Published online Jan 18, 2025. doi: 10.5312/wjo.v16.i1.102120
Revised: November 29, 2024
Accepted: December 20, 2024
Published online: January 18, 2025
Processing time: 96 Days and 0.2 Hours
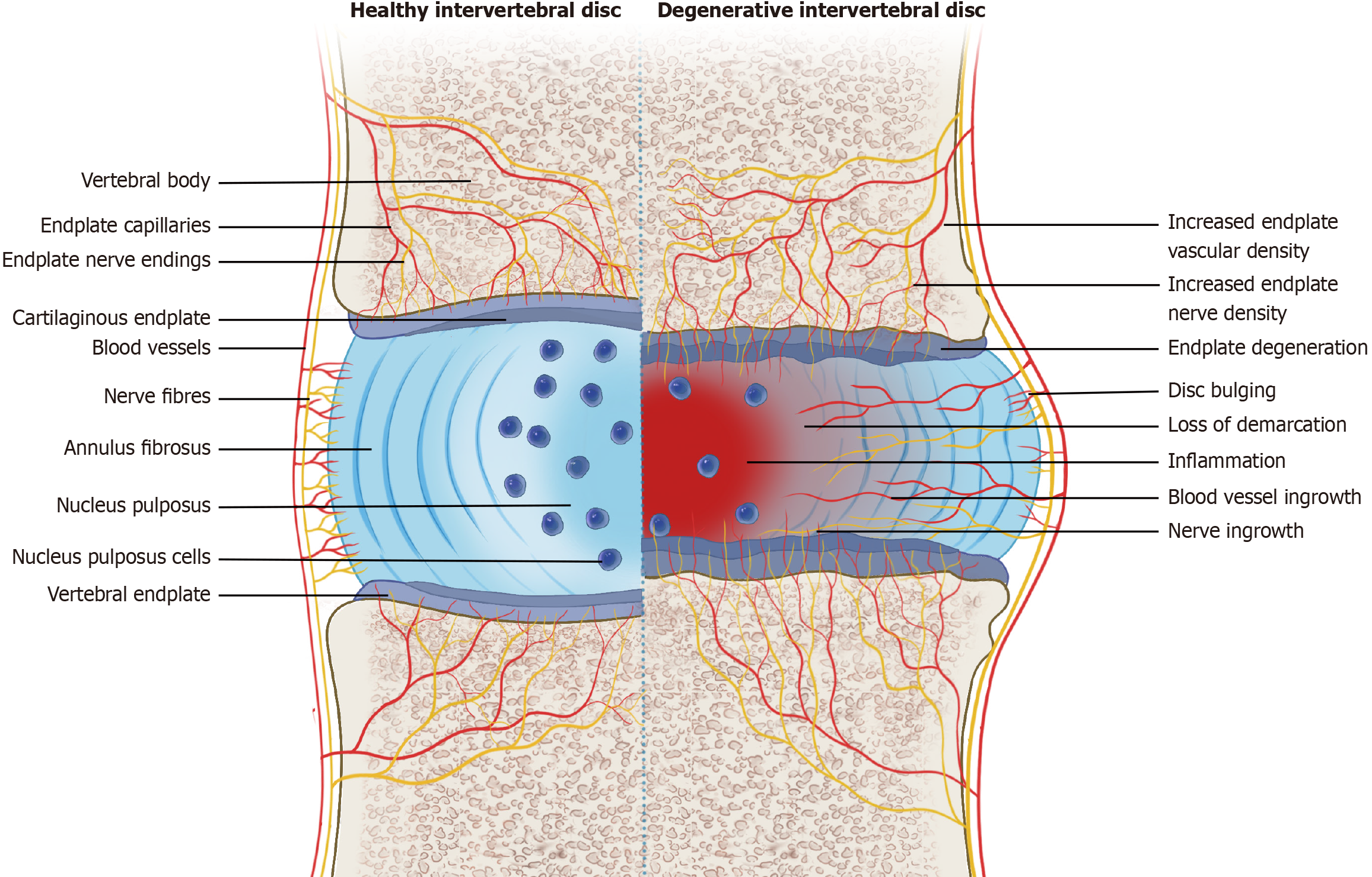
In healthy intervertebral discs (IVDs), nerves and blood vessels are present only in the outer annulus fibrosus, while in degenerative IVDs, a large amount of nerve and blood vessel tissue grows inward. Evidence supports that neurogenic inflammation produced by neuropeptides such as substance P and calcitonin gene re
Core Tip: The outer layer of the annulus fibrosus and the vertebral endplate are innervated by nociceptive nerves fibers. Injury to the annulus fibrosus or endplate excites nociceptive stimuli, releasing neuropeptides such as substance P and calcitonin gene related peptide, which results in neurogenic inflammation within the intervertebral disc. Neurogenic inflammation further leads to progressive disc degeneration and chronic discogenic pain through complex interactions between the nervous and immune systems.
- Citation: Peng BG, Li YC, Yang L. Role of neurogenic inflammation in intervertebral disc degeneration. World J Orthop 2025; 16(1): 102120
- URL: https://www.wjgnet.com/2218-5836/full/v16/i1/102120.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i1.102120
The research history of neurogenic inflammation dates back to 1874 when Goltz realized that the sciatic nerve in dogs contains vasodilator fibers in addition to normal constrictor nerves. The term neurogenic inflammation is now widely used to describe the mechanism by which sensory nerve contributes to inflammation[1,2]. Neurogenic inflammation exists not only in the skin and joints but also in the viscera, trachea, heart, bladder, and reproductive organs[3]. Although the term neurogenic inflammation has hardly been mentioned in published literature on the intervertebral disc (IVD) pathology, a large number of studies have implicated the role of neurogenic inflammation in IVD degeneration (IVDD). Neurogenic inflammation of the IVD is a complex process in which neuropeptides like substance P (SP) and calcitonin gene related peptide (CGRP) are released from afferent neurons innervating the IVD in response to IVD injury or degeneration[4-6]. This process is characterized by complex interactions between blood vessels, the immune system, and the nervous system, leading to changes in the structure and function of the IVD and ultimately to discogenic low back pain (DLBP)[7-12].
Low back pain (LBP) is one of the most important medical and social issues in the world today[13]. It is estimated that up to 84% of the population suffers from LBP at some point in their lives, while 10% are chronically disabled[14]. The components that make up the lumbar spine, such as muscles, ligaments, vertebrae, facet and sacroiliac joints, IVDs, and neurovascular structures, are susceptible to different stressors, and each of these factors, individually or in combination, can contribute to LBP[15]. Multiple studies have shown that LBP caused by IVDD, also known as DLBP, is the most common type of LBP. It is one of the main causes of disability and has significant socioeconomic impacts[16].
The development of IVDD is a complex and multifaceted situation. Numerous studies have suggested that multiple factors such as aging, abnormal mechanical stress, trauma, infection, nutrition, genetics, obesity, and gender are associated with IVDD[17-21]. Due to the complex and multifactorial nature of IVDD, little is known about its pathoge
The IVD is a functional unit that connects the vertebrae of the spine. Each IVD consists of three structures. A soft gelatinous nucleus pulposus (NP) in the center is surrounded by a tough peripheral lamellar annulus fibrosus (AF) and sandwiched between two cartilage endplates (CEP). The components of the disc work together to promote the movement of the spine and act as a shock absorber between the two vertebral bodies[22]. The cell density of the human IVD is very low and its renewal is very slow. As a result, they heal slowly and limited after injury and exhibit progressive and age-related changes[12].
The AF consists of 15-25 concentric angle-ply layers that cross each other obliquely in space. The AF is divided into inner and outer zones with different biochemical and cellular compositions as well as biomechanical properties. The outer layer of the AF is composed of fibroblasts that produce type I collagen, along with small amounts of collagen types III, V, and VI. The cells in the inner layer of AF are more like chondrocytes, producing mainly type II collagen and proteo
The NP is a jelly-like structure rich in aggrecans restricted within the endplate and AF. It consists of chondrocyte-like cells that produce polysaccharide/mucoprotein molecules such as chondroitin sulfate, collagen, and elastin fibers[24]. The main function of the NP is to redistribute the applied load to the rest of the surrounding IVD.
The CEP is a thin, incomplete layer of cartilage that covers the area of the vertebral body surrounded by a ring apophysis[25]. Its thickness is between 0.1 and 1.6 mm, and it is the largest near the NP and inner AF and absent in the outer AF[26]. The CEP adjacent to the IVD consists mainly of fibrocartilage and is closely bound to the nuclear and annulus regions. However, the portion adjacent to the vertebral body consists mainly of hyaline cartilage, which is weakly attached to the subchondral bone[25].
Because the blood supply within the IVD is limited to the outer AF, and the nearest blood supply to most disc tissue in adults is located in the subchondral bone of the vertebral body, metabolite exchange mainly comes from the diffusion of the vertebral body through CEP[25,27]. In this way, the IVD microenvironment is often described as harsh because of its limited nutrients (glucose and oxygen), low pH, and large changes in osmolarity. The dense extracellular matrix of IVD mechanically inhibits the ingrowth of blood vessels through high physical pressure and chemically through high aggrecan content[12]. The combination of high aggrecan content and secretory inhibitors can prevent the ingrowth of nerves and blood vessels in non-degenerative IVDs[28,29]. AF, CEP, and immunosuppressive molecular factors are defined as the blood-NP barrier, which separates NP from the host immune system[30].
The prerequisite for DLBP is that IVD has innervation, but it is generally believed that normal NP and inner annular zones do not have innervation[7]. However, early opinions were divided as to whether the outer annulus was innervated or not[31]. Malinsky[32] found a variety of free nerve endings and some button-like terminals in the outer layers of the lumbar AF and noted that partially and fully encapsulated mechanoreceptors were confined to the AF surface. Since then, researchers have generally agreed with Malinsky’s classic observation that the superficial layers of the normal AF have sensory nerve endings[33], that the nerve penetration of the human AF is about 3 mm deep[34], and that it involves three outer lamellar layers[35].
Numerous animal studies have shown that the nerve fibers that innervate the IVD come from multiple dorsal root ganglion (DRG) neurons. There are two pathways between the AF and the DRGs: One through the sinuvertebral nerve (SVN) and the other through the sympathetic trunk[36-40]. It was agreed that SVN consists of a fine sympathetic branch from the grey ramus communicans and a fine sensory spinal branch from the ventral ramus[41]. The SVN re-enters the vertebral canal through each intervertebral foramen and is located in front of the nerve root with segmental blood vessels[42]. It is commonly believed that up to three segments of the lumbar SVN overlap, which may explain the poor loca
In degenerative IVDs in humans and animals, especially in painful IVDs in humans, a significant increase in inner
In addition to the peripheral portion of the AF, the vertebral endplate is innervated by nociceptive nerves[45]. The basivertebral nerve (BVN) plays an important role in the transmission of pain sensation in the endplate[46,47]. BVN is a branch of SVN that enters the vertebral body through the central vascular foramen along with the basivertebral vessels and branches around the vertebral endplate[48]. With the degeneration of the IVD, IVD cells and infiltrating immune cells release inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor (TNF)-α, and chemokines such as CC motif chemokine ligand (CCL) 2, CCL3, and C-X-C motif chemokine 10[4-10]. Through convection, crosstalk occurs between the IVD and subchondral bone marrow, and the vertebral endplate gradually undergoes degenerative changes, displaying Modic changes on magnetic resonance imaging (Figure 2)[49,50]. Inflammatory cytokines, especially IL-1, stimulate the expression of angiogenic factors and neurotrophic factors, which trigger the ingrowth of nociceptive nerves into the vertebral endplate (Figure 1)[7].
Studies have shown that the degree of endplate lesions is related to the severity of back pain and is closely related to the degree of IVDD[51]. The density of vertebral endplate innervation is directly proportional to the degree of vertebral endplate injury, further providing evidence for its role in chronic LBP[52]. A histopathological study found that those pathologically altered vertebral endplates, such as structural defects or endplate lesions, had a higher innervation density than unaltered endplate nerves[53]. The reports on the improvement of LBP after radiofrequency ablation of the BVNs and lumbar fusion surgery in patients with endplate-related LBP reinforce this important viewpoint[54-57].
It is widely recognized that normal IVDs are innervated by sensory (mainly nociceptive) and postganglionic sympathetic (vasomotor efferent) nerve fibers[5,7]. The sensory nerve fibers innervating the lumbar disc consist mainly of small myelinated A-delta fibers and unmyelinated C-fibers[33]. These tiny fibers express SP and CGRP, which are nociceptors associated with inflammatory pain[7]. They are nerve growth factor (NGF)-dependent and express high-affinity tyrosine receptor kinase (Trk) A[5]. After experimental IVD puncture and inflammation induction, the number of CGRP-positive activated DRG neurons innervating lumbar IVD increases[36]. Studies have indicated that almost all nociceptive nerve fibers in human and rat IVDs are peptide-containing nerve fibers, suggesting that nerve fibers associated with inflammation may transmit pain originating from degenerative IVDs[58,59].
An early study showed that nerve fibers observed in human painful discs express growth associated protein 43 (GAP-43)[60]. GAP-43 is associated with axonal growth and synaptic plasticity and plays an important role in neuronal development and regeneration[61]. During progressive degeneration of IVD, GAP-43-positive nerve fibers from DRG neurons penetrate to different depths of IVD. The inflammation of the IVD in rats induced by complete Freund’s adjuvant[62,63] or exposure of the NP to the outside of the AF[62] can increase the expression of GAP-43 in DRG neurons innervating the IVD, suggesting that IVD inflammation may promote axonal growth of DRG neurons. In addition, the studies also found in rats[64] and humans[65] that mRNA and protein levels of GAP-43 were significantly higher in the degenerative IVDs than in the control group. These results suggest that GAP-43 may be an effective marker for iden
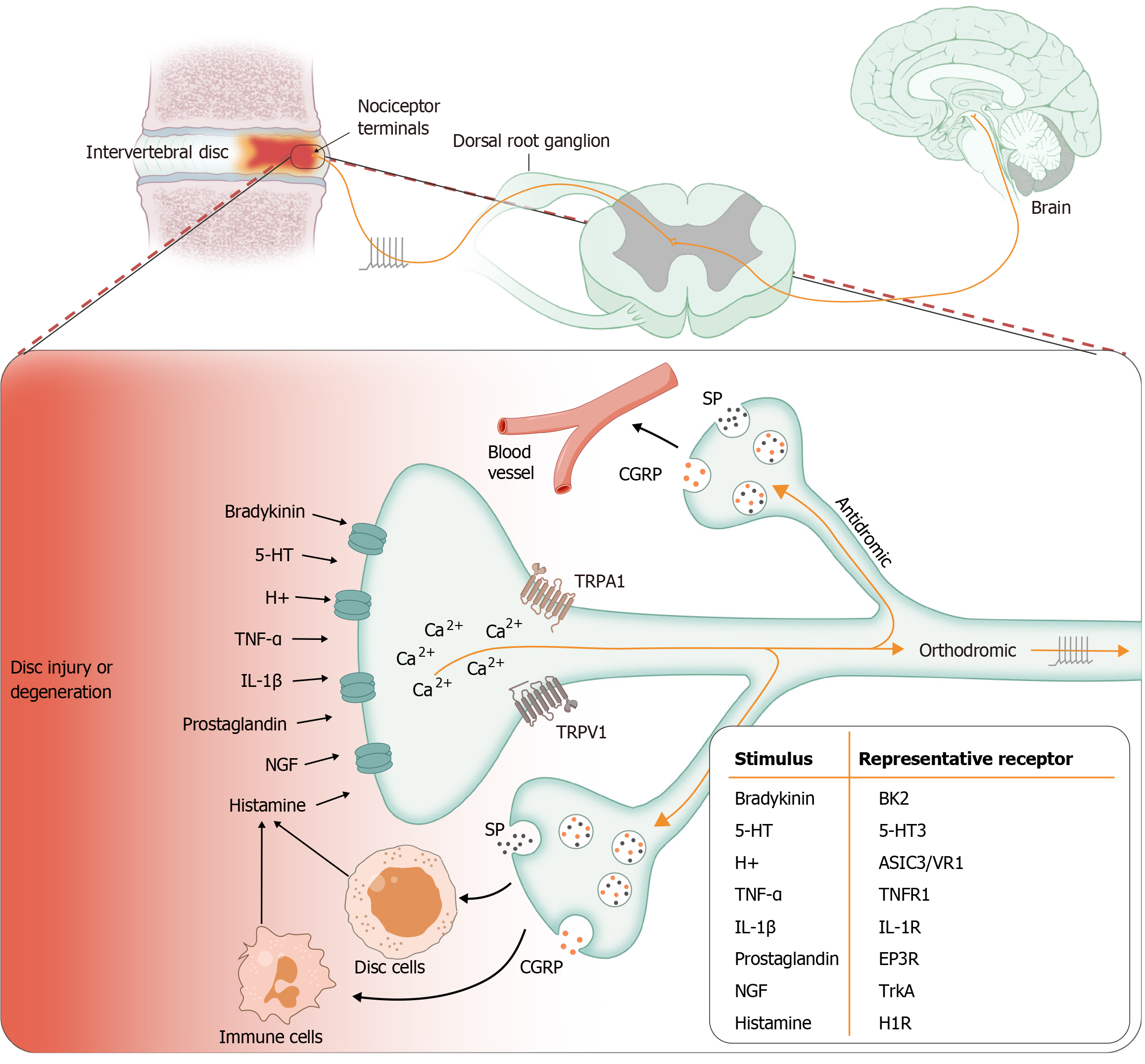
Neurogenic inflammation is mediated by the release of neuropeptides such as SP and CGRP from sensory nerve fibers[1-3]. In rodents, A-delta and C-fiber afferents cannot be distinguished according to the mode of stimulation. Both types of afferents can be distinguished based on the electrophysiological and chemical properties of DRG neurons. The C-fiber population is characterized by a sensitivity to capsaicin, associated with expression of the TRPV1 receptor, a high acti
SP and CGRP are classical neuropeptides that act directly on vascular endothelial cells and smooth muscle cells, thereby mediating vascular events. SP increases vascular permeability, followed by plasma extravasation and edema[1]. The release of SP induces mast cells to release vascular endothelial growth factor (VEGF), which promotes angiogenesis and inflammatory cell infiltration[67]. CGRP is an extremely potent microvascular vasodilator, which contributes to most neurogenic vasodilation and participates in the recruitment of inflammatory cells[67]. SP and CGRP both act through their respective G-protein-coupled receptors: Neurokinin-1 receptor (NK1R) for SP and CGRP receptor complex for CGRP[4].
Nerve fibers expressing CGRP and SP are distributed in the outer AF of healthy IVDs, and in degenerative or painful IVDs, these nerve fibers reach the inner AF and NP[33,60]. In the IVD, sensory nerve fibers are mixed with autonomic nerve fibers that contain additional neuropeptides such as neuropeptide Y, noradrenaline and vasoactive intestinal peptide, which may be involved in vascular regulatory function or IVD cell biology[6,68].
Clinically, neurogenic inflammation of degenerative lumbar IVDs may be present in patients with DLBP. Histological studies of painful IVDs have shown many features of neurogenic inflammation, such as infiltration of mast cells and macrophages, nerve fiber ingrowth, and new angiogenesis[69,70]. Immunostaining studies have shown that the expre
SP is an 11-amino acid protein that belongs to the tachykinin protein family. SP is typically described as a neurotransmitter that preferentially binds to the NK1R, which is expressed in two splicing variants: The full-length isoform and the truncated isoform[75,76]. The interaction between SP and its preferred receptor NK1R leads to nuclear factor κB (NF-κB) activation and production of proinflammatory cytokines[76]. In IVD, SP has been used as a specific marker for identifying ingrowth of nerves, which may transmit nociceptive signals in degenerative IVDs[69]. SP-treated disc cells showed significant upregulation of IL-1β, IL-6, IL-8, and TNF-α in NP and AF cells. AF and NP cells expressed SP at low levels, but the expression was significantly upregulated after IL-1β or TNF-α treatment. Both SP receptor isoforms are expressed in NP and AF cells[77].
Further studies showed increased SP production in the discs of patients with LBP compared to patients without back pain, leading Richardson et al[71] to suggest that SP plays a central regulatory role in the development of painful disc degeneration. Treatment of IVD cells with NK1R antagonists inhibited IL-1β, IL-6, and IL-8 expression in a dose-dependent manner. This suggests that SP mediates signaling in IVD cells via NK1R. The interaction of SP with its preferred receptor NK1R leads to the activation of p38 mitogen-activated protein kinase and extracellular signal-regulated kinase 1/2 (ERK1/2), suggesting that p38 mitogen-activated protein kinase and ERK1/2 control SP-induced cytokine expression. Inhibition of p38 and ERK1/2 activation reduces SP-induced IL-6 production in human IVD cells[78].
CGRP is a 37-amino acid neuropeptide. It is widely produced in the central and peripheral nervous systems. However, it is primarily released from sensory nerves and thus is implicated in pain pathways. CGRP works through the CGRP receptor complex. This complex requires the colocalization of the G-protein component calcitonin-like receptor with a single transmembrane component receptor activity-modifying protein 1 and an intracellular signaling component receptor component protein[79]. The regulation of CGRP synthesis is still unclear. CGRP synthesis is enhanced in tissues undergoing an inflammatory reaction[80].
In a rat model of lumbar IVD injury, CGRP is persistently upregulated in DRG neurons innervating the IVD[4,81]. This may be related to the local release of NGF from disc cells, macrophages, or mast cells. NGF is essential for the growth of sensory nerves and the maintenance of mature nerve function. After treatment with the TRPV1 agonist capsaicin, the sensory neuropeptide in nerve endings is exhausted, and NGF is required to synthesize new peptide[80].
Similar to SP, normal disc cells express low levels of CGRP and its receptor. The expression levels of CGRP and its receptor are significantly increased in degenerative or painful discs[9,74]. In vitro experiments have shown that CGRP can increase the production of inflammatory mediators, such as cyclooxygenase-2, inducible nitric oxide synthase, IL-1β, and IL-6 in human NP cells in a dose-dependent manner. In addition, the CGRP receptor antagonist rimegepant can improve the adverse reaction of CGRP to NP cells, which has been demonstrated both in vitro and in vivo[82]. Data from patients with disc degeneration suggest that NF-κB signaling of peptides, including CGRP, may be involved in the mechanism of peripheral pain[9,74]. Studies have found higher levels of NF-κB and CGRP in disc tissue from patients with degeneration compared to controls[82]. NF-κB activity has been shown to be associated with the degree of disc degeneration. NF-κB may modulate the expression of pain-related neuropeptides in disc cells and the sensory neurons that innervate IVD. This relationship may be a key link in the pain and inflammation cycles that persist in chronic LBP[74].
The release of SP and CGRP from sensory nerves or disc cells into the matrix also upregulate the production of matrix-degrading enzymes. In fact, studies have shown that SP and CGRP can increase the expression of matrix metalloproteinase-3[82,83]. Thus, these neurotransmitters exhibit dual mechanisms of action, including action potential neurotransmission to the central nervous system while inducing or perpetuating peripheral inflammation. These findings provide evidence that SP and CGRP contribute to a degenerative phenotype within the IVD, sensitize ingrowing nerves, and potentially serve as a mechanism for cross-talk between the disc cells and pain fibers to enable transmission of painful stimuli.
NGF has many similarities in structure and physiological functions with other neurotrophins (NTs). These NTs include brain-derived neurotrophic factor (BDNF), NT-3, NT-4/5, NT-6, and NT-7[84]. They are secreted proteins that promote the survival and growth of different elements of the peripheral nervous system. NTs can bind to two types of receptors: Trks and p75NTR, a member of the TNF receptor superfamily. There are three different Trk receptors (TrkA, TrkB, and TrkC), each with its own preference for a specific NT. TrkA has high selectivity towards NGF and NT-3 to a lesser extent, while TrkC binds NT-3 and TrkB BDNF and NT-4/5. On the contrary, p75NTR is promiscuous, binding all NTs, including NGF, with low affinity[85]. NTs are composed of different subgroups in neuronal and non-neuronal tissues, with diffe
The production of NGF is tightly controlled in all areas of the organism innervated by sensory and sympathetic neurons. Normally low basal NGF production is substantially upregulated in the inflammatory response. It is reasonable to assume that mediators regulating local NGF concentrations are released during inflammation due to tissue damage. Many studies have shown that cytokines involved in inflammation, such as IL-1β, TNF-α, and IL-6, are promoters of NGF synthesis in a variety of cell types[86]. NGF is an important cytokine-like neurotrophic mediator. This molecule regulates the survival and differentiation of embryonic sensory and sympathetic neurons from the neuronal crest[86]. In adults, it regulates neuronal regeneration for injury and pain perception and induces neurogenic inflammation by stimulating neuropeptide overexpression and activating immune cells[86,87].
Since TrkA is the main receptor of NGF, it may be considered one of the most important receptors for pain regulation. The expression of TrkA in the basal forebrain of rats is stimulated by NGF itself, suggesting that the upregulation of NGF may lead to amplified pain, accompanied by hyperesthesia and allodynia[88]. Instead, proinflammatory neurotransmitters induce overexpression of NGF, which may lead to chronic self-perpetuating pain sensation mechanisms[85]. In addition, NGF may mediate mechanical and thermal hyperesthesia in systemic applications in animals and humans[84].
NGF and BDNF have been identified in human IVD and have been implicated in mechanisms related to nerve ingrowth and pain perception in IVDD[89,90]. In addition, TrkA and TrkB expression have been demonstrated in both normal and degenerative human IVDs[90]. p75NTR has been found in the AF superficial layers of rat IVD[91]. It has been reported that a large number of TrkA-positive and p75NTR-positive neurons colocalize with CGRP-positive sensory neurons in rat DRG[92]. NGF regulates SP in adult sensory neurons through TrkA and p75NTR[93]. NGF, BDNF, TrkA, and TrkB are expressed in all stages of IVDD[88]. It is generally believed that NGF, TrkA, and p75NTR are always expressed at elevated levels in painful IVD[8]. The expression of NTs and their receptors in AF and NP cells suggests that these molecules have an autocrine or paracrine role in regulating IVD biology in addition to neurotrophic functions, whose function may be regulated by proinflammatory cytokines, which are known to be upregulated during IVDD. For example, the expression of the BDNF gene in cultured AF cells was significantly positively correlated with IVDD[94].
NGF binding to TrkA initiates complex signal transduction pathways, leading to the activation of transcription factors such as NF-κB and inducing the expression of a large number of proinflammatory cytokines[8]. In addition, receptor proteins, such as TRPV1 and bradykinin receptor, voltage-gated sodium channels (NaV1.7, NaV1.8, and NaV1.9), acid-sensing ion channel proteins (ASIC3), and G-protein-coupled receptors are involved in sensory transmission processes and are also regulated by NGF[5,7]. There is experimental evidence that peripheral inflammation leads to increased BDNF content in DRGs, which correlates with increased and widespread BDNF release in the dorsal horn of the spinal cord[7].
Studies have shown that the level of NGF in IVDs is positively correlated with innervation density[95,96]. NGF from human degenerative NP extraction medium promoted the axon growth of DRG neurons in rats and induced SP ex
IVDD is usually related to inflammation, immune cell infiltration, and neovascularization. The pathogenesis of immune cell infiltration into the degenerative IVD is not yet clear, especially since healthy IVDs have immune privileges to a large extent. It is likely that an event secondary to AF injury or tear results in recruitment of immune cells to the painful degenerative discs (Figure 3)[70]. The response of immune cells to acute tissue injury and their role in inflammation suggest that they may play a key role in promoting catabolic remodeling within IVD, thereby enhancing neovascularization and neoinnervation[98].
In painful IVDs, repeated stimulation of nociceptive fibers can induce antidromic transport, followed by the release of SP or CGRP at nociceptors. These neuropeptides modulate the immune response through receptor binding[96,99-101], activating lymphocytes, mast cells, or macrophages, which in turn lead to further upregulation of inflammation and nociceptive sensations[102]. In addition, because NGF receptors TrkA and p75NTR are present on the surface of immune cells, NGF is able to induce immune cells to synthesize neuropeptides with immunomodulatory functions, such as CGRP in monocytes and B cells[84]. Furthermore, immune cells express neuropeptides such as SP, which further recruit leuco
Mast cells are the first responders to tissue damage. Extensive infiltration of mast cells in painful IVDs has been found[70,98]. The mechanism of mast cell infiltration into degenerative IVD is not yet clear, but it may be related to the upregulation of mast cell chemoattractant stem cell factor by degenerative IVD cells[98]. The role of mast cells in DLBP and IVDD may be related to the mediators and bioactive substances released during degranulation, including histamine, proteases, TNF-α, IL-6, NGF, VEGF, SP, etc.[70,95,98,104]. Sensory nerve fibers are usually in close contact with mast cells. Mast cells are particularly relevant to the nervous system in neurogenic inflammation[67].
Neuropeptide SP released from sensory nerve endings acts on mast cells via Mas-related G-protein coupled receptor member B2, inducing mast cell degranulation and promoting proinflammatory effects of subsequent mediators such as histamine[99]. Histamine released from mast cells, in turn, stimulates the release of neuropeptides that act on histamine receptors on sensory nerve endings, thereby establishing a bidirectional loop between mast cells and sensory nerves[105]. In addition, SP induces mast cells to release VEGF, which promotes vascular endothelial cell proliferation and vascularization and promotes inflammatory processes. However, VGEF can also stimulate neurite growth in vitro, promote Schwann cell migration and survival, and may play a role in axon guidance[106,107]. In addition, mast cells promote matrix remodeling by releasing chemokines such as CCL2/monocyte chemotactic protein-1, which are responsible for recruiting other types of cells, such as macrophages[98]. Since DLBP is considered a chronic pain state, it is reasonable to think that mast cells may play an indispensable role in the degeneration and sensitization of IVD.
Macrophage infiltration has been identified in herniated[108,109] and painful IVDs[70], or animal models of IVD injury[10,110]. In humans, levels of macrophage markers were positively associated with degeneration within the NP and endplate, and in cadaveric specimens, macrophage markers were more strongly associated with unhealthy areas of disc structural damage[111]. In addition, the prevalence of CCR7+ M1 macrophages (proinflammatory) and CD163+ M2 macrophages (anti-inflammatory) increases with age and degeneration[111]. A mouse model of disc injury also supports the association between macrophage infiltration and IVDD[112]. Macrophages in injured IVDs produce inflammatory cytokines TNF-α and IL-1β, and macrophage-derived inflammatory factors further regulate the production of NGF and VGEF in IVDs[110,113]. Furthermore, when CD14+ cells (a monocyte marker expressed mainly by macrophages) were isolated from degenerative human IVD tissues and stimulated with TNF-α and IL-1β, production of CGRP and NGF increased[71]. Thus, the influx of macrophages into the degenerative IVD can initiate and/or maintain neurogenic inflammation, exacerbate inflammation and degeneration and promote painful stimulation[114].
The immune privilege of the NP tissue occurs through vascular isolation and the biochemical phenotype of Fas ligands leading to apoptosis of infiltrating T lymphocytes[30]. T lymphocytes and B lymphocytes have been identified in herniated human disc tissue[115-117] and animal models of disc injury[118,119]. Detection of autoantibodies against degenerative or injured IVD and induction of T lymphocytes into IL-4-producing CD4+ Th2 cells further support the hypothesis that contact of NP tissue with systemic circulation in IVD herniation or injury leads to lymphocyte activation and subsequent autoimmune responses[120].
Populations of different types of immune cells express neuronal receptors and respond to neuropeptides and neurotransmitters, suggesting that peripheral neurons are part of a local effector system involved in inflammatory responses to tissue irritation and injury[86]. Thus, the nervous system communicates with the immune system through neuropeptides that are involved in IVD neurogenic inflammation. It is becoming increasingly clear that the nervous system plays a key role in regulating tissue immunity. Given its rapid response and reflex circuits, the ability of the nervous system to sense environmental stimuli and transmit signals to the immune system is essential for an efficient and effective anticipatory immune response. However, our current understanding of the complex dialogue between the nervous system and the immune system is not advanced enough. Addressing these gaps in knowledge will inform the development of new targeted therapeutic strategies to treat chronic inflammation and DLBP in degenerative IVDs[99].
TRP channels are a cation-selective transmembrane receptor superfamily. More than 50 TRP channels have been found in many species, with 28 TRP channels found in mammals[121]. The TRP channel consists of six transmembrane domains, intracellular N-terminal and C-terminal, and a pore domain between segments 5 and 6, which is particularly permeable to Ca2+ ions. There are seven families of mammalian TRPs, including TRPC, TRPV, TRPM, TRPA, TRPP, TRPML, and TRPN. In yeast, an eighth TRP family was recently discovered and named TRPY[122]. TRP channels act as cellular sensors that sense a variety of stimuli, including temperature, membrane voltage, oxidative stress, mechanical stimulation, pH, and endogenous and exogenous ligands, illustrating their versatility[123]. Therefore, TRP channels regulate various functions of excitable and non-excitable cells mainly by mediating Ca2+ homeostasis. Dysregulation of TRP channels is associated with many disorders, including cardiovascular disease, muscular dystrophy, and hyperalgesia[121]. However, the importance of expression, physiological function, and regulation of TRP channels in IVD cells has been largely unex
The release of neuropeptides from sensory nerves is triggered by an increase in cytoplasmic Ca2+ concentration. Cationic channels expressed on sensory nerve endings include some TRP channels involved in neuropeptide release. TRPV1, also known as the capsaicin receptor, is one of six members of the TRPV family (TRPV1-6). TRPV1 is a nociceptive cation channel that is sensitive to high temperature (> 43 °C), and capsaicin is its natural agonist[121]. Most TRPV1-expressing neurons innervating the IVD coexpress CGRP[36]. When TRPV1 is activated by capsaicin and other vanilloids, heat stimuli, and protons, Ca2+ influx is initiated, and neuropeptides are released[124].
Interestingly, TRPV1 is also expressed in non-neuronal cells such as chondrocytes[125] and IVD cells[126,127], but its role in relation to inflammation and pain in these tissues is unclear. One possibility is that it works by mediating acidic microenvironments[128]. IVD is composed of avascular tissue, and its metabolism is mainly through glycolysis. TRPV1 is activated by an acidic pH, which is an important factor in IVD homeostasis[128]. Lactic acid builds up in degenerative IVD due to impaired liquid transport, alters cellular repair capacity, and promotes pain[129]. As a presumed sensor of acidic pH, overstimulated or upregulated TRPV1 may be involved in the IVD cell response to acidity. Thus, inhibition or desensitization of TRPV1 may be beneficial for pH-induced hyperalgesia.
Increased expression and activity of TRPV1 in DRGs after inflammatory stimulation may lead to chronic inflammatory pain in rats, including thermal hyperalgesia and mechanical allodynia[130]. In addition, TRPV1 responds to proinflammatory agents, such as TNF-α, which are known to be highly expressed in painful IVD tissues. Therefore, the inflammatory microenvironment associated with IVDD may increase the likelihood of channel opening[131]. Immune cells such as macrophages, lymphocytes, and mast cells also express TRPV1, and activation of TRPV1 directly affects the function of these cells to increase the expression of inflammatory genes that may be involved in neurogenic inflammation[124].
There is evidence that another member of the TRPV family, TRPV4, is a major osmolarity-regulated ion channel in disc tissue. In bovine disc cells, decreased osmolarity activates TRPV4 expression and induces TRPV4-mediated Ca2+ signaling and proinflammatory cytokine gene expression[132]. In addition, cytokine IL-1β rather than TNF-α stimulation signi
TRPV2 was originally described as a noxious heat sensor, but it now appears to have nothing to do with temperature sensing and instead has more complex physiological characteristics[131]. Cytokine TNF-α stimulation can significantly induce the expression of the TRPV2 gene in human IVD cells[126]. TRPV2 expression was upregulated in DRG induced by peripheral inflammation[133] and may be involved in the release of CGRP[134].
TRPA1 is a ligand-gated non-selective Ca2+ channel, which in contrast to TRPV1 responds to cold thermal sensation (< 17 °C). TRPA1 is located in approximately 60%-75% of sensory C-fibers, which are also positive for TRPV1[124]. TRPA1 is the only known member of the TRPA family and may be involved in the development of chronic pain and mechanical hyperalgesia. TRPA1 is widely expressed in sensory neurons, such as nociceptive DRG neurons, and in non-neuronal cells, including epithelial cells, keratinocytes, and chondrocytes[121].
Both neuronal and non-neuronal TRPA1 may be involved in the occurrence of pain. TRPA1 mediated mechanical hyperalgesia in mice after plantar injection of TNF-α[135], and mechanical hyperalgesia was alleviated after application of the TRPA1 antagonist HC-030031[136]. TRPA1 is expressed in degenerative IVD but not in healthy mature IVD tissues, and its expression in IVD cells is upregulated by proinflammatory cytokines[126]. The expression and activity of TRPA1 in sensory and non-neuronal cells are regulated not only by proinflammatory cytokines but also by neuropeptides and reactive oxygen species, all of which are generally elevated in degenerative IVD[121]. There is a growing hypothesis that TRPA1 is involved in neurogenic inflammation. TRPA1 activation leads to the release of neuropeptides from sensory neurons in a Ca2+-dependent manner, followed by signs of neurogenic inflammation such as edema and leukocyte infiltration[118]. These data suggest that blocking TRPA1 may help reduce chronic pain and inflammation associated with IVDD.
A recent study found that 26 of the 28 currently known TRP channels in mammals are expressed in IVD at the mRNA level. This study supports the importance of TRP channels in IVD homeostasis and pathology and their potential as pharmacological targets for the treatment of IVDD and LBP. However, the exact function and activation of the prominent TRP channels will be determined in future studies[137].
According to our search, the term neurogenic inflammation has barely appeared in the literature in the field of IVD pathology research. In fact, the role of neurogenic inflammation in IVDD and DLBP has been widely involved, but no studies have summarized and generalized it. Data from animal models of IVDD have provided important insights. IVD injury is characterized by an early transient inflammatory response to the initial event with increased production of proinflammatory molecules, including IL-1β, IL-6, IL-8, TNF-α, and NGF[4,81,138,139], followed by a delayed phase that occurs simultaneously with the morphological and biochemical features of progressive IVDD[140,141]. The initial event of increased expression of inflammatory mediators after acute IVD injury is unknown.
It has been shown that IVDs in rats can heal rapidly after a single-stab injury, and the fibrous cap covers the wound track. This process corresponds to the subside of the inflammatory response after the nociceptive stimulus has disa
Neurogenic inflammation in the IVDs may involve complex communication between IVD cells and infiltrating immune cells and afferent nerve endings. The initial release of neuropeptides SP and CGRP by nociceptive nerve fibers can trigger a variety of processes, including vascular events, immune cell infiltration, IVD cells and immune cell release mediators, and sensitization of primary afferent neurons (Figure 4). The upregulation of NGF is believed to play an important role in the development and maintenance of neurogenic inflammation.
Emerging evidence highlights the potential role of TRP channels in IVDD, DLBP, and peripheral signal amplification associated with neurogenic inflammation. It is conceivable that some of the effective drugs currently used to treat various inflammatory diseases, especially osteoarthritis, may be beneficial to the IVD-sensory conduction process[102,144]. Pharmacological interventions targeting IVDD-related neurogenic inflammation may provide new research avenues and potential new strategies for the clinical treatment of DLBP[145].
| 1. | Sousa-Valente J, Brain SD. A historical perspective on the role of sensory nerves in neurogenic inflammation. Semin Immunopathol. 2018;40:229-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Yaksh TL, Di Nardo A. Complexity of systems and actions underlying neurogenic inflammation. Semin Immunopathol. 2018;40:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Sorkin LS, Eddinger KA, Woller SA, Yaksh TL. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin Immunopathol. 2018;40:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Miyagi M, Ishikawa T, Orita S, Eguchi Y, Kamoda H, Arai G, Suzuki M, Inoue G, Aoki Y, Toyone T, Takahashi K, Ohtori S. Disk injury in rats produces persistent increases in pain-related neuropeptides in dorsal root ganglia and spinal cord glia but only transient increases in inflammatory mediators: pathomechanism of chronic diskogenic low back pain. Spine (Phila Pa 1976). 2011;36:2260-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Zhang S, Hu B, Liu W, Wang P, Lv X, Chen S, Shao Z. The role of structure and function changes of sensory nervous system in intervertebral disc-related low back pain. Osteoarthritis Cartilage. 2021;29:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Sun K, Jiang J, Wang Y, Sun X, Zhu J, Xu X, Sun J, Shi J. The role of nerve fibers and their neurotransmitters in regulating intervertebral disc degeneration. Ageing Res Rev. 2022;81:101733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 7. | García-Cosamalón J, del Valle ME, Calavia MG, García-Suárez O, López-Muñiz A, Otero J, Vega JA. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Diwan AD, Melrose J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine. 2023;6:e1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 9. | Krock E, Rosenzweig DH, Chabot-Doré AJ, Jarzem P, Weber MH, Ouellet JA, Stone LS, Haglund L. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. J Cell Mol Med. 2014;18:1213-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Mohd Isa IL, Teoh SL, Mohd Nor NH, Mokhtar SA. Discogenic Low Back Pain: Anatomy, Pathophysiology and Treatments of Intervertebral Disc Degeneration. Int J Mol Sci. 2022;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 166] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 11. | Lama P, Le Maitre CL, Harding IJ, Dolan P, Adams MA. Nerves and blood vessels in degenerated intervertebral discs are confined to physically disrupted tissue. J Anat. 2018;233:86-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Bermudez-Lekerika P, Crump KB, Tseranidou S, Nüesch A, Kanelis E, Alminnawi A, Baumgartner L, Muñoz-Moya E, Compte R, Gualdi F, Alexopoulos LG, Geris L, Wuertz-Kozak K, Le Maitre CL, Noailly J, Gantenbein B. Immuno-Modulatory Effects of Intervertebral Disc Cells. Front Cell Dev Biol. 2022;10:924692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Zhao L, Manchikanti L, Kaye AD, Abd-Elsayed A. Treatment of Discogenic Low Back Pain: Current Treatment Strategies and Future Options-a Literature Review. Curr Pain Headache Rep. 2019;23:86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 1264] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 15. | Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet. 2021;398:78-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 673] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 16. | Fujii K, Yamazaki M, Kang JD, Risbud MV, Cho SK, Qureshi SA, Hecht AC, Iatridis JC. Discogenic Back Pain: Literature Review of Definition, Diagnosis, and Treatment. JBMR Plus. 2019;3:e10180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 17. | Teraguchi M, Yoshimura N, Hashizume H, Muraki S, Yamada H, Minamide A, Oka H, Ishimoto Y, Nagata K, Kagotani R, Takiguchi N, Akune T, Kawaguchi H, Nakamura K, Yoshida M. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: the Wakayama Spine Study. Osteoarthritis Cartilage. 2014;22:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 344] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 18. | Li W, Lai K, Chopra N, Zheng Z, Das A, Diwan AD. Gut-disc axis: A cause of intervertebral disc degeneration and low back pain? Eur Spine J. 2022;31:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Parenteau CS, Lau EC, Campbell IC, Courtney A. Prevalence of spine degeneration diagnosis by type, age, gender, and obesity using Medicare data. Sci Rep. 2021;11:5389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 20. | Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88 Suppl 2:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | MacLean MA, Touchette CJ, Han JH, Christie SD, Pickett GE. Gender differences in the surgical management of lumbar degenerative disease: a scoping review. J Neurosurg Spine. 2020;32:799-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Chan WC, Sze KL, Samartzis D, Leung VY, Chan D. Structure and biology of the intervertebral disk in health and disease. Orthop Clin North Am. 2011;42:447-464, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 23. | Peredo AP, Gullbrand SE, Smith HE, Mauck RL. Putting the Pieces in Place: Mobilizing Cellular Players to Improve Annulus Fibrosus Repair. Tissue Eng Part B Rev. 2021;27:295-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Watanabe H, Yamada Y, Kimata K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J Biochem. 1998;124:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 226] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Beattie PF. Current understanding of lumbar intervertebral disc degeneration: a review with emphasis upon etiology, pathophysiology, and lumbar magnetic resonance imaging findings. J Orthop Sports Phys Ther. 2008;38:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Ghannam M, Jumah F, Mansour S, Samara A, Alkhdour S, Alzuabi MA, Aker L, Adeeb N, Massengale J, Oskouian RJ, Tubbs RS. Surgical anatomy, radiological features, and molecular biology of the lumbar intervertebral discs. Clin Anat. 2017;30:251-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine (Phila Pa 1976). 2004;29:2700-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 728] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 28. | Johnson WE, Caterson B, Eisenstein SM, Hynds DL, Snow DM, Roberts S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46:2658-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Mima Y, Suzuki S, Fujii T, Morikawa T, Tamaki S, Takubo K, Shimoda M, Miyamoto T, Watanabe K, Matsumoto M, Nakamura M, Fujita N. Potential involvement of semaphorin 3A in maintaining intervertebral disc tissue homeostasis. J Orthop Res. 2019;37:972-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Sun Z, Liu B, Luo ZJ. The Immune Privilege of the Intervertebral Disc: Implications for Intervertebral Disc Degeneration Treatment. Int J Med Sci. 2020;17:685-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 31. | Jackson HC, Winkelmann RK, Bickel WH. Nerve Endings in the Human Lumbar Spinal Column and Related Structures. The Journal of Bone & Joint Surgery. 1966;48:1272-1281. [DOI] [Full Text] |
| 32. | Malinský J. The Ontogenetic Development of Nerve Terminations in the Intervertebral Discs of Man (Histology of Intervertebral Discs, 11th Communication). Cells Tissues Organs. 1959;38:96-113. [RCA] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Edgar MA. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg Br. 2007;89:1135-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Ashton IK, Walsh DA, Polak JM, Eisenstein SM. Substance P in intervertebral discs. Binding sites on vascular endothelium of the human annulus fibrosus. Acta Orthop Scand. 1994;65:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Palmgren T, Grönblad M, Virri J, Kääpä E, Karaharju E. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine (Phila Pa 1976). 1999;24:2075-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Aoki Y, Takahashi Y, Ohtori S, Moriya H, Takahashi K. Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review. Life Sci. 2004;74:2627-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Morinaga T, Takahashi K, Yamagata M, Chiba T, Tanaka K, Takahashi Y, Nakamura S, Suseki K, Moriya H. Sensory innervation to the anterior portion of lumbar intervertebral disc. Spine (Phila Pa 1976). 1996;21:1848-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Ohtori S, Takahashi Y, Takahashi K, Yamagata M, Chiba T, Tanaka K, Hirayama J, Moriya H. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine (Phila Pa 1976). 1999;24:2295-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Sensory innervation of the dorsal portion of the lumbar intervertebral discs in rats. Spine (Phila Pa 1976). 2001;26:946-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Zhang Y, Kerns JM, Anderson DG, Lee YS, Chen EY, Tannoury C, An HS. Sensory neurons and fibers from multiple spinal cord levels innervate the rabbit lumbar disc. Am J Phys Med Rehabil. 2006;85:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Quinones S, Konschake M, Aguilar LL, Simon C, Aragones P, Hernández LM, Abramovic A, Tubbs RS, Bouzada J, Valderrama-Canales FJ, Vazquez T, Sanudo J. Clinical anatomy of the lumbar sinuvertebral nerve with regard to discogenic low back pain and review of literature. Eur Spine J. 2021;30:2999-3008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Breemer MC, Malessy MJA, Notenboom RGE. Origin, branching pattern, foraminal and intraspinal distribution of the human lumbar sinuvertebral nerves. Spine J. 2022;22:472-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Brisby H. Pathology and possible mechanisms of nervous system response to disc degeneration. J Bone Joint Surg Am. 2006;88 Suppl 2:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Ohtori S, Inoue G, Miyagi M, Takahashi K. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015;15:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 45. | Brown MF, Hukkanen MV, McCarthy ID, Redfern DR, Batten JJ, Crock HV, Hughes SP, Polak JM. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Fras C, Kravetz P, Mody DR, Heggeness MH. Substance P-containing nerves within the human vertebral body. an immunohistochemical study of the basivertebral nerve. Spine J. 2003;3:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Santifort KM, Glass EN, Meij BP, Bergknut N, Pumarola M, Gil VA. Anatomic description of the basivertebral nerve and meningeal branch of the spinal nerve in the dog. Ann Anat. 2023;245:152000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Fischgrund JS, Rhyne A, Franke J, Sasso R, Kitchel S, Bae H, Yeung C, Truumees E, Schaufele M, Yuan P, Vajkoczy P, Depalma M, Anderson DG, Thibodeau L, Meyer B. Intraosseous Basivertebral Nerve Ablation for the Treatment of Chronic Low Back Pain: 2-Year Results From a Prospective Randomized Double-Blind Sham-Controlled Multicenter Study. Int J Spine Surg. 2019;13:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 49. | Järvinen J, Karppinen J, Niinimäki J, Haapea M, Grönblad M, Luoma K, Rinne E. Association between changes in lumbar Modic changes and low back symptoms over a two-year period. BMC Musculoskelet Disord. 2015;16:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 50. | Ohtori S, Miyagi M, Inoue G. Sensory nerve ingrowth, cytokines, and instability of discogenic low back pain: A review. Spine Surg Relat Res. 2018;2:11-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Wang Y, Videman T, Battié MC. ISSLS prize winner: Lumbar vertebral endplate lesions: associations with disc degeneration and back pain history. Spine (Phila Pa 1976). 2012;37:1490-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 52. | Morisako T, Nakamae T, Kamei N, Tamura T, Tsuchikawa Y, Harada T, Maruyama T, Adachi N. Development of a rat model with lumbar vertebral endplate lesion. Eur Spine J. 2022;31:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 53. | Fields AJ, Liebenberg EC, Lotz JC. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014;14:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 54. | Kim HS, Wu PH, Jang IT. Lumbar Degenerative Disease Part 1: Anatomy and Pathophysiology of Intervertebral Discogenic Pain and Radiofrequency Ablation of Basivertebral and Sinuvertebral Nerve Treatment for Chronic Discogenic Back Pain: A Prospective Case Series and Review of Literature. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 55. | Schnapp W, Martiatu K, Delcroix GJ. Basivertebral Nerve Ablation for the Treatment of Chronic Low Back Pain: A Scoping Review of the Literature. Pain Physician. 2022;25:E551-E562. [PubMed] |
| 56. | Nwosu M, Agyeman WY, Bisht A, Gopinath A, Cheema AH, Chaludiya K, Khalid M, Yu AK. The Effectiveness of Intraosseous Basivertebral Nerve Ablation in the Treatment of Nonradiating Vertebrogenic Pain: A Systematic Review. Cureus. 2023;15:e37114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 57. | Peng B, Chen J, Kuang Z, Li D, Pang X, Zhang X. Diagnosis and surgical treatment of back pain originating from endplate. Eur Spine J. 2009;18:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Aoki Y, Ohtori S, Takahashi K, Ino H, Takahashi Y, Chiba T, Moriya H. Innervation of the lumbar intervertebral disc by nerve growth factor-dependent neurons related to inflammatory pain. Spine (Phila Pa 1976). 2004;29:1077-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Ozawa T, Ohtori S, Inoue G, Aoki Y, Moriya H, Takahashi K. The degenerated lumbar intervertebral disc is innervated primarily by peptide-containing sensory nerve fibers in humans. Spine (Phila Pa 1976). 2006;31:2418-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 60. | Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 643] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 61. | Koshi T, Ohtori S, Inoue G, Ito T, Yamashita M, Yamauchi K, Suzuki M, Aoki Y, Takahashi K. Lumbar posterolateral fusion inhibits sensory nerve ingrowth into punctured lumbar intervertebral discs and upregulation of CGRP immunoreactive DRG neuron innervating punctured discs in rats. Eur Spine J. 2010;19:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Aoki Y, Ohtori S, Ino H, Douya H, Ozawa T, Saito T, Moriya H, Takahashi K. Disc inflammation potentially promotes axonal regeneration of dorsal root ganglion neurons innervating lumbar intervertebral disc in rats. Spine (Phila Pa 1976). 2004;29:2621-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Inoue G, Ohtori S, Aoki Y, Ozawa T, Doya H, Saito T, Ito T, Akazawa T, Moriya H, Takahashi K. Exposure of the nucleus pulposus to the outside of the anulus fibrosus induces nerve injury and regeneration of the afferent fibers innervating the lumbar intervertebral discs in rats. Spine (Phila Pa 1976). 2006;31:1433-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Jiang H, Wang J, Xu B, Yang Q, Liu Y. Study on the expression of nerve growth associated protein-43 in rat model of intervertebral disc degeneration. J Musculoskelet Neuronal Interact. 2017;17:104-107. [PubMed] |
| 65. | Staszkiewicz R, Gralewski M, Gładysz D, Bryś K, Garczarek M, Gadzieliński M, Marcol W, Sobański D, Grabarek BO. Evaluation of the concentration of growth associated protein-43 and glial cell-derived neurotrophic factor in degenerated intervertebral discs of the lumbosacral region of the spine. Mol Pain. 2023;19:17448069231158287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 66. | Birder LA, Kullmann FA. Role of neurogenic inflammation in local communication in the visceral mucosa. Semin Immunopathol. 2018;40:261-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018;40:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 68. | Ahmed M, Bjurholm A, Kreicbergs A, Schultzberg M. Neuropeptide Y, tyrosine hydroxylase and vasoactive intestinal polypeptide-immunoreactive nerve fibers in the vertebral bodies, discs, dura mater, and spinal ligaments of the rat lumbar spine. Spine (Phila Pa 1976). 1993;18:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 95] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Peng B, Wu W, Hou S, Li P, Zhang C, Yang Y. The pathogenesis of discogenic low back pain. The Journal of Bone and Joint Surgery British volume. 2005;87-B:62-67. [RCA] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 70. | Peng B, Hao J, Hou S, Wu W, Jiang D, Fu X, Yang Y. Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976). 2006;31:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 71. | Richardson SM, Doyle P, Minogue BM, Gnanalingham K, Hoyland JA. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res Ther. 2009;11:R126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 72. | Binch AL, Cole AA, Breakwell LM, Michael AL, Chiverton N, Cross AK, Le Maitre CL. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res Ther. 2014;16:416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 73. | Miyagi M, Uchida K, Takano S, Nakawaki M, Sekiguchi H, Nakazawa T, Imura T, Saito W, Shirasawa E, Kawakubo A, Akazawa T, Inoue G, Takaso M. Role of CD14-positive cells in inflammatory cytokine and pain-related molecule expression in human degenerated intervertebral discs. J Orthop Res. 2021;39:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 74. | Ahmed AS, Berg S, Alkass K, Druid H, Hart DA, Svensson CI, Kosek E. NF-κB-Associated Pain-Related Neuropeptide Expression in Patients with Degenerative Disc Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 75. | Chernova I, Lai JP, Li H, Schwartz L, Tuluc F, Korchak HM, Douglas SD, Kilpatrick LE. Substance P (SP) enhances CCL5-induced chemotaxis and intracellular signaling in human monocytes, which express the truncated neurokinin-1 receptor (NK1R). J Leukoc Biol. 2009;85:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 76. | Douglas SD, Leeman SE. Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci. 2011;1217:83-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 77. | Kepler CK, Markova DZ, Hilibrand AS, Vaccaro AR, Risbud MV, Albert TJ, Anderson DG. Substance P stimulates production of inflammatory cytokines in human disc cells. Spine (Phila Pa 1976). 2013;38:E1291-E1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Kepler CK, Markova DZ, Koerner JD, Mendelis J, Chen CM, Vaccaro AR, Risbud MV, Albert TJ, Anderson DG. Substance P Receptor Antagonist Suppresses Inflammatory Cytokine Expression in Human Disc Cells. Spine (Phila Pa 1976). 2015;40:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 877] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 80. | Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 335] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 81. | Miyagi M, Ishikawa T, Kamoda H, Suzuki M, Murakami K, Shibayama M, Orita S, Eguchi Y, Arai G, Sakuma Y, Kubota G, Oikawa Y, Ozawa T, Aoki Y, Toyone T, Takahashi K, Inoue G, Kawakami M, Ohtori S. ISSLS prize winner: disc dynamic compression in rats produces long-lasting increases in inflammatory mediators in discs and induces long-lasting nerve injury and regeneration of the afferent fibers innervating discs: a pathomechanism for chronic discogenic low back pain. Spine (Phila Pa 1976). 2012;37:1810-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 82. | Sun K, Zhu J, Yan C, Li F, Kong F, Sun J, Sun X, Shi J, Wang Y. CGRP Regulates Nucleus Pulposus Cell Apoptosis and Inflammation via the MAPK/NF-κB Signaling Pathways during Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2021;2021:2958584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Fong G, Backman LJ, Hart DA, Danielson P, McCormack B, Scott A. Substance P enhances collagen remodeling and MMP-3 expression by human tenocytes. J Orthop Res. 2013;31:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 84. | Wise BL, Seidel MF, Lane NE. The evolution of nerve growth factor inhibition in clinical medicine. Nat Rev Rheumatol. 2021;17:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 85. | Denk F, Bennett DL, McMahon SB. Nerve Growth Factor and Pain Mechanisms. Annu Rev Neurosci. 2017;40:307-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 86. | Minnone G, De Benedetti F, Bracci-Laudiero L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 232] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 87. | Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 709] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 88. | Kojima M, Ikeuchi T, Hatanaka H. Role of nerve growth factor in the expression of trkA mRNA in cultured embryonic rat basal forebrain cholinergic neurons. J Neurosci Res. 1995;42:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Abe Y, Akeda K, An HS, Aoki Y, Pichika R, Muehleman C, Kimura T, Masuda K. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine (Phila Pa 1976). 2007;32:635-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 90. | Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10:R99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 91. | Sugiura A, Ohtori S, Yamashita M, Inoue G, Yamauchi K, Koshi T, Suzuki M, Norimoto M, Orita S, Eguchi Y, Takahashi Y, Watanabe TS, Ochiai N, Takaso M, Takahashi K. Existence of nerve growth factor receptors, tyrosine kinase a and p75 neurotrophin receptors in intervertebral discs and on dorsal root ganglion neurons innervating intervertebral discs in rats. Spine (Phila Pa 1976). 2008;33:2047-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Mousa SA, Cheppudira BP, Shaqura M, Fischer O, Hofmann J, Hellweg R, Schäfer M. Nerve growth factor governs the enhanced ability of opioids to suppress inflammatory pain. Brain. 2007;130:502-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Skoff AM, Adler JE. Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp Neurol. 2006;197:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Gruber HE, Ingram JA, Hoelscher G, Zinchenko N, Norton HJ, Hanley EN Jr. Brain-derived neurotrophic factor and its receptor in the human and the sand rat intervertebral disc. Arthritis Res Ther. 2008;10:R82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 95. | Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MT, Ross ER, O'Brien JP, Hoyland JA. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 336] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 96. | Kokubo Y, Uchida K, Kobayashi S, Yayama T, Sato R, Nakajima H, Takamura T, Mwaka E, Orwotho N, Bangirana A, Baba H. Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J Neurosurg Spine. 2008;9:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 97. | Yamauchi K, Inoue G, Koshi T, Yamashita M, Ito T, Suzuki M, Eguchi Y, Orita S, Takaso M, Nakagawa K, Aoki Y, Ochiai N, Kishida S, Endo M, Yamashita T, Takahashi K, Ohtori S. Nerve growth factor of cultured medium extracted from human degenerative nucleus pulposus promotes sensory nerve growth and induces substance p in vitro. Spine (Phila Pa 1976). 2009;34:2263-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Wiet MG, Piscioneri A, Khan SN, Ballinger MN, Hoyland JA, Purmessur D. Mast Cell-Intervertebral disc cell interactions regulate inflammation, catabolism and angiogenesis in Discogenic Back Pain. Sci Rep. 2017;7:12492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 99. | Chu C, Artis D, Chiu IM. Neuro-immune Interactions in the Tissues. Immunity. 2020;52:464-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 100. | Mikami N, Matsushita H, Kato T, Kawasaki R, Sawazaki T, Kishimoto T, Ogitani Y, Watanabe K, Miyagi Y, Sueda K, Fukada S, Yamamoto H, Tsujikawa K. Calcitonin gene-related peptide is an important regulator of cutaneous immunity: effect on dendritic cell and T cell functions. J Immunol. 2011;186:6886-6893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 101. | Suvas S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J Immunol. 2017;199:1543-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 237] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 102. | Seidel MF, Hügle T, Morlion B, Koltzenburg M, Chapman V, MaassenVanDenBrink A, Lane NE, Perrot S, Zieglgänsberger W. Neurogenic inflammation as a novel treatment target for chronic pain syndromes. Exp Neurol. 2022;356:114108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 103. | Zieglgänsberger W. Substance P and pain chronicity. Cell Tissue Res. 2019;375:227-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 104. | Freemont AJ, Jeziorska M, Hoyland JA, Rooney P, Kumar S. Mast cells in the pathogenesis of chronic back pain: a hypothesis. J Pathol. 2002;197:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 105. | Rosa AC, Fantozzi R. The role of histamine in neurogenic inflammation. Br J Pharmacol. 2013;170:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 106. | Rosenstein JM, Mani N, Khaibullina A, Krum JM. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23:11036-11044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 107. | Rosenstein JM, Krum JM. New roles for VEGF in nervous tissue--beyond blood vessels. Exp Neurol. 2004;187:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 223] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 108. | Djuric N, Yang X, El Barzouhi A, Ostelo R, van Duinen SG, Lycklama À Nijeholt GJ, van der Kallen BFW, Peul WC, Vleggeert-Lankamp CLA. Lumbar disc extrusions reduce faster than bulging discs due to an active role of macrophages in sciatica. Acta Neurochir (Wien). 2020;162:79-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 109. | Yu P, Mao F, Chen J, Ma X, Dai Y, Liu G, Dai F, Liu J. Characteristics and mechanisms of resorption in lumbar disc herniation. Arthritis Res Ther. 2022;24:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 110. | Nakawaki M, Uchida K, Miyagi M, Inoue G, Kawakubo A, Satoh M, Takaso M. Changes in Nerve Growth Factor Expression and Macrophage Phenotype Following Intervertebral Disc Injury in Mice. J Orthop Res. 2019;37:1798-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 111. | Nakazawa KR, Walter BA, Laudier DM, Krishnamoorthy D, Mosley GE, Spiller KL, Iatridis JC. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J. 2018;18:343-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 112. | Lee S, Millecamps M, Foster DZ, Stone LS. Long-term histological analysis of innervation and macrophage infiltration in a mouse model of intervertebral disc injury-induced low back pain. J Orthop Res. 2020;38:1238-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 113. | Miyagi M, Uchida K, Takano S, Fujimaki H, Aikawa J, Sekiguchi H, Nagura N, Ohtori S, Inoue G, Takaso M. Macrophage-derived inflammatory cytokines regulate growth factors and pain-related molecules in mice with intervertebral disc injury. J Orthop Res. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 114. | Koroth J, Buko EO, Abbott R, Johnson CP, Ogle BM, Stone LS, Ellingson AM, Bradley EW. Macrophages and Intervertebral Disc Degeneration. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 115. | Shamji MF, Setton LA, Jarvis W, So S, Chen J, Jing L, Bullock R, Isaacs RE, Brown C, Richardson WJ. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62:1974-1982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 116. | Virri J, Grönblad M, Seitsalo S, Habtemariam A, Kääpä E, Karaharju E. Comparison of the prevalence of inflammatory cells in subtypes of disc herniations and associations with straight leg raising. Spine (Phila Pa 1976). 2001;26:2311-2315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Habtemariam A, Grönblad M, Virri J, Seitsalo S, Karaharju E. A comparative immunohistochemical study of inflammatory cells in acute-stage and chronic-stage disc herniations. Spine (Phila Pa 1976). 1998;23:2159-65; discussion 2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 118. | Kanerva A, Kommonen B, Grönblad M, Tolonen J, Habtemariam A, Virri J, Karaharju E. Inflammatory cells in experimental intervertebral disc injury. Spine (Phila Pa 1976). 1997;22:2711-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Habtemariam A, Virri J, Grönblad M, Holm S, Kaigle A, Karaharju E. Inflammatory cells in full-thickness anulus injury in pigs. An experimental disc herniation animal model. Spine (Phila Pa 1976). 1998;23:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 120. | Ye F, Lyu FJ, Wang H, Zheng Z. The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine. 2022;5:e1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 121. | Krupkova O, Zvick J, Wuertz-Kozak K. The role of transient receptor potential channels in joint diseases. Eur Cell Mater. 2017;34:180-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 122. | Li C, Wang W. Molecular Biology of Aquaporins. Adv Exp Med Biol. 2017;969:1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 123. | Liu C, Montell C. Forcing open TRP channels: Mechanical gating as a unifying activation mechanism. Biochem Biophys Res Commun. 2015;460:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 124. | Gouin O, L'Herondelle K, Lebonvallet N, Le Gall-Ianotto C, Sakka M, Buhé V, Plée-Gautier E, Carré JL, Lefeuvre L, Misery L, Le Garrec R. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell. 2017;8:644-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 125. | Gavenis K, Schumacher C, Schneider U, Eisfeld J, Mollenhauer J, Schmidt-Rohlfing B. Expression of ion channels of the TRP family in articular chondrocytes from osteoarthritic patients: changes between native and in vitro propagated chondrocytes. Mol Cell Biochem. 2009;321:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 126. | Kameda T, Zvick J, Vuk M, Sadowska A, Tam WK, Leung VY, Bölcskei K, Helyes Z, Applegate LA, Hausmann ON, Klasen J, Krupkova O, Wuertz-Kozak K. Expression and Activity of TRPA1 and TRPV1 in the Intervertebral Disc: Association with Inflammation and Matrix Remodeling. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 127. | Aripaka SS, Bech-Azeddine R, Jørgensen LM, Mikkelsen JD. Transient receptor potential (TRP) channels mRNA transcripts in the lumbar intervertebral discs: biomarkers for inflammation, pain, disability, and clinical outcome. Mol Cell Biochem. 2023;478:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 128. | Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, Patapoutian A. TRPV1 is activated by both acidic and basic pH. J Neurosci. 2009;29:153-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 129. | Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9:R45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 350] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 130. | Yu L, Yang F, Luo H, Liu FY, Han JS, Xing GG, Wan Y. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund's adjuvant. Mol Pain. 2008;4:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 131. | Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol. 2013;716:61-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 132. | Walter BA, Purmessur D, Moon A, Occhiogrosso J, Laudier DM, Hecht AC, Iatridis JC. Reduced tissue osmolarity increases TRPV4 expression and pro-inflammatory cytokines in intervertebral disc cells. Eur Cell Mater. 2016;32:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 133. | Shimosato G, Amaya F, Ueda M, Tanaka Y, Decosterd I, Tanaka M. Peripheral inflammation induces up-regulation of TRPV2 expression in rat DRG. Pain. 2005;119:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 134. | Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231-6238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 135. | Fernandes ES, Russell FA, Spina D, McDougall JJ, Graepel R, Gentry C, Staniland AA, Mountford DM, Keeble JE, Malcangio M, Bevan S, Brain SD. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor α-induced inflammatory hyperalgesia and Freund's complete adjuvant-induced monarthritis. Arthritis Rheum. 2011;63:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 136. | Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, Henze DA, Kane SA, Urban MO. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 344] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 137. | Sadowska A, Hitzl W, Karol A, Jaszczuk P, Cherif H, Haglund L, Hausmann ON, Wuertz-Kozak K. Differential regulation of TRP channel gene and protein expression by intervertebral disc degeneration and back pain. Sci Rep. 2019;9:18889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 138. | Rousseau MA, Ulrich JA, Bass EC, Rodriguez AG, Liu JJ, Lotz JC. Stab incision for inducing intervertebral disc degeneration in the rat. Spine (Phila Pa 1976). 2007;32:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 139. | Ulrich JA, Liebenberg EC, Thuillier DU, Lotz JC. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine (Phila Pa 1976). 2007;32:2812-2819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 140. | Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, Kang JD. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 141. | Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J Bone Joint Surg Am. 2006;88 Suppl 2:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 142. | Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1699] [Article Influence: 70.8] [Reference Citation Analysis (1)] |
| 143. | Li Y, Dai C, Wu B, Yang L, Yan X, Liu T, Chen J, Zheng Z, Peng B. Intervertebral disc injury triggers neurogenic inflammation of adjacent healthy discs. Spine J. 2024;24:1527-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 144. | Fine N, Lively S, Séguin CA, Perruccio AV, Kapoor M, Rampersaud R. Intervertebral disc degeneration and osteoarthritis: a common molecular disease spectrum. Nat Rev Rheumatol. 2023;19:136-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 145. | Canseco JA, Levy HA, Karamian BA, Blaber O, Chang M, Patel N, Curran J, Hilibrand AS, Schroeder GD, Vaccaro AR, Markova DZ, Surrey DE, Kepler CK. Inhibition of Neurogenic Inflammatory Pathways Associated with the Reduction in Discogenic Back Pain. Asian Spine J. 2023;17:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |