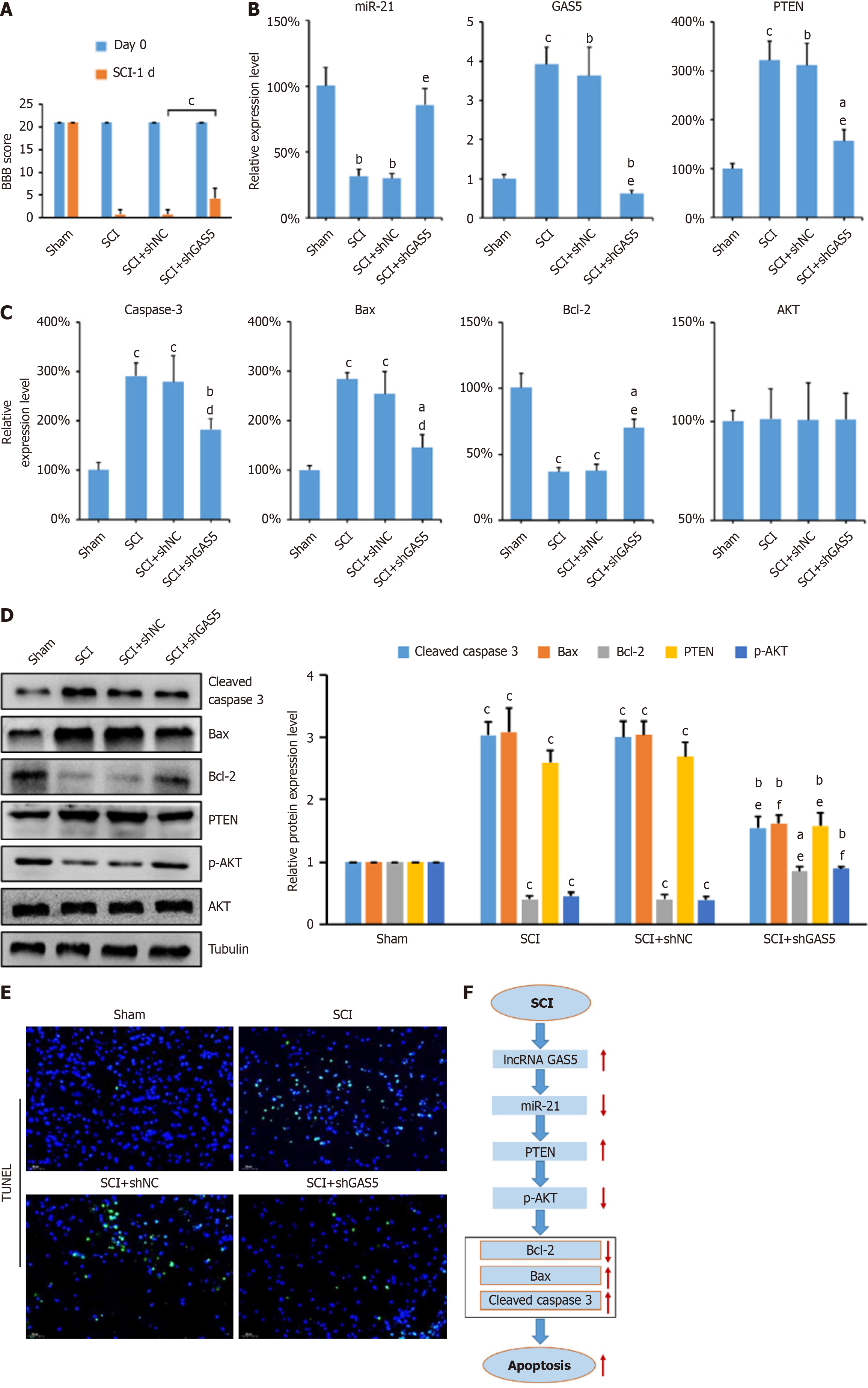
The locomotor function of SCI rats was assessed using the BBB score on postoperative days 0, 1, 3, 7, and 14. SCI rats showed significantly lower BBB scores than the sham group (Figure 1A). Even after 14 days, a significant difference remained between the SCI and sham groups. Spinal cord tissues were collected for qPCR, Western blotting, and the TUNEL assays. GAS5 and PTEN expression levels were significantly higher in SCI rats, whereas miR-21 expression was lower compared to the sham group (Figure 1B). The levels of apoptosis-related factors (caspase-3, Bax, Bcl-2) were measured, revealing increased pro-apoptotic factors (caspase-3 and Bax) and decreased anti-apoptotic factor (Bcl-2) in SCI rats. Total AKT expression remained unchanged (Figure 1C and D). However, p-AKT levels were significantly decreased in SCI rats, indicating inhibition of the AKT signaling pathway. TUNEL staining showed a significant increase in apoptotic cells during the early stages of SCI, particularly on days 1 and 3 (Figure 1E). These results suggest a close relationship between GAS5 expression and SCI-induced neuronal apoptosis.
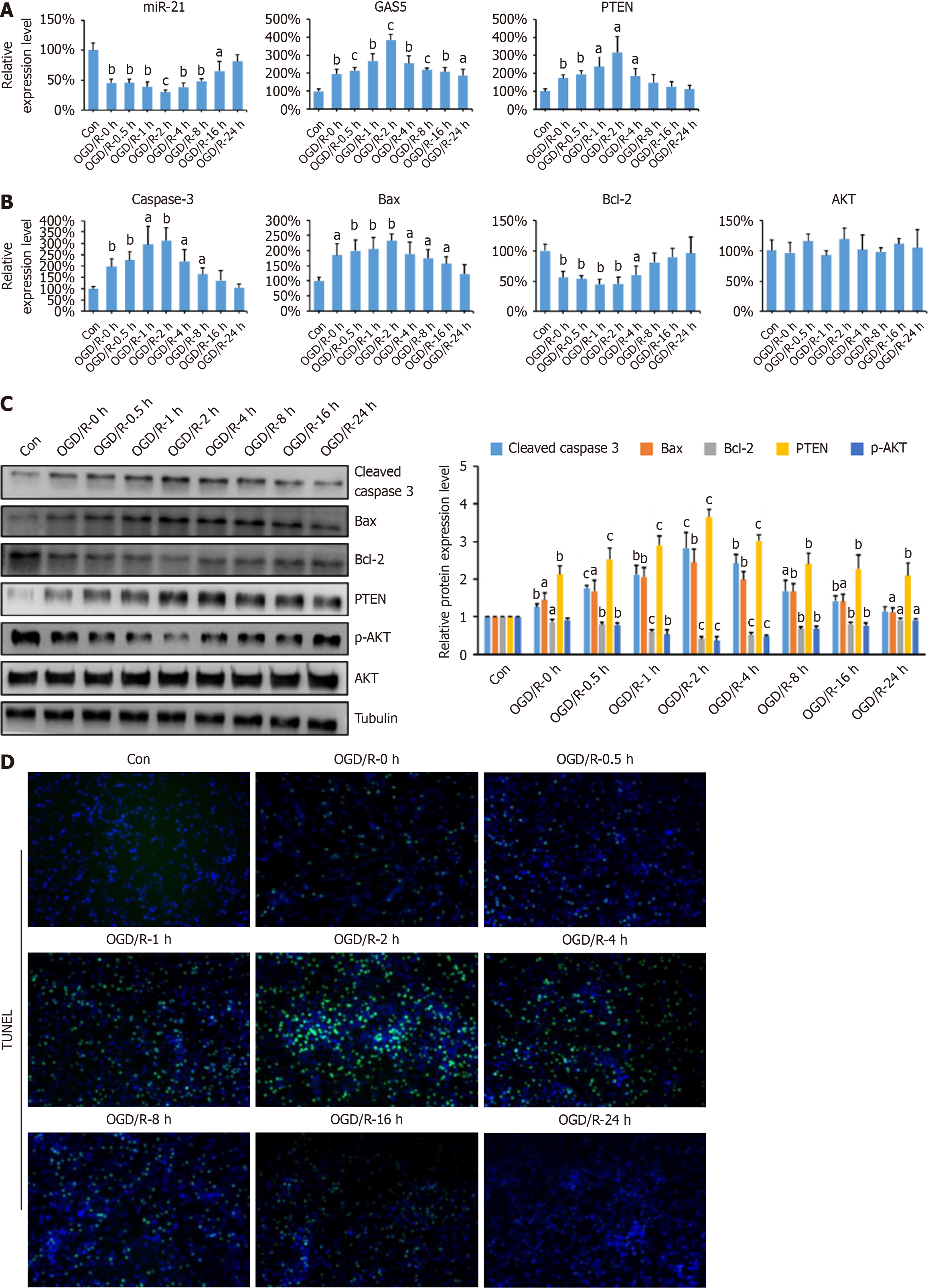
Hypoxia and tissue ischemia are common secondary damages in SCI, causing severe neuronal dysfunction. Therefore, hypoxic cell models are often used to simulate SCI[21]. PC-12 cells were subjected to OGD/R treatment to establish an in vitro SCI model. Initial miR-21 expression decreased and then gradually increased, returning to control levels after 24 hours of reoxygenation (Figure 2A). Similar trends were observed for Bcl-2, GAS5, PTEN, Bax, and caspase-3 mRNA levels, with maximum changes at 2 hours of reoxygenation (Figure 2A and B). Protein levels exhibited similar changes. p-AKT levels decreased initially and then increased, consistent with Bcl-2 protein levels (Figure 2C). TUNEL staining showed that cell apoptosis peaked at 2 hours of reoxygenation (Figure 2D). These findings indicate that GAS5 promotes neuronal apoptosis in the PC12 OGD/R model, with 2 hours of reoxygenation being optimal for subsequent experiments.
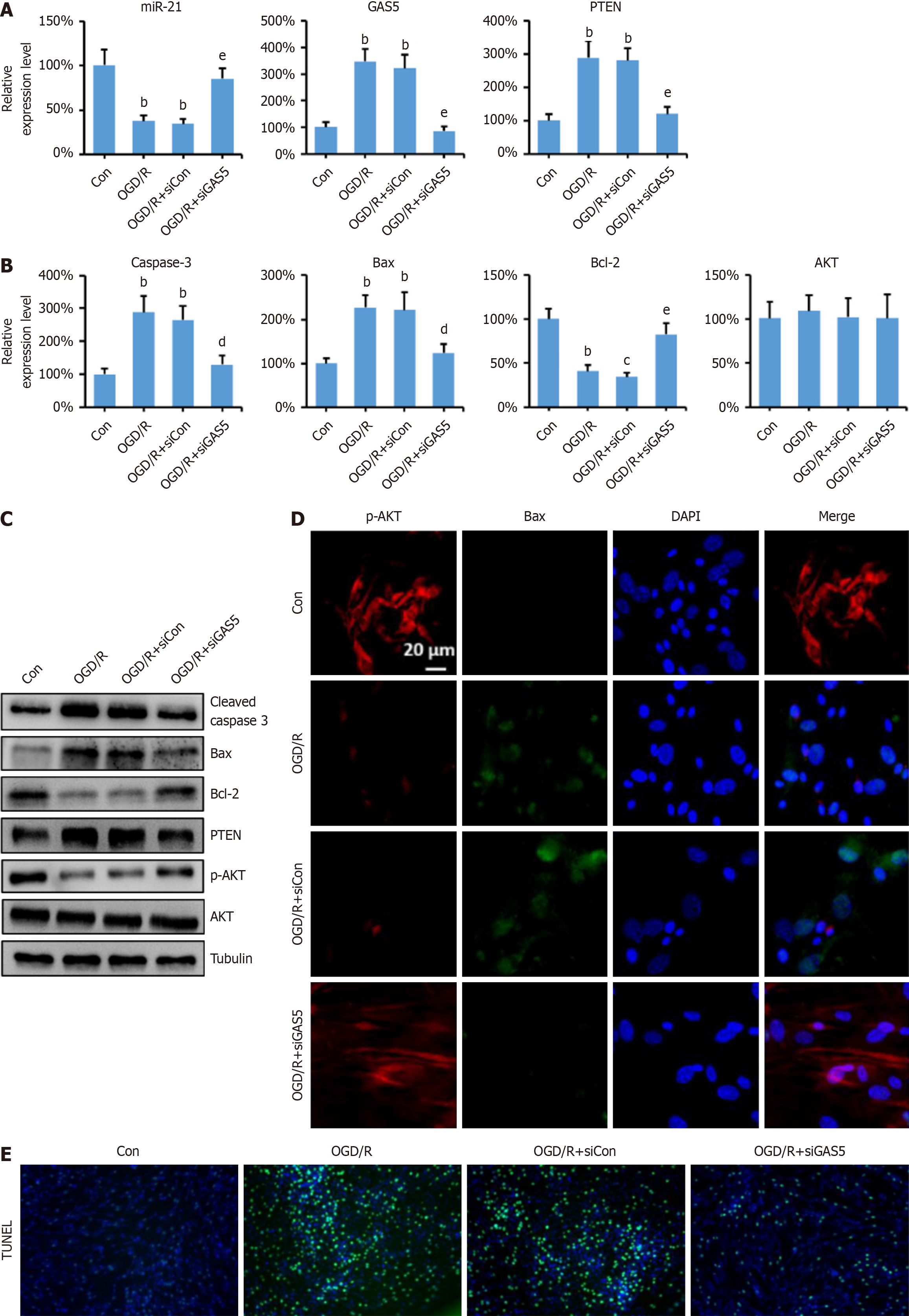
To investigate the effects of GAS5 on neuronal apoptosis, GAS5 expression was silenced in PC-12 cells using LV-siRNA transfection. qPCR results showed that GAS5 knockdown restored miR-21 Levels, which decreased after OGD/R treatment (Figure 3A). Similar restoration was observed for PTEN, Bcl-2, Bax, and caspase-3 mRNA levels (Figure 3A and B). Western blot analysis confirmed the regulation of GAS5 on protein levels (Figure 3C). Immunofluorescence results were consistent with Western blot findings (Figure 3D). TUNEL staining demonstrated that GAS5 knockdown signifi
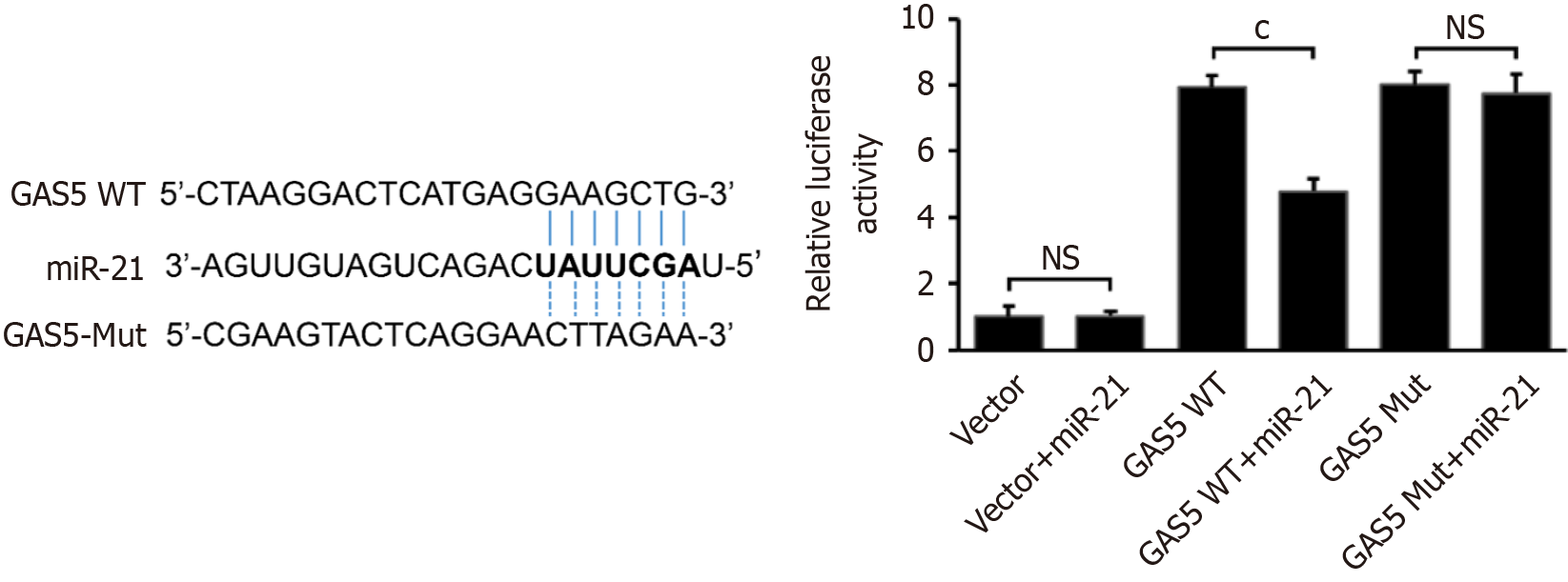
LncRNAs can regulate miR expression through the ceRNA mechanism, impacting target gene expression[13]. Numerous studies have shown that this regulatory mechanism significantly contributes to the physiological and pathological changes observed in a variety of diseases, including tumors, cardiovascular diseases, and cerebrovascular diseases[22-26]. Given the opposite trends of GAS5 and miR-21 in the SCI model, we hypothesized that GAS5 and miR-21 interact to regulate neuronal apoptosis. To elucidate the interaction between GAS5 and miR-21, we first predicted the binding sites using TargetScan and constructed wild-type (GAS5 WT) and mutant (GAS5 Mut) plasmids containing the miR-21 binding sites. Dual-luciferase reporter assays showed that miR-21 significantly reduced luciferase activity in cells transfected with GAS5 WT, but not in those with GAS5 Mut (Figure 4A). This confirmed that GAS5 directly binds to miR-21, acting as a molecular sponge.
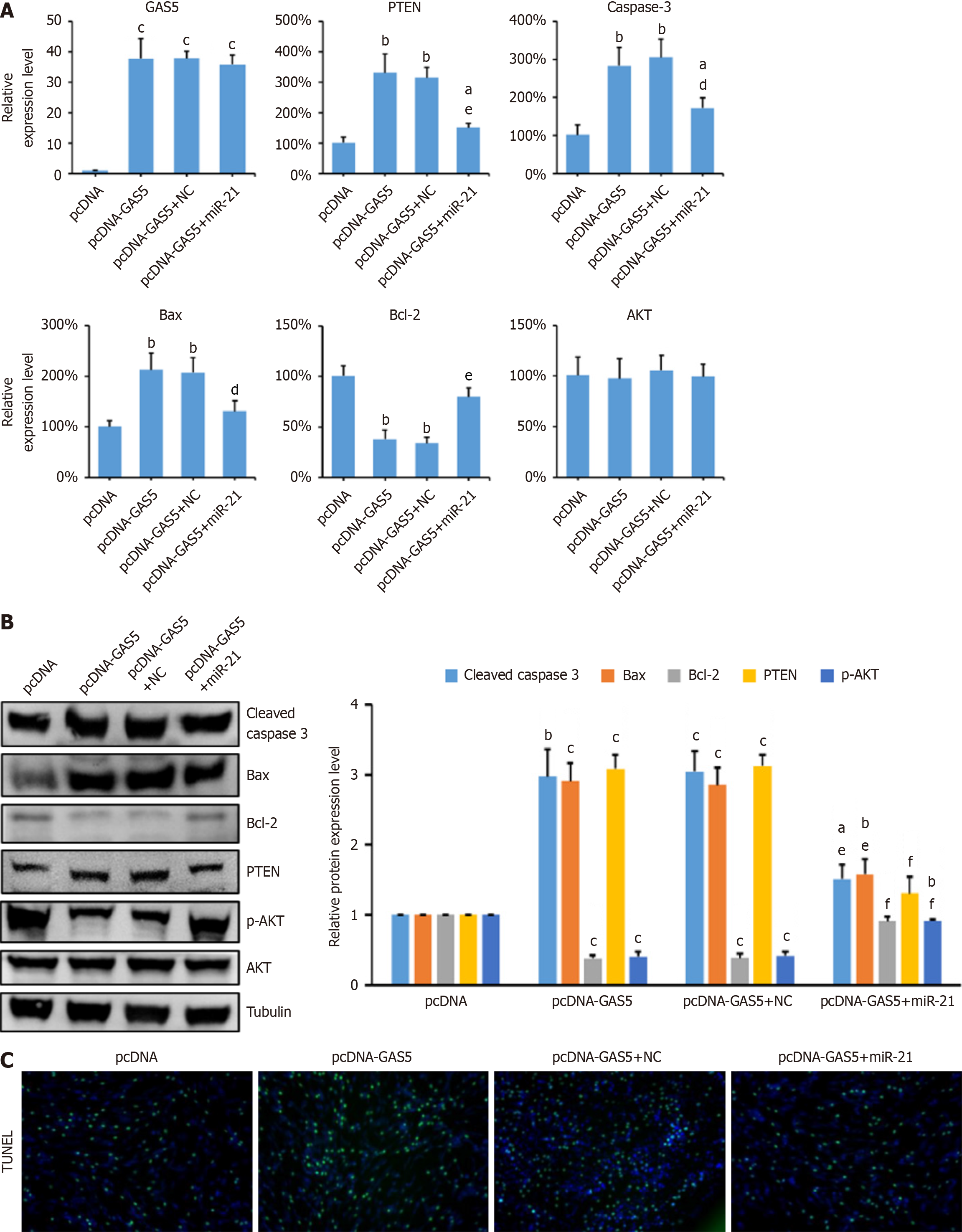
Given that miR-21 is known to target PTEN, we further investigated whether GAS5 regulates PTEN expression through miR-21. PC-12 cells were transfected with pcDNA-GAS5 alone or in combination with miR-21 mimics. Overexpression of GAS5 increased PTEN expression and reduced p-AKT levels, indicative of AKT pathway inhibition. How
In order to determine if GAS5 regulates PTEN through targeting miR-21 in neuronal cells, pcDNA-GAS5 and pcDNA-GAS5 combined with miR-21 mimic were transfected into PC-12 cells. The results showed that the changes in the expression levels of PTEN, Bcl-2, Bax, and Caspase 3 caused by overexpression of GAS5 can be inhibited by concurrently overexpressed miR-21 (Figure 5A). The results of the western blot and qRT-PCR showed consistency. The protein expression level of p-AKT significantly decreased when GAS5 was overexpressed, but it was significantly restored when miR-21 was overexpressed (Figure 5B). The staining results of TUNEL demonstrated that overexpression of miR-21 was capable of considerably reducing the levels of cell apoptosis caused by the overexpression of GAS5 (Figure 5C). The results mentioned above indicated that GAS5 could act as a lure for miR-21, resulting in the release of PTEN expression, which led to the suppression of AKT signaling pathway and ultimately neuronal apoptosis.
To confirm the anti-apoptotic effects of GAS5 knockdown in SCI rats, shGAS5 and shNC plasmids were synthesized and injected into the spinal cord of SCI rats. Motor function significantly improved in GAS5-knockdown rats one day post-injury (Figure 6A). qPCR and Western blot analyses showed that GAS5 knockdown reduced PTEN expression and restored p-AKT levels (Figure 6B-D). TUNEL staining indicated decreased apoptosis in the spinal cord tissues of GAS5-knockdown rats (Figure 6E). These findings suggest that GAS5 enhances neuronal apoptosis via the miR-21/PTEN axis (Figure 6F), and its inhibition may be a potential therapeutic target for SCI treatment.
This study provides novel insights into the molecular mechanisms underlying neuronal apoptosis following SCI by elucidating the role of lncRNAs GAS5. Our findings demonstrate that GAS5 is significantly upregulated in SCI rats and promotes neuronal apoptosis through its interaction with miR-21 and the downstream PTEN/AKT signaling pathway. Specifically, GAS5 acts as a molecular sponge for miR-21, leading to increased PTEN expression and inhibition of the AKT signaling pathway, which ultimately facilitates neuronal apoptosis. These results highlight the potential therapeutic value of targeting GAS5 in SCI treatment.
SCI is characterized by a cascade of secondary pathological processes, including inflammation, hypoxia, and neuronal apoptosis, which exacerbate tissue damage and functional deficits[27]. Neuronal apoptosis is a critical component of secondary injury, contributing significantly to the long-term disability observed in SCI patients[28]. Our study reveals that GAS5 is significantly upregulated in SCI rats, coinciding with increased neuronal apoptosis and decreased AKT activation. This finding is consistent with previous studies demonstrating the role of GAS5 in promoting apoptosis in other neurological conditions, such as ischemic brain injury[11] and acute kidney injury[29]. GAS5 has been shown to function as a molecular sponge for miRs, thereby regulating gene expression at the post-transcriptional level[30]. In SCI, GAS5 binds to miR-21, a well-known anti-apoptotic miR, leading to increased PTEN expression and inhibition of the AKT signaling pathway. This interaction ultimately promotes neuronal apoptosis and worsens secondary injury.
miR-21 is a well-studied miR with established roles in inhibiting apoptosis across various tissues, including neurons[31,32]. Our study confirms that miR-21 overexpression reduces neuronal apoptosis and enhances functional recovery in SCI rats. This finding is consistent with recent studies demonstrating the therapeutic potential of miR-21 in SCI. For example, exosome-derived miR-21 from mesenchymal stem cells reduces neuronal apoptosis and promotes functional recovery in SCI rats[17]. Additionally, a recent study by Liu et al[33] demonstrated that miR-21 delivery via exosomes could further enhance neuronal survival and functional recovery in sciatic nerve injury models[33]. These studies collectively highlight the importance of miR-21 in neuron injury and suggest that its upregulation may be a viable therapeutic strategy.
PTEN is a well-known tumor suppressor that negatively regulates the AKT signaling pathway by dephosphorylating AKT[34]. In SCI, PTEN upregulation is associated with increased neuronal apoptosis and functional deficits[35]. Our study demonstrates that GAS5-mediated PTEN upregulation inhibits AKT activation, leading to increased neuronal apoptosis. This finding is supported by previous studies showing that PTEN inhibition can promote neuronal survival and functional recovery in SCI[36]. A recent study reported that pharmacological inhibition of PTEN significantly improved motor function in SCI rats by enhancing AKT signaling[37]. Additionally, a study showed that PTEN inhibition reduces neuronal apoptosis and promotes axonal regeneration in SCI models[38]. These studies collectively highlight the importance of PTEN in SCI and suggest that targeting PTEN may be a promising therapeutic strategy.
Despite our findings, this study has several limitations. First, our in vitro experiments used the PC-12 cell line, which may not fully represent primary neurons. Future studies should validate these findings in primary neuronal cultures. Second, our study focused primarily on neuronal apoptosis and did not comprehensively address other aspects of secondary injury, such as inflammation and glial scar formation. Future research should explore the role of GAS5 in these processes. Third, our study lacked histopathological analysis of SCI in rats, which could provide additional insights into the mechanisms of GAS5 in SCI. Finally, the therapeutic potential of targeting GAS5 should be further explored in preclinical and clinical settings.
The lncRNA GAS5 promotes neuronal apoptosis in SCI by binding to miR-21 and upregulating PTEN, inhibiting AKT phosphorylation. Silencing GAS5 significantly reduces neuronal apoptosis and improves SCI outcomes. Our findings suggest that the GAS5/miR-21/PTEN axis could offer new perspectives for SCI prevention and treatment.
| 1. | McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 805] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 2. | Karsy M, Hawryluk G. Modern Medical Management of Spinal Cord Injury. Curr Neurol Neurosci Rep. 2019;19:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 3. | Eckert MJ, Martin MJ. Trauma: Spinal Cord Injury. Surg Clin North Am. 2017;97:1031-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 4. | Mourelo Fariña M, Salvador de la Barrera S, Montoto Marqués A, Ferreiro Velasco ME, Galeiras Vázquez R. Update on traumatic acute spinal cord injury. Part 2. Med Intensiva. 2017;41:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Sterner RC, Sterner RM. Immune response following traumatic spinal cord injury: Pathophysiology and therapies. Front Immunol. 2022;13:1084101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 100] [Reference Citation Analysis (0)] |
| 6. | Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220:e202009045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 528] [Cited by in RCA: 976] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 7. | Zhou J, Li Z, Wu T, Zhao Q, Zhao Q, Cao Y. LncGBP9/miR-34a axis drives macrophages toward a phenotype conducive for spinal cord injury repair via STAT1/STAT6 and SOCS3. J Neuroinflammation. 2020;17:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Yang J, Gong Z, Dong J, Bi H, Wang B, Du K, Zhang C, Chen L. lncRNA XIST inhibition promotes M2 polarization of microglial and aggravates the spinal cord injury via regulating miR-124-3p / IRF1 axis. Heliyon. 2023;9:e17852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 9. | Bai G, Jiang L, Meng P, Li J, Han C, Wang Y, Wang Q. LncRNA Neat1 Promotes Regeneration after Spinal Cord Injury by Targeting miR-29b. J Mol Neurosci. 2021;71:1174-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Han X, Xu J, Chen Z, Li P, Zhao L, Tao J, Shen Y, Zhu S, Yu B, Zhu J, Cao Q, Zhou S. Gas5 inhibition promotes the axon regeneration in the adult mammalian nervous system. Exp Neurol. 2022;356:114157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 11. | Zhao RB, Zhu LH, Shu JP, Qiao LX, Xia ZK. GAS5 silencing protects against hypoxia/ischemia-induced neonatal brain injury. Biochem Biophys Res Commun. 2018;497:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Zhang XC, Gu AP, Zheng CY, Li YB, Liang HF, Wang HJ, Tang XL, Bai XX, Cai J. YY1/LncRNA GAS5 complex aggravates cerebral ischemia/reperfusion injury through enhancing neuronal glycolysis. Neuropharmacology. 2019;158:107682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2305] [Cited by in RCA: 3097] [Article Influence: 281.5] [Reference Citation Analysis (0)] |
| 14. | Peng T, Ji D, Jiang Y. Long non-coding RNA GAS5 suppresses rheumatoid arthritis progression via miR-128-3p/HDAC4 axis. Mol Cell Biochem. 2021;476:2491-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Zhu C, Zhang H, Wei D, Sun Z. Silencing lncRNA GAS5 alleviates apoptosis and fibrosis in diabetic cardiomyopathy by targeting miR-26a/b-5p. Acta Diabetol. 2021;58:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Li J, Liu S. LncRNA GAS5 suppresses inflammatory responses and apoptosis of alveolar epithelial cells by targeting miR-429/DUSP1. Exp Mol Pathol. 2020;113:104357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Kang J, Li Z, Zhi Z, Wang S, Xu G. MiR-21 derived from the exosomes of MSCs regulates the death and differentiation of neurons in patients with spinal cord injury. Gene Ther. 2019;26:491-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Wu X, Wei H, Wu JQ. Coding and long non-coding gene expression changes in the CNS traumatic injuries. Cell Mol Life Sci. 2022;79:123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Zhang T, Ni S, Luo Z, Lang Y, Hu J, Lu H. The protective effect of microRNA-21 in neurons after spinal cord injury. Spinal Cord. 2019;57:141-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Scheff SW, Saucier DA, Cain ME. A statistical method for analyzing rating scale data: the BBB locomotor score. J Neurotrauma. 2002;19:1251-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Cao Y, Jiang C, Lin H, Chen Z. Silencing of Long Noncoding RNA Growth Arrest-Specific 5 Alleviates Neuronal Cell Apoptosis and Inflammatory Responses Through Sponging microRNA-93 to Repress PTEN Expression in Spinal Cord Injury. Front Cell Neurosci. 2021;15:646788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3:1113-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 681] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 23. | Chen L, Wei K, Li J, Li Y, Cao H, Zheng Z. Integrated Analysis of LncRNA-Mediated ceRNA Network in Calcific Aortic Valve Disease. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Li S, Cao Y, Zhang H, Lu X, Wang T, Xu S, Kong T, Bo C, Li L, Ning S, Wang J, Wang L. Construction of lncRNA-Mediated ceRNA Network for Investigating Immune Pathogenesis of Ischemic Stroke. Mol Neurobiol. 2021;58:4758-4769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Mao M, Zhang J, Xiang Y, Gong M, Deng Y, Ye D. Role of exosomal competitive endogenous RNA (ceRNA) in diagnosis and treatment of malignant tumors. Bioengineered. 2022;13:12156-12168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 26. | Basera A, Hull R, Demetriou D, Bates DO, Kaufmann AM, Dlamini Z, Marima R. Competing Endogenous RNA (ceRNA) Networks and Splicing Switches in Cervical Cancer: HPV Oncogenesis, Clinical Significance and Therapeutic Opportunities. Microorganisms. 2022;10:1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front Neurol. 2019;10:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 808] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 28. | Gao G, Duan Y, Chang F, Zhang T, Huang X, Yu C. METTL14 promotes apoptosis of spinal cord neurons by inducing EEF1A2 m6A methylation in spinal cord injury. Cell Death Discov. 2022;8:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Geng X, Song N, Zhao S, Xu J, Liu Y, Fang Y, Liang M, Xu X, Ding X. LncRNA GAS5 promotes apoptosis as a competing endogenous RNA for miR-21 via thrombospondin 1 in ischemic AKI. Cell Death Discov. 2020;6:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Nguyen LNT, Pyburn JS, Nguyen NL, Schank MB, Zhao J, Wang L, Leshaodo TO, El Gazzar M, Moorman JP, Yao ZQ. Epigenetic Regulation by lncRNA GAS5/miRNA/mRNA Network in Human Diseases. Int J Mol Sci. 2025;26:1377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 31. | Jenike AE, Halushka MK. miR-21: a non-specific biomarker of all maladies. Biomark Res. 2021;9:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 32. | Han T, Song P, Wu Z, Liu Y, Ying W, Shen C. Inflammation Modifies miR-21 Expression Within Neuronal Extracellular Vesicles to Regulate Remyelination Following Spinal Cord Injury. Stem Cell Rev Rep. 2023;19:2024-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 33. | Liu YP, Yang YD, Mou FF, Zhu J, Li H, Zhao TT, Zhao Y, Shao SJ, Cui GH, Guo HD. Exosome-Mediated miR-21 Was Involved in the Promotion of Structural and Functional Recovery Effect Produced by Electroacupuncture in Sciatic Nerve Injury. Oxid Med Cell Longev. 2022;2022:7530102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Worby CA, Dixon JE. PTEN. Annu Rev Biochem. 2014;83:641-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 35. | Liu S, Jia J, Zhou H, Zhang C, Liu L, Liu J, Lu L, Li X, Kang Y, Lou Y, Cai Z, Ren Y, Kong X, Feng S. PTEN modulates neurites outgrowth and neuron apoptosis involving the PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 2019;20:4059-4066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | He X, Li Y, Deng B, Lin A, Zhang G, Ma M, Wang Y, Yang Y, Kang X. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities. Cell Prolif. 2022;55:e13275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 149] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 37. | Metcalfe M, Steward O. PTEN deletion in spinal pathways via retrograde transduction with AAV-RG enhances forelimb motor recovery after cervical spinal cord injury; Sex differences and late-onset pathophysiologies. Exp Neurol. 2023;370:114551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Shabanzadeh AP, D'Onofrio PM, Magharious M, Choi KAB, Monnier PP, Koeberle PD. Modifying PTEN recruitment promotes neuron survival, regeneration, and functional recovery after CNS injury. Cell Death Dis. 2019;10:567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |