Published online Apr 18, 2025. doi: 10.5312/wjo.v16.i4.104489
Revised: March 19, 2025
Accepted: March 20, 2025
Published online: April 18, 2025
Processing time: 116 Days and 5.6 Hours
Crush syndrome refers to the traumatic rhabdomyolysis leading to a spectrum of disorders culminating in acute kidney injury. The burden of crush syndrome is high, and mortality can be as high as 20%. The significant bulk of knowledge is from old articles. Over the last 10 years new research has occurred on diagnosis and treatment in animal models.
To overview of crush syndrome and discuss the newer advances related to the pathogenesis and management of a patient with crush syndrome.
The search of databases such as MEDLINE, Google Scholar, Web of Science, and EMBASE revealed 8226 articles. A thorough screening culminated in 83 crush syndrome articles included in this study.
Acute kidney injury in crush syndrome is currently thought to be due to iron retention. The management of crush syndrome has also been updated with antioxidants, and several gases are being used to treat crush syndrome. In the end, treatment of crush syndrome also includes mental, social, and physical rehabilitation for better outcomes.
The outcomes of crush syndrome have significantly improved with the intro
Core Tip: Crush syndrome is a life-threatening condition caused by traumatic rhabdomyolysis, resulting in systemic complications, particularly acute kidney injury. Pathophysiologically, muscle damage releases toxins such as myoglobin, potassium, and lactic acid into the bloodstream, leading to renal tubule damage, hyperkalemia-induced cardiac arrhythmias, metabolic acidosis, and coagulopathy. Modern insights highlight the role of ferroptosis, oxidative stress, and macrophage activity in acute kidney injury progression. Management emphasizes early recognition, fluid resuscitation, renal protection, and innovative therapies like antioxidants, hyperbaric oxygen, and erythropoietin. Comprehensive treatment also includes pre-hospital care, electrolyte correction, renal replacement therapy, and long-term rehabilitation to improve outcomes and quality of life.
- Citation: Khan S, Neradi D, Unnava N, Jain M, Tripathy SK. Pathophysiology and management of crush syndrome: A narrative review. World J Orthop 2025; 16(4): 104489
- URL: https://www.wjgnet.com/2218-5836/full/v16/i4/104489.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i4.104489
Crush syndrome occurs when prolonged pressure on muscles leads to the release of toxins into the bloodstream, potentially causing systemic complications such as renal failure[1]. This term gained popularity during World War II when the victims trapped under the fallen buildings appeared normal but later succumbed to renal failure. Also known as Bywaters’ syndrome, this condition arises from traumatic injury to muscles leading to rhabdomyolysis[2]. About 10% of patients with crush injuries end up with crush syndrome[2,3]. Since most of the patients end up in renal failure, the mortality due to crush syndrome can be as high as 20%[4]. Hence, a healthcare provider needs to understand the details of crush syndrome.
The bulk of our knowledge about crush syndrome comes from old articles. Much work has occurred in animal models in the last decade, and the results are promising. This review discussed these advances in the diagnosis and treatment of crush syndrome.
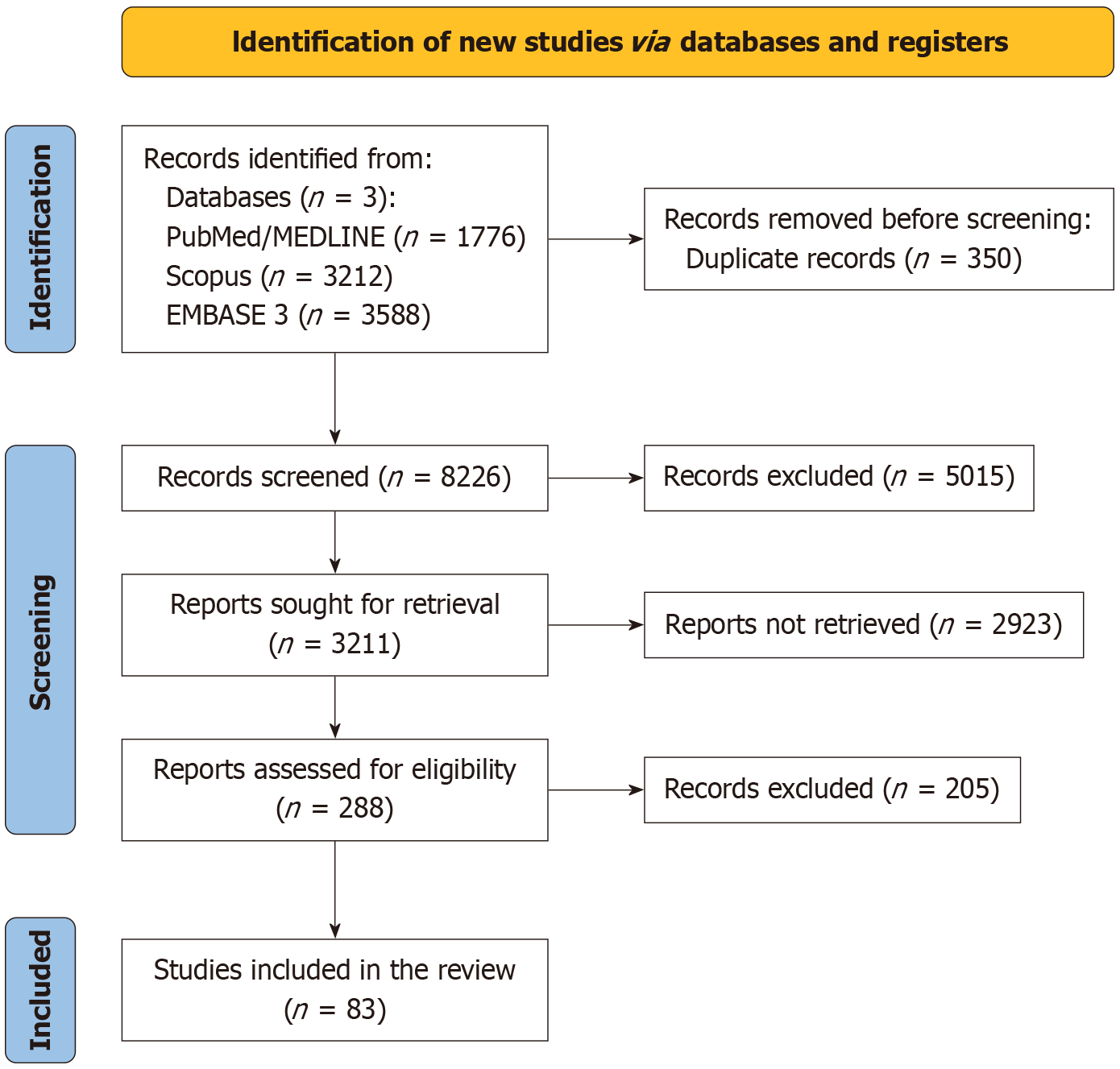
A thorough search was conducted in databases like MEDLINE, Google Scholar, Web of Science, and EMBASE using keywords like “crush syndrome,” “crush injury,” and “traumatic rhabdomyolysis” on December 1, 2024 by three authors (SK, DN, and NU). The search was limited to English-language literature. We meticulously evaluated the titles and abstracts of the retrieved articles for potential inclusion in this review. Furthermore, we examined the references of relevant articles and reviews to uncover additional studies related to our topic. Any disagreements in study selection were resolved through discussion among the authors, and if consensus was not reached, we consulted a senior author for guidance (SKT). We included all the original articles, review articles, case reports, case series, and in vitro studies (Figure 1). PRISMA guidelines were used to screen the available literature. Articles in languages other than English were excluded. Furthermore, we also excluded articles in which the full text was not available.
The search revealed 8226 results, and 83 articles were selected for this study.
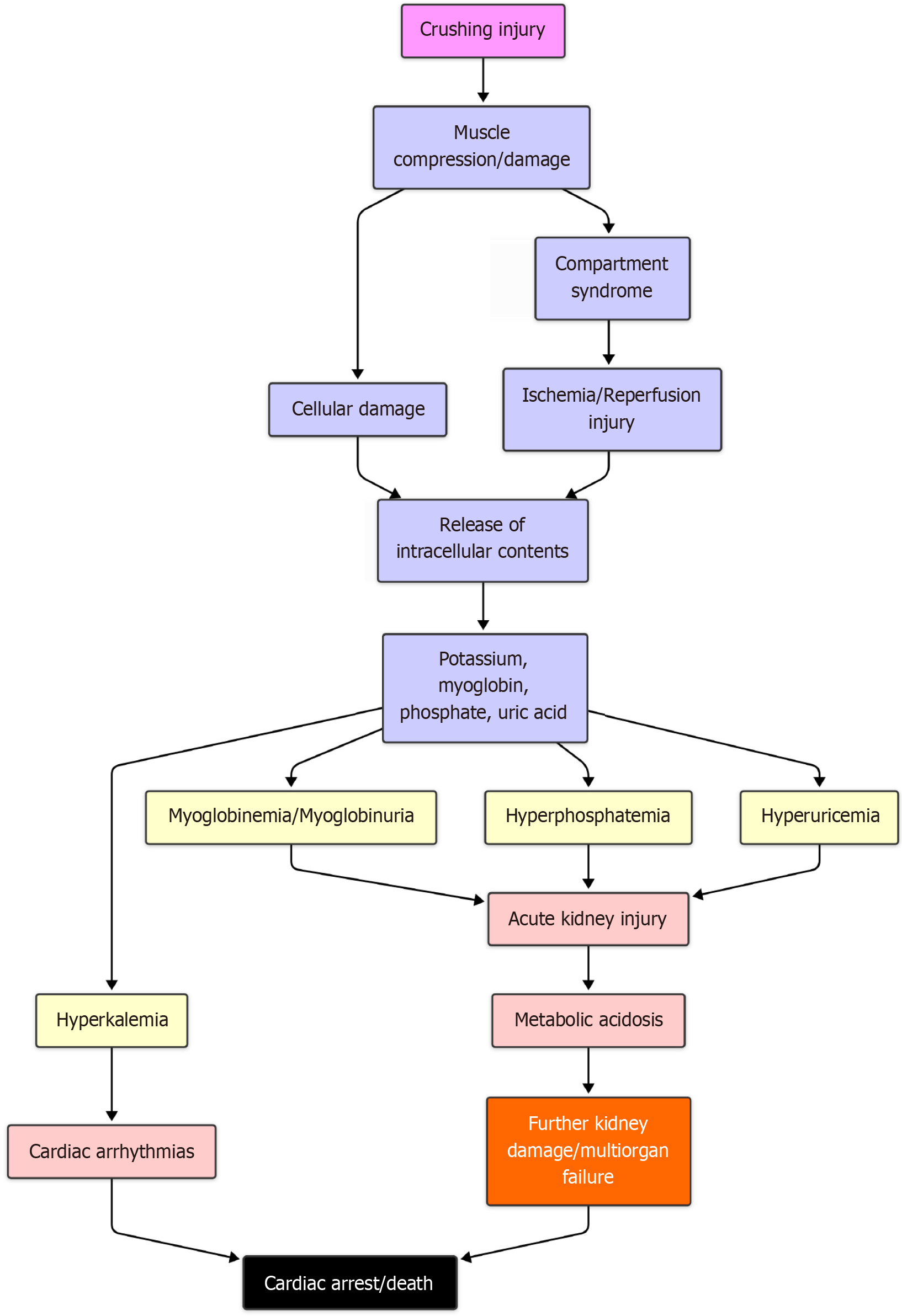
The rupture of muscle due to a crushing injury is the central cause of crush syndrome. This rupture could be directly due to trauma or indirectly due to ischemia and reperfusion injury. The rupture of sarcolemma triggers a cascade of events that release several substances and toxic metabolites. The pathophysiology is summarized in Figure 2.
The myoglobin released from an injured muscle is usually filtered by the kidneys. However, beyond a certain threshold myoglobin damages the distal convoluted tubules. The rapid destruction of these tubules leads to acute kidney injury (AKI). The release of uric acid leads to hyperuricemia, which further damages the kidneys.
A variety of electrolyte imbalances follow the rupture of muscle fibers. These electrolytes have intracellular storage. Due to the rupture of the sarcolemma, these are released into circulation. The potassium released from the skeletal muscles is responsible for cardiac arrhythmia and cardiac arrest. The phosphate released from the muscles combines with the calcium and forms calcium phosphate crystals, which further leads to hypocalcemia. This interferes with neural conduction and muscle action.
Thromboplastin and other products lead to disseminated intravascular coagulation. The release of lactic acid leads to metabolic acidosis, which frequently occurs during presentation. The presence of shock further compounds the situation.
The involvement of nitric oxide in the pathogenesis of crush syndrome is also significant. It has been seen that the inducible nitric oxide synthase is expressed in crushed limbs, which causes vasodilation, leading to hyperperfusion and edema[5].
The most essential cell form involved in the pathogenesis of crush syndrome is the M1 macrophages[6,7]. A rapid influx of macrophages and monocytes into the kidney leads to AKI[8]. This can be a potential target for drugs used to treat renal failure.
The above-mentioned mechanisms along with an inflammatory milieu resulting from injury lead to multiple organ dysfunction syndrome, which is ultimately fatal.
Ferroptosis: In a recent article by Qiao et al[9], ferroptosis was popularized as the cause of AKI. Though described earlier, this term describes a type of cell death caused by increased iron retention and reduced glutathione in a cell[9,10]. The iron overload caused by the degradation of myoglobin in crush syndrome is responsible for ferroptosis. This type of cell death is characterized by the contraction of mitochondria and increased membrane density of mitochondria[11,12]. This mechanism is now a target for therapy.
The primary cause of all clinical features is the external compression injury, which leads not only to local symptoms but also systemic complications. Traumatic rhabdomyolysis occurs when skeletal muscle cells release their contents into the circulatory system and includes electrolytes, enzymes, and myoglobin. Due to its systemic involvement, patients may present with nonspecific features like nausea, vomiting, confusion, fever, anuria, etc. Dehydration is a common feature, and myoglobinuria may cause a reddish-brown discoloration in the urine. Severe pain upon passive movements of the limb may be due to compartment syndrome. Thus, a high index of suspicion is required to accurately and quickly diagnose the complications of crush syndrome. A swollen, injured limb with reddish urine should raise suspicion of crush syndrome.
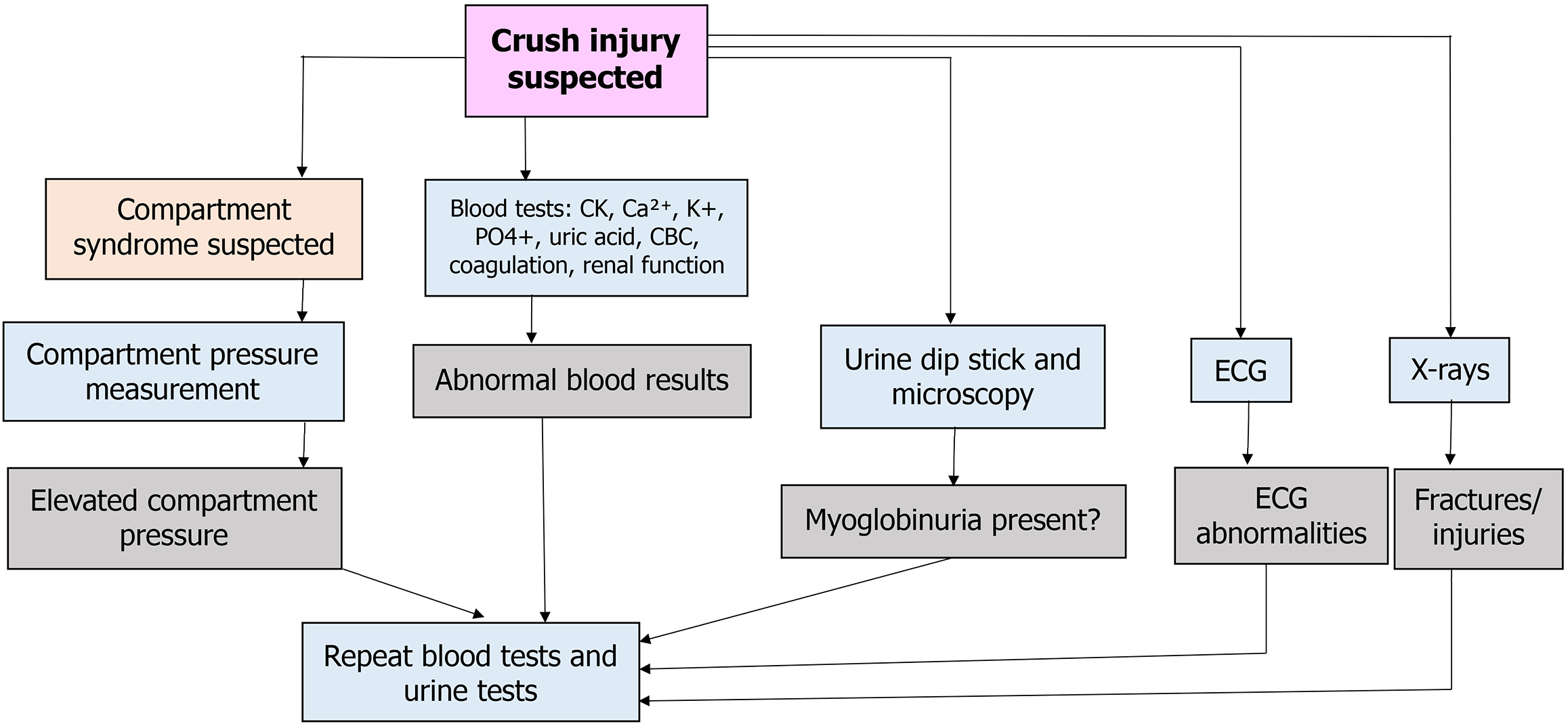
Figure 3 summarizes the investigations that are performed in the case of crush syndrome. We discuss them in detail.
Blood investigations: Blood investigations are done routinely in all cases of trauma. However, specific investigations aid in the diagnosis of crush syndrome. We will discuss some of the tests routinely performed in such a scenario: (1) Creatinine kinase (CK). CK is an important molecule regulating energy transfer in skeletal, smooth, and cardiac muscle tissues. Thus, it is widely used to diagnose diseases of the skeletal and cardiac muscles. CK is one of the earliest and most sensitive markers used in patients with crush injuries. It is crucial to identify that typical values vary with age, sex, and race to estimate test results better. Smith et al[13] showed that a CK level > 8500 predicted renal failure and that CK > 5000 had a worse outcome compared with lower levels[15]. It is generally accepted that values > 1000 or 5 times the upper limit of normal indicate rhabdomyolysis. Thus, CK values have a proportionate rise with an increasing risk of crush syndrome AKI. While CK values rise and peak only after 2-3 days, a high initial value at admission may help triage patients. A sequentially rising trend of CK also helps in understanding the progression of AKI; (2) Serum uric acid. Uric acid rises steadily due to purine metabolism resulting from rhabdomyolysis. Higher uric acid levels for a prolonged time can damage the renal tubules leading to AKI; (3) Renal function test. This test is essential in prognosticating the patient’s condition. Both renal and pre-renal AKI are observed in crush syndrome. Both serum urea and creatinine rise due to renal damage. A urea:creatinine ratio of more than 20:1 suggests pre-renal AKI. This type of AKI usually responds to hydration. A urea:creatinine ratio less than 20:1 suggests renal AKI resulting from tubular damage. This type of AKI does not respond to hydration and requires hemodialysis; (4) Serum electrolytes. Hyperkalemia is commonly seen in crush syndrome. This is due to the release of intracellular potassium. Hypocalcemia and hyperphosphatemia are also not uncommon in crush syndrome; (5) Other investigations. Other blood investigations that supplement the diagnosis of crush syndrome include serum lactate dehydrogenase levels. Lactate dehydrogenase is a marker of skeletal muscle injury and shows a steady rise in crush syndrome. Stress related hyperglycemia may be seen among the patients. High serum lactate levels with a high base deficit indicate hypoxia at cellular level with metabolic acidosis; and (6) Arterial blood gas (ABG). ABG is an easy test that can give much information about the pH and the electrolyte status. Most patients with crush syndrome have metabolic acidosis, which may be compensated by respiratory alkalosis.
Electrocardiogram: The hypovolemic shock and direct myocardial toxicity from the electrolyte imbalances lead to cardiovascular instability. Hyperkalemia-related arrhythmias are common causes of death. Electrocardiogram (ECG) changes may show the progression of this state with tall T waves, ST segment depression, and flatter P waves. Patients may also succumb to ventricular fibrillation during the progression of hyperkalemia.
A state of metabolic acidosis and coagulopathy is initiated due to the above events, and it becomes increasingly important to protect the patient from hypothermia and the triad of death.
Urine dipstick tests: Due to the noninvasiveness and ease of collection, urinary dipstick tests serve as a functional pre-hospital screening test for patients with crushing injuries. In non-erythrocytic urine, a positive test is an indirect marker for myoglobin. It helps in the effective triaging of patients who may further deteriorate to a state of renal failure.
Radiographs: Regional radiographs are necessary to localize fractures, which may necessitate bony stabilization by splinting or surgery.
Compartment pressure monitoring: Compartment syndrome occurs due to fracture and swelling in a localized limb compartment. Though it is commonly seen in limbs, it can also be seen in the abdomen in patients with crush syndrome. Though it is a clinical diagnosis, measuring compartment pressure can support the diagnosis. However, this test is not done routinely. Pressure more than 30 mmHg or pressure difference between diastolic blood pressure and compartment pressure less than 30 mmHg is diagnostic of compartment syndrome and necessitates fasciotomy.
Other tests under investigation that may aid in diagnosis: (1) Neutrophil-gelatinase-associated lipocalin is a member of the lipocalin family. Also known as 24p3, this molecule is secreted from the ascending loop of Henle at an early stage of kidney injury. While it has a role in multiple complex processes like inflammation, immunity, and chemotaxis, it is secreted early in renal injury. It serves as a sensitive and specific marker for detecting acute renal failure. Routine tests like serum urea and creatinine take much longer to rise, and significant renal damage has already occurred by this time; (2) Alpha-1-acid glycoprotein is an acute phase protein made in the liver. It has roles as an inflammatory marker but also helps regulate immunological processes. Intraperitoneal injections in mice have been shown to protect against ischemia-reperfusion injury. Alpha-1-acid glycoprotein may serve as the mediator in developing and progressing crush-related kidney injury. Values may also indicate the severity of the disease. Research is being conducted to identify a potential role in management options; (3) MicroRNAs are recently discovered small non-coding segments of RNA that regulate proteins post-transcriptionally. They regulate multiple intracellular processes, and their abnormal expression has been used to identify many diseases. miR-122 and miR-133a illustrated specificity for liver and muscle toxicity, respectively, in a study by Laterza et al[14]. Bailey et al[15] comprehensively assessed skeletal-muscle-specific microRNAs and found that miR-1, miR-133a, miR-133b, and miR-206 in skeletal muscle outperformed CK as a skeletal muscle injury marker[14]. Thus, these circulating microRNAs may be helpful as clinical biomarkers in estimating skeletal injury in crush syndrome; and (4) Kidney injury molecule 1 is a novel biomarker for diagnosing renal tubular injury. It is not usually present in urine. The presence of kidney injury molecule 1 in urine is specific for proximal convoluted tubule injury[16,17].
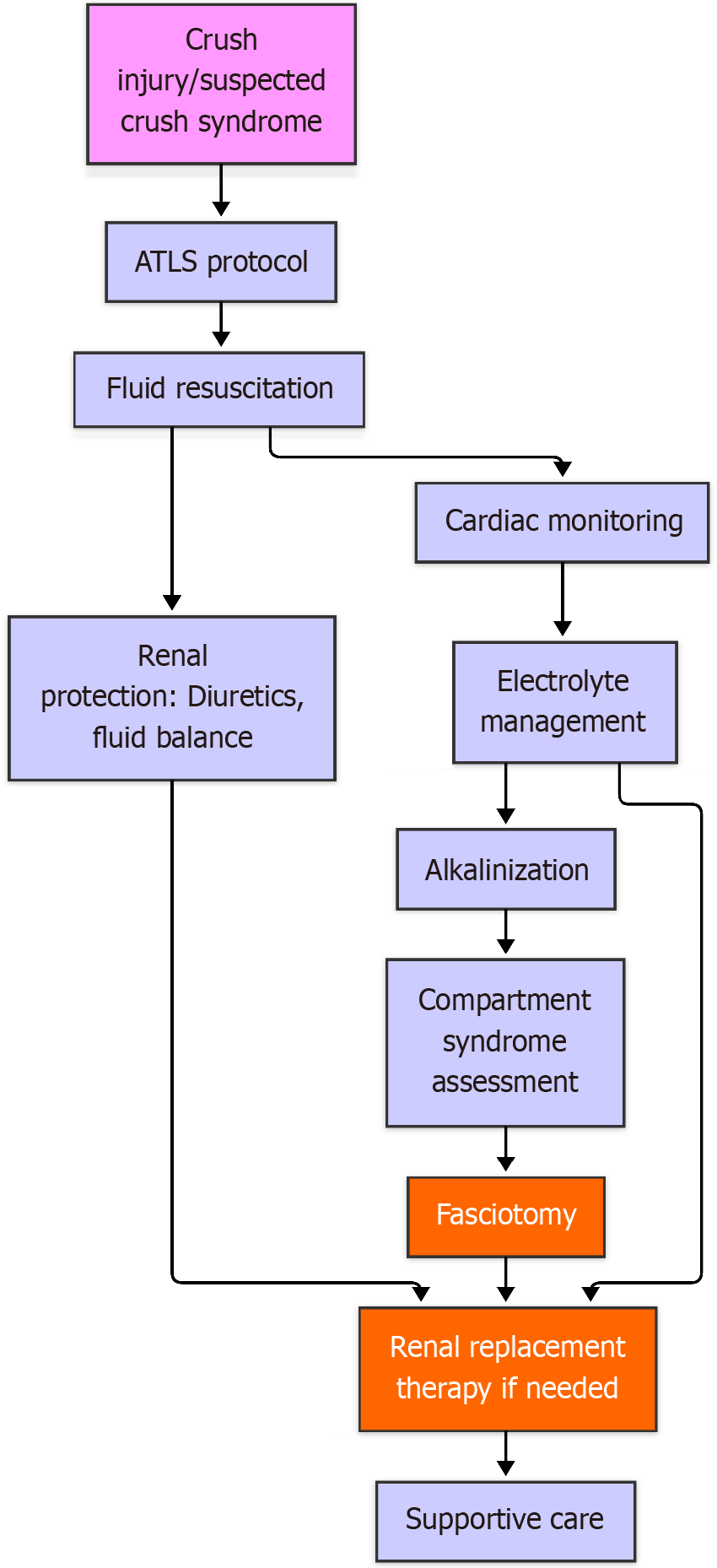
Early suspicion and identification of crush syndrome is necessary to reduce mortality in crush syndrome. It is essential to have a multidisciplinary approach involving emergency medicine, critical care, nephrology, and other specialties to reduce the mortality and morbidity due to crush syndrome. Treatment strategies may vary based on the severity of the crush injury, the presence of different injuries, and the patient’s overall health (Figure 4).
Patients with suspected crush syndrome need to be transferred to a healthcare facility where dialysis facilities are available. Most commonly, the patients are in a state of shock due to dehydration and third space losses.
Pre-hospital management: Pre-hospital interventions emphasize the importance of swift extrication, assessment of crush injury severity, and the initiation of basic life support measures. Special attention is given to the challenges first responders face in resource-limited settings and strategies to optimize patient outcomes.
Initial assessment and diagnosis: One must follow the Advanced Trauma Life Support protocol. Early recognition and triage of patients is necessary, and patients who are at high risk of developing crush syndrome are given more priority in cases of natural calamities. Timely and accurate diagnosis is pivotal in crush syndrome management.
Fluid resuscitation: Fluid resuscitation remains the mainstay of treatment. It should start before the patient is extricated. Securing intravenous access is difficult in such patients due to shock, and proper fluid resuscitation remains a challenge. Rapid hydration flushes the toxic metabolites out of the body and preserves the renal perfusion.
The fluid resuscitation protocol is individualized, varies from center to center, and is based on the patient’s status. Crystalloids are the mainstay of the treatment. The standard rate of administration of normal saline is 1 L/hour in the first 2 h, followed by maintenance of 500 mL per hour[18]. Lower doses of 10 mL/kg crystalloids are recommended in patients with heart failure. The urine output should provide a guide to fluid resuscitation. Urine output should be maintained at 0.5 mL/kg/hour[19]. If the patient can take orally and has adequate urine output, oral intake of fluids must be encouraged. Without a response to a crystalloid replacement, blood and blood products are transfused. Recently, the use of plasmalytes has been studied in rat models by Olivier et al[20]. The efficacy was similar to normal saline but with no hyperchloremic acidosis.
Alkalinization of urine: Adding 50 mmol of sodium bicarbonate to 1000 mL of normal saline increases the solubility of myoglobin in urine, reducing the risk of renal damage[21]. The addition of mannitol to cause diuresis has not shown any additional benefit[22]. However, in rat models, mannitol has been shown to reduce oxygen free radicals[23].
Management of electrolyte imbalance: Hyperkalemia is a common problem in crush syndrome. Early diagnosis can prevent cardiac complications. ABG and renal function tests can identify hyperkalemia. In the absence of such tests at the calamity site, portable ECG monitors can help. Tall T waves, widening of QRS, and shrinking P waves are the common manifestations of hyperkalemia on ECG. Calcium gluconate should be given intravenously at doses of 1000 mg over 2-3 min to stabilize cardiac membranes as soon as possible. This is followed by 10 units of regular insulin with 50 mL of 50% dextrose over 5 min. Salbutamol nebulization is also effective in reducing hyperkalemia. Potassium-binding resins can be used but have shown to be less efficacious due to very slow action. ECG monitoring and ABG should be monitored during correction.
Renal replacement therapy: Renal replacement therapy is necessary in patients with renal shutdown. This should be initiated if potassium levels are more than 7 mEq/L. Vanholder et al[24] described prophylactic dialysis in patients with hyperkalemia.
Role of surgery: Aggressive debridement of necrosed muscles is necessary to prevent crush syndrome. In acute settings, fasciotomies are encouraged to treat crush-induced compartment syndrome[25]. It is necessary to decompress all the compartments in a limb.
Aggressive debridement of necrotic muscles and amputations may be performed in life-threatening injuries. In many cases, extrication is facilitated by amputation[26]. In cases where limb salvage surgeries are planned, external fixators are useful.
Hyperbaric oxygen therapy: Hyperbaric oxygen therapy involves inhalation of 100% oxygen in a pressurized cabin. It acts by reducing edema and free radical formation. It also reduces the risk of anaerobic infections[27]. It increases collagen formation and reduces the risk of reperfusion injuries, which increases the chances of healing in crush injuries. Vasoconstriction induced by hyperbaric oxygen also reduces tissue edema. However, this modality is not very common due to the limited availability of specialized centers. The most common complications include trauma to the middle ear, eye damage, and lung failure.
Other therapies in experiment stage: (1) Carbon monoxide (CO) therapy. CO-enriched red blood cells are now used to treat rhabdomyolysis and crush syndrome[28]. Being an endogenous signaling molecule produced by heme oxygenase-mediated metabolism of heme, this agent suppresses the reactive oxygen species generated by the degradation of myoglobin[29,30]. CO is a highly diffusible gas administered in very low doses through gas, metal-based carriers, and prodrugs. CO-RBCs can be prepared by bubbling CO over RBCs for 5 min. The level of carboxyhemoglobin should be monitored and kept below 10% to ensure safety[29]. A rat experiment found that CO-RBC infusion significantly reduced the severity of AKI in rats with crush syndrome, contributing to increased life expectancy. However, human studies are yet to be done[30]; (2) Role of antioxidants. The role of antioxidants has recently been popularized in the treatment of crush syndrome. Murata et al[31] studied the effect of an antioxidant, Astragalus saponin, in animal models and observed a significant improvement in the survival of rodents with crush syndrome. Selenium is beneficial in malignancies, but the evidence for the treatment of crush syndrome is limited[32]. Vitamin E and N-acetylcysteine have also reduced free radical formation and rates of rhabdomyolysis. Vitamin C inhibits the oxidation of myoglobin and enhances its excretion[33]. L-carnitine also preserves renal histology and prevents kidney injury by inhibiting nitric oxide production in rat models with rhabdomyolysis[34]. Thioredoxin-1 is an antioxidant that inhibits macrophage aggregation and protects the kidneys from injury[35]. A similar agent, stevioside, has shown renoprotective effects in rat models[36]. It inhibits peroxisome proliferator-activated receptor gamma, a key factor in the pathogenesis of rhabdomyolysis-induced kidney injury[36]. Umbelliferone also acts through the peroxisome proliferator-activated receptor gamma pathway and protects against kidney injury[37]. Quercetin, a naturally occurring flavonoid in many vegetables, inhibits ferroptosis and reduces the risk of AKI[10]. Curcumin inhibits ferroptosis and is a potential drug for preventing AKI in crush syndrome[38]; (3) Role of anti-inflammatory drugs. Although nonsteroidal anti-inflammatory drugs are not encouraged in crush syndrome due to nephrotoxicity, the use of paracetamol is safe owing to its hepatic excretion. Paracetamol provides analgesia, reduces inflammation, and improves renal tubular histology as shown in rat models[23]. However, the efficacy in humans needs to be studied. High-dose intramuscular dexamethasone also reduces reperfusion injury and may increase short-term survival[39]; (4) Role of erythropoietin. Recombinant erythropoietin is now being used to treat AKI. Initially used for erythropoiesis, this drug has regenerative potential and has shown promise in the treatment of nerve injuries and AKI. The immunomodulatory role of this drug is believed to be mediated by inhibition of the toll-like receptor 4/nuclear factor kappa B pathways and inducible nitric oxide synthase in macrophages[40-42]. Since most of these studies are in rat models, translation of the same to human beings will significantly help in the future. Erythropoietin also inhibits M1 macrophage-mediated cell injury and promotes the M2 macrophages, inhibiting inflammation and repair[42-44]; (5) Role of pentoxifylline. Studies have reported that pentoxifylline reduces the rates of muscle damage[45]. It has also been shown to reduce the reperfusion injury rates at various human body sites[46,47]. Being a potent vasodilator, this drug probably increases blood flow and reduces the sequestration of neutrophils at different sites, thereby reducing injury[48]; and (6) Hydrogen sulfide. Hydrogen sulfide is a potent vasodilator and increases glomerular filtration rate and renal blood flow[43,49]. This property has been utilized for the treatment of diabetic nephropathy, ischemic injuries, and drug-induced AKI[50,51]. Hydrogen sulfide also has antioxidant and antiapoptotic effects[49].
Supportive treatment: Other supportive treatments, such as vasopressors like fenoldopam, are necessary to maintain blood pressure and renal output[52]. Broad-spectrum antibiotics are not excreted by the kidneys and are needed to control the infection[3]. The use of tetanus prophylaxis is encouraged when contamination is suspected. The use of regional blocks and opioids can manage pain. Ketamine is an effective drug that can be used during extrication[53]. It provides adequate analgesia and does not depress the cardiovascular center. Other electrolyte imbalances also need to be corrected to improve the outcomes.
Rehabilitation and long-term follow-up: Rehabilitation forms a major aspect of the management of patients. This should be commenced as soon as possible after recovery from crush syndrome. The patients should be encouraged to exercise joint range of motion to prevent stiffness[54]. Cold application reduces pain by causing numbness and reduces tissue swelling due to vasoconstriction[55]. Regular dressings and antibiotics are necessary for patients who underwent fasciotomy patients[56].
The patients undergoing amputations should be mobilized as early as possible with immediate postoperative prostheses. Stump exercises should be encouraged right from postoperative day 1.
In crush syndrome, most of the healing occurs by fibrosis. Repeated stretching of the scar tissue can lead to hypersensitization of nerve tissues. This can be countered by desensitization therapy and reduced anxiety levels in patients[56].
Mental rehabilitation also forms an essential part of the management of patients with crush syndrome[57]. Natural calamities often have long-lasting effects on patients’ minds. A psychiatrist and psychology expert must be included in the treating team as part of a multidisciplinary approach. Patients undergoing amputation also need special attention when it comes to mental rehabilitation. The prevalence of psychiatric disorders can range from 32% to 84% in patients undergoing amputations, with anxiety, grief, and post-traumatic stress disorder being the most common[57,58]. Cognitive behavior therapy, group psychotherapy, and meditation can help people lead a meaningful life after trauma[57].
Social rehabilitation is also essential as the patients should be able to earn a living and contribute to society for the rest of their lives. Since most of the patients who have experienced trauma end up being disabled, a change of profession is required to meet the physical abilities of the patients. Re-training and rehabilitation programs must be encouraged to help individuals integrate into everyday life[59].
This study has a few limitations. On one hand, this narrative review included all types of studies, making the results heterogeneous. On the other hand, many treatment modalities are successful in animal and in vitro models, but the role of the same in human subjects has not been established.
Crush syndrome is a condition that leads to significant mortality during natural and man-made calamities. Preventing this condition should remain the focus of healthcare workers. A high degree of suspicion is needed to diagnose and effectively manage crush syndrome. The addition of new tests will help in early diagnosis of crush syndrome. Newer management techniques and newer drugs, though in the experimental stage, have shown promise in preventing the severity of the disease. There are multiple potential areas for future research, including novel therapeutic approaches, advanced monitoring techniques, and the development of standardized protocols to enhance crush syndrome management globally. Early diagnosis of crush syndrome is an area that is entirely unexplored and needs more research. Pharmacological agents for preventing crush syndrome will be beneficial in reducing morbidity. Agents that bind to myoglobin in circulation will prevent renal injury. Many studies are successful in animal and in vitro models, but translating the results into humans remains challenging.
| 1. | Rajagopalan S. Crush Injuries and the Crush Syndrome. Med J Armed Forces India. 2010;66:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Bywaters EG. 50 years on: the crush syndrome. BMJ. 1990;301:1412-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Sever MS, Vanholder R. Management of crush syndrome casualties after disasters. Rambam Maimonides Med J. 2011;2:e0039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Stewart IJ, Faulk TI, Sosnov JA, Clemens MS, Elterman J, Ross JD, Howard JT, Fang R, Zonies DH, Chung KK. Rhabdomyolysis among critically ill combat casualties: Associations with acute kidney injury and mortality. J Trauma Acute Care Surg. 2016;80:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Filippin LI, Cuevas MJ, Lima E, Marroni NP, Gonzalez-Gallego J, Xavier RM. Nitric oxide regulates the repair of injured skeletal muscle. Nitric Oxide. 2011;24:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Kawakami T, Lichtnekert J, Thompson LJ, Karna P, Bouabe H, Hohl TM, Heinecke JW, Ziegler SF, Nelson PJ, Duffield JS. Resident renal mononuclear phagocytes comprise five discrete populations with distinct phenotypes and functions. J Immunol. 2013;191:3358-3372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Stamatiades EG, Tremblay ME, Bohm M, Crozet L, Bisht K, Kao D, Coelho C, Fan X, Yewdell WT, Davidson A, Heeger PS, Diebold S, Nimmerjahn F, Geissmann F. Immune Monitoring of Trans-endothelial Transport by Kidney-Resident Macrophages. Cell. 2016;166:991-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 167] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 8. | Okubo K, Kurosawa M, Kamiya M, Urano Y, Suzuki A, Yamamoto K, Hase K, Homma K, Sasaki J, Miyauchi H, Hoshino T, Hayashi M, Mayadas TN, Hirahashi J. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat Med. 2018;24:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 9. | Qiao Y, Sun C, Kan S, He L, Wang Y, Gao H, Zhang Y, Cheng Y, Wang S, Zhao L, Niu W. SRS 16-86 promotes diabetic nephropathy recovery by regulating ferroptosis. Exp Physiol. 2024;109:1199-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Quan F, Cao Q, Lin Y, Yue C, Bi R, Cui X, Yang H, Yang Y, Birnbaumer L, Li X, Gao X. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2021;28:231-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 395] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 11. | Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, Jiang X. Role of Mitochondria in Ferroptosis. Mol Cell. 2019;73:354-363.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 1372] [Article Influence: 196.0] [Reference Citation Analysis (0)] |
| 12. | Musheshe N, Oun A, Sabogal-Guáqueta AM, Trombetta-Lima M, Mitchel SC, Adzemovic A, Speek O, Morra F, van der Veen CHJT, Lezoualc'h F, Cheng X, Schmidt M, Dolga AM. Pharmacological Inhibition of Epac1 Averts Ferroptosis Cell Death by Preserving Mitochondrial Integrity. Antioxidants (Basel). 2022;11:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Smith W, Hardcastle T. A crushing experience: The spectrum and outcome of soft tissue injury and myonephropathic syndrome at an Urban South African University Hospital. Afr J Emerg Med. 2011;1:17-24. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Laterza OF, Lim L, Garrett-Engele PW, Vlasakova K, Muniappa N, Tanaka WK, Johnson JM, Sina JF, Fare TL, Sistare FD, Glaab WE. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009;55:1977-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 15. | Bailey WJ, Barnum JE, Erdos Z, LaFranco-Scheuch L, Lane P, Vlasakova K, Sistare FD, Glaab WE. A Performance Evaluation of Liver and Skeletal Muscle-Specific miRNAs in Rat Plasma to Detect Drug-Induced Injury. Toxicol Sci. 2019;168:110-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a specific and sensitive biomarker of kidney injury. Scand J Clin Lab Invest Suppl. 2008;241:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1240] [Cited by in RCA: 1312] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 18. | Better OS, Rubinstein I. Management of shock and acute renal failure in casualties suffering from the crush syndrome. Ren Fail. 1997;19:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15:58-69. [PubMed] |
| 20. | Olivier PY, Beloncle F, Seegers V, Tabka M, Renou de La Bourdonnaye M, Mercat A, Cales P, Henrion D, Radermacher P, Piquilloud L, Lerolle N, Asfar P. Assessment of renal hemodynamic toxicity of fluid challenge with 0.9% NaCl compared to balanced crystalloid (PlasmaLyte(®)) in a rat model with severe sepsis. Ann Intensive Care. 2017;7:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Moore KP, Holt SG, Patel RP, Svistunenko DA, Zackert W, Goodier D, Reeder BJ, Clozel M, Anand R, Cooper CE, Morrow JD, Wilson MT, Darley-Usmar V, Roberts LJ 2nd. A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure. J Biol Chem. 1998;273:31731-31737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 196] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Holt SG, Moore KP. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensive Care Med. 2001;27:803-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 177] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Çelikmen MF, Sarıkaya S, Özüçelik DN, Sever MŞ, Açıksarı K, Çelikmen DM, Yazıcıoğlu M, Kandemir A, Doğan H, Ayvacı BM, Özaşır Abuşka D, Sadıllıoğlu S. Effects of acetaminophen and mannitol on crush injuries in rats: An experimental study. Ulus Travma Acil Cerrahi Derg. 2016;22:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 464] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 25. | Oda Y, Shindoh M, Yukioka H, Nishi S, Fujimori M, Asada A. Crush syndrome sustained in the 1995 Kobe, Japan, earthquake; treatment and outcome. Ann Emerg Med. 1997;30:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Michaelson M. Crush injury and crush syndrome. World J Surg. 1992;16:899-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Myers RA. Hyperbaric oxygen therapy for trauma: crush injury, compartment syndrome, and other acute traumatic peripheral ischemias. Int Anesthesiol Clin. 2000;38:139-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Taguchi K, Ogaki S, Nagasaki T, Yanagisawa H, Nishida K, Maeda H, Enoki Y, Matsumoto K, Sekijima H, Ooi K, Ishima Y, Watanabe H, Fukagawa M, Otagiri M, Maruyama T. Carbon Monoxide Rescues the Developmental Lethality of Experimental Rat Models of Rhabdomyolysis-Induced Acute Kidney Injury. J Pharmacol Exp Ther. 2020;372:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Yang X, de Caestecker M, Otterbein LE, Wang B. Carbon monoxide: An emerging therapy for acute kidney injury. Med Res Rev. 2020;40:1147-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 674] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 31. | Murata I, Abe Y, Yaginuma Y, Yodo K, Kamakari Y, Miyazaki Y, Baba D, Shinoda Y, Iwasaki T, Takahashi K, Kobayashi J, Inoue Y, Kanamoto I. Astragaloside-IV prevents acute kidney injury and inflammation by normalizing muscular mitochondrial function associated with a nitric oxide protective mechanism in crush syndrome rats. Ann Intensive Care. 2017;7:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Kiełczykowska M, Kocot J, Paździor M, Musik I. Selenium - a fascinating antioxidant of protective properties. Adv Clin Exp Med. 2018;27:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 33. | Boutaud O, Roberts LJ 2nd. Mechanism-based therapeutic approaches to rhabdomyolysis-induced renal failure. Free Radic Biol Med. 2011;51:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Aydogdu N, Atmaca G, Yalcin O, Taskiran R, Tastekin E, Kaymak K. Protective effects of L-carnitine on myoglobinuric acute renal failure in rats. Clin Exp Pharmacol Physiol. 2006;33:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Nishida K, Watanabe H, Ogaki S, Kodama A, Tanaka R, Imafuku T, Ishima Y, Chuang VT, Toyoda M, Kondoh M, Wu Q, Fukagawa M, Otagiri M, Maruyama T. Renoprotective effect of long acting thioredoxin by modulating oxidative stress and macrophage migration inhibitory factor against rhabdomyolysis-associated acute kidney injury. Sci Rep. 2015;5:14471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Kaur T, Singh D, Singh AP, Pathak D, Arora S, Singh B, Kaur S, Singh B. Stevioside protects against rhabdomyolysis-induced acute kidney injury through PPAR-γ agonism in rats. Drug Dev Res. 2021;82:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Kaur T, Singh D, Pathak D, Singh AP, Singh B. Umbelliferone attenuates glycerol-induced myoglobinuric acute kidney injury through peroxisome proliferator-activated receptor-γ agonism in rats. J Biochem Mol Toxicol. 2021;35:e22892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Guerrero-Hue M, García-Caballero C, Palomino-Antolín A, Rubio-Navarro A, Vázquez-Carballo C, Herencia C, Martín-Sanchez D, Farré-Alins V, Egea J, Cannata P, Praga M, Ortiz A, Egido J, Sanz AB, Moreno JA. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 2019;33:8961-8975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 39. | Murata I, Goto M, Komiya M, Motohashi R, Hirata M, Inoue Y, Kanamoto I. Early Therapeutic Intervention for Crush Syndrome: Characterization of Intramuscular Administration of Dexamethasone by Pharmacokinetic and Biochemical Parameters in Rats. Biol Pharm Bull. 2016;39:1424-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Zhou J, Bai Y, Jiang Y, Tarun P, Feng Y, Huang R, Fu P. Immunomodulatory role of recombinant human erythropoietin in acute kidney injury induced by crush syndrome via inhibition of the TLR4/NF-κB signaling pathway in macrophages. Immunopharmacol Immunotoxicol. 2020;42:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Yang FL, Subeq YM, Chiu YH, Lee RP, Lee CJ, Hsu BG. Recombinant human erythropoietin reduces rhabdomyolysis-induced acute renal failure in rats. Injury. 2012;43:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Wang S, Zhang C, Li J, Niyazi S, Zheng L, Xu M, Rong R, Yang C, Zhu T. Erythropoietin protects against rhabdomyolysis-induced acute kidney injury by modulating macrophage polarization. Cell Death Dis. 2017;8:e2725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide. 2014;41:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 44. | Nairz M, Schroll A, Moschen AR, Sonnweber T, Theurl M, Theurl I, Taub N, Jamnig C, Neurauter D, Huber LA, Tilg H, Moser PL, Weiss G. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-κB-inducible immune pathways. Immunity. 2011;34:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 45. | Adams JG Jr, Dhar A, Shukla SD, Silver D. Effect of pentoxifylline on tissue injury and platelet-activating factor production during ischemia-reperfusion injury. J Vasc Surg. 1995;21:742-8; discussion 748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Reignier J, Mazmanian M, Detruit H, Chapelier A, Weiss M, Libert JM, Hervé P. Reduction of ischemia-reperfusion injury by pentoxifylline in the isolated rat lung. Paris-Sud University Lung Transplantation Group. Am J Respir Crit Care Med. 1994;150:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Seibert AF, Haynes J, Taylor A. Ischemia-reperfusion injury in the isolated rat lung. Role of flow and endogenous leukocytes. Am Rev Respir Dis. 1993;147:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Kamphuis J, Smits P, Thien T. Vascular effects of pentoxifylline in humans. J Cardiovasc Pharmacol. 1994;24:648-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Tekşen Y, Kadıoğlu E, Koçak C, Koçak H. Effect of Hydrogen Sulfide on Kidney Injury in Rat Model of Crush Syndrome. J Surg Res. 2019;235:470-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Lobb I, Sonke E, Aboalsamh G, Sener A. Hydrogen sulphide and the kidney: important roles in renal physiology and pathogenesis and treatment of kidney injury and disease. Nitric Oxide. 2015;46:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 51. | Zhou X, Feng Y, Zhan Z, Chen J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J Biol Chem. 2014;289:28827-28834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 52. | Mathur VS, Swan SK, Lambrecht LJ, Anjum S, Fellmann J, McGuire D, Epstein M, Luther RR. The effects of fenoldopam, a selective dopamine receptor agonist, on systemic and renal hemodynamics in normotensive subjects. Crit Care Med. 1999;27:1832-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Chesters A, Webb T. Ketamine for procedural sedation by a doctor-paramedic prehospital care team: a 4-year description of practice. Eur J Emerg Med. 2015;22:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Matsuoka T, Yoshioka T, Tanaka H, Ninomiya N, Oda J, Sugimoto H, Yokota J. Long-term physical outcome of patients who suffered crush syndrome after the 1995 Hanshin-Awaji earthquake: prognostic indicators in retrospect. J Trauma. 2002;52:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Murata I, Imanari M, Komiya M, Kobayashi J, Inoue Y, Kanamoto I. Icing treatment in rats with crush syndrome can improve survival through reduction of potassium concentration and mitochondrial function disorder effect. Exp Ther Med. 2020;19:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Altan L. Postoperative rehabilitation of compartment syndrome following fasciotomy. Turk J Phys Med Rehabil. 2023;69:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 57. | Jo SH, Kang SH, Seo WS, Koo BH, Kim HG, Yun SH. Psychiatric understanding and treatment of patients with amputations. Yeungnam Univ J Med. 2021;38:194-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 58. | Sahu A, Sagar R, Sarkar S, Sagar S. Psychological effects of amputation: A review of studies from India. Ind Psychiatry J. 2016;25:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 59. | Sheikhbardsiri H, Yarmohammadian MH, Rezaei F, Maracy MR. Rehabilitation of vulnerable groups in emergencies and disasters: A systematic review. World J Emerg Med. 2017;8:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |