Published online Sep 18, 2024. doi: 10.5312/wjo.v15.i9.882
Revised: July 19, 2024
Accepted: August 5, 2024
Published online: September 18, 2024
Processing time: 73 Days and 20.1 Hours
The use of opioids for pain is linked to an increased risk of developing opioid use disorder, and has resulted in the emergence of the opioid crisis over the last few years.
The systematic review question is “How does the use of opioid medications in pain management, compared with non-opioid medications, affect pain intensity over the short, intermediate, and long-term in adults with acute traumatic pain?”.
The protocol was prospectively registered on the International Prospective Re
After full-text screening, we included 14 studies in the qualitative synthesis. Of these 14 studies, 12 were rando
Non-opioids can be considered an alternative to opioids for short-term pain management of acute musculoskeletal injury. Intravenous ketamine may cause more adverse events than other routes of administration.
Core Tip: Opioid use is linked to an increased risk of developing opioid use disorder. This systematic review question is “How does the use of opioid medications in pain management, compared with non-opioid medications, affect pain intensity over the short, intermediate, and long-term in adults with acute traumatic pain?”. The search was performed using Medline and Google Scholar. We included 14 studies in the final synthesis [12 were randomized clinical trials (RCTs) and 2 were pseudo-RCTs]. Most retrieved studies on the use of non-opioids concluded that non-opioid drugs are non-inferior to opioids for the control of acute pain in acute musculoskeletal injury.
- Citation: Fiore M, Nasto LA, McCaffery E, Barletta F, Visconti A, Gargano F, Pola E, Pace MC. Pain management in acute musculoskeletal injury: Effect of opioid vs nonopioid medications. World J Orthop 2024; 15(9): 882-890
- URL: https://www.wjgnet.com/2218-5836/full/v15/i9/882.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i9.882
In the late 90s, the pharmaceutical industry assured the medical community that opioids prescribed for pain relief would not result in addiction. In turn, medical doctors increased their rates of opioid prescription, with the well-intentioned belief that they were effectively treating pain with little risk of harm. Increased awareness of the addictive quality of these medications has led to a reduction in the prescription of opiates in recent years. However, paradoxically, although the prescription rate has been reduced, deaths from overdoses associated with opioids continue to increase[1]. Opioid over
The question being addressed in this systematic review is “How does the use of opioid medications in pain manage
This systematic review was conducted in accordance with the updated guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[5].
The protocol was prospectively registered on the International Prospective Register of Systematic Reviews (PROSPERO): CRD42021279639 on November 18, 2021 after searching the main electronic registers (the Cochrane database of systematic reviews, the JBI database of systematic reviews, and implementation reports and PROSPERO) to exclude existing systematic reviews on the same subject.
The participants, intervention, comparison, outcomes, study design method was utilized to conduct the search strategy (Table 1). The databases utilized were Medline through PubMed and Google Scholar via Publish or Perish software[6].
| Participants | Intervention | Comparison | Outcomes | Study design |
| Adult patients with catastrophic orthopedic trauma | Opioid medications | Non-opioid medications | A minimum clinically important difference in pain | Randomized controlled trials and observational studies |
The search strategy used Boolean operators to combine selected keywords in detail (Supplementary Table 1). A first comprehensive search was performed, which began at the inception of the search strategy and ceased in December 2021. The search was re-run, updating the data collection definitively until May 15, 2024.
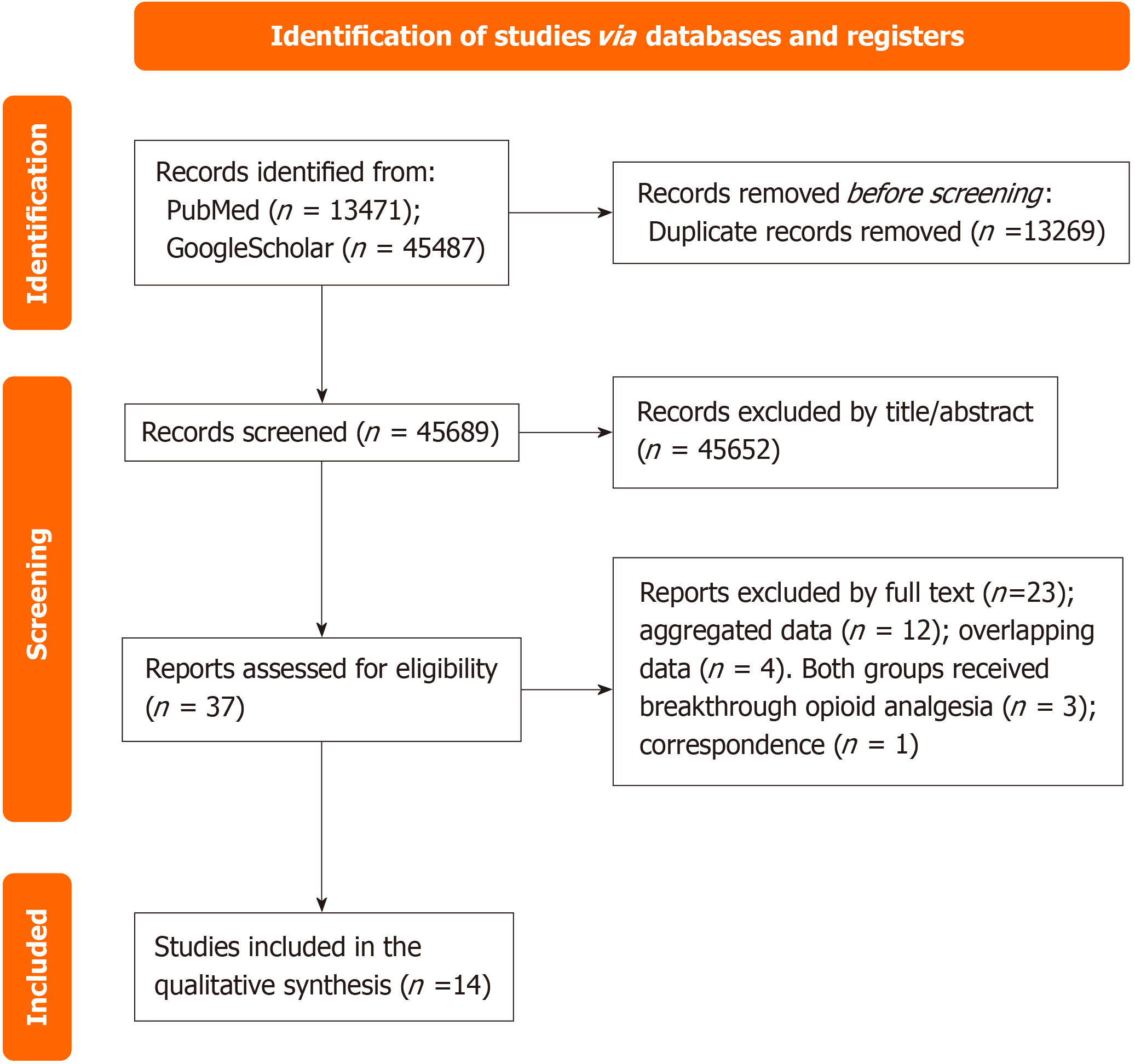
After searching, we eliminated duplicate studies utilizing Endnote VX9 (Clarivate Analytics, Philadelphia, PA, United States), a citation management software. Randomized clinical trials (RCTs) and non-RCTs with a control group, were considered eligible studies. Two authors (Barletta and Visconti) evaluated the eligible studies independently through initial screening based on the title and abstract, without any restrictions. The authors (Barletta and Visconti) conducted full-text screening of the selected articles to ensure their suitability for final inclusion. Any disagreements about study eligibility or data extraction was resolved by a third author (McCaffery). Two independent reviewers (Barletta and Visconti) reviewed the entire text of the selected citations and documented the reason for excluding full-text studies that did not meet the inclusion criteria; McCaffery performed a final check as well. Each step of the search is represented in the PRISMA flow diagram (Figure 1).
For this study, catastrophic orthopedic trauma was defined as any trauma that necessitated surgery or hospitalization. We excluded trauma that necessitated access to and evaluation in the emergency department (ED) but did not require surgery or hospitalization, such as whiplash or sprained ankle. The primary outcome was a minimum clinically important difference (MCID) in pain intensity. The secondary outcome was a reduction in pain as defined by the authors of the primary studies. Patients with a trauma-related pain diagnosis were evaluated for all outcomes.
The Cochrane data collection form for intervention reviews in RCT and non-RCT studies was used by two authors (McCaffery and Nasto) to extract data independently from the included studies. The quality of the methodology and risk of bias were evaluated by the authors (McCaffery and Nasto) using the Cochrane Collaboration Revised Assessment Tool[7].
Overall, 58958 papers were retrieved: 13471 on Medline and 45487 on Google Scholar, and 13269 duplicates were identi
| Ref. | Country/year | Injury | Opioid(s)-ROA | Nonopioid(s)-ROA | Patients /study type | Outcome (minimum clinically important difference) | Evaluation time | Efficacy result | Safety results |
| Soave et al[8] | Italy/1983 | Severe pain due to fractures | Pentazocine-IV | Indoprofen-IV | The 40/pseudo-RCT | Any reduction on VAS | The 0 minute, 30 minutes, 1 hour, 2 hours, 4 hours, 6 hours | Pain relief in Pentazocine group | No differences |
| Chang et al[9] | United States/2017 | Acute extremity pain | OXY/APAP-PO, HYCOD/APAP-PO, COD/APAP-PO | IBP/APAP-PO | 416/RCT | Reduction of 1.3 on the NRS | 2 hours | No differences | ADR in opioid |
| Bijur et al[10] | United States/2021 | Acute musculo-skeletal pain | OXY/APAP-PO, HYCOD/APAP-PO, COD/APAP-PO | IBP/APAP-PO | 600/RCT | Any reduction on NRS | The 0 hour, 1 hour or 2 hours | No differences | ADR in opioid |
| Buccelletti et al[11] | Italy/2014 | Acute musculo-skeletal traumatic pain | COD/APAP-PO | Ketorolac-PO | 134/pseudo-RCT | Any reduction on NRS | The 0 minute, 30 minutes, 2 hours | Pain relief in COD/APAP group | Not available |
| Craig et al[12] | United Kingdom/2012 | Severe traumatic limb pain | MORPH-IV | APAP-IV | 55/RCT | ≥ 13 mm reduction on VAS | The 0 minute, 5 minutes, 15 minutes, 30 minutes, 60 minutes | No differences | ADR in opioid |
| Jalili et al[13] | Iran/2016 | Severe traumatic limb pain | MORPH-IV | APAP-IV | 60/RCT | Any reduction on NRS | The 0 minute, 15 minutes, 30 minutes | Pain relief in APAP group | No differences |
| Farahmand et al[14] | Iran/2018 | Severe traumatic limb pain | MORPH-IV | Lidocaine-IV | 50/RCT | Reduction of 1.3 on the NRS | The 0 minute, 15 minutes, 30 minutes, 45 minutes, 60 minutes | No differences | Heart rate/respiratory rate reduction in opioid |
| Gurnani et al[15] | India/1996 | Acute musculo-skeletal traumatic pain | MORPH-IV | KET-subcutaneous | 40/RCT | Any reduction on VAS | The 0 minute, 15 minutes, 1 hour, 2 hours, 4 hours, 8 hours, 12 hours, 24 hours | Pain relief in KET group | ADR in opioid |
| Shimonovich et al[16] | Israel/2016 | Moderate-severe acute traumatic pain | MORPH-IV/MORPH-IM | KET-IN | 90/RCT | ≥ 15 mm reduction and max pain reduction on VAS | Time to onset in min | No differences | No differences |
| Tongbua et al[17] | Thailand/2022 | Moderate-severe Musculo-skeletal pain | MORPH-IV | KET-IN | 74/RCT | Reduction of 1.3 on the NRS | The 0 minute, 15 minutes, 30 minutes, 45 minutes, 60 minutes, 75 minutes, 90 minutes, 105 minutes and 120 minutes | No differences | No differences |
| Kampan et al[18] | Thailand/2024 | Moderate-severe Musculo-skeletal pain (≥ 65) | MORPH-IV | KET-IN | 92/RCT | Reduction of 1.3 on the NRS | The 0 minute, 30 minutes | No differences | ADR in opioid |
| Esfahani et al[19] | Iran/2021 | Severe traumatic limb pain | MORPH-IV | KET-IV | 76/RCT | Any reduction on NRS | The 0 minute, 5 minutes, 10 minutes, 15 minutes, 20 minutes, 25 minutes, 30 minutes | No differences | ADR in KET |
| Le Cornec et al[20] | France/2024 | Out-of-hospital traumatic pain | MORPH-IV | KET-IV | 251/RCT | Reduction of 1.3 on the NRS | The 0 minute, 30 minutes | No differences | ADR in KET |
| Lim et al[21] | Singapore/ 2021 | Out-of-hospital traumatic pain | Tramadol-IM | MTX-inhalation | 369/RCT | ≥ 3-point reduction in NRS | The 0 minute, 5 minutes, 10 minutes, 15 minutes, 20 minutes | Pain relief in MTX group | ADR in MTX |
The first published study on the topic, written by Soave et al[8], dates back more than 40 years. In this double-blind, randomized, parallel-group study, the analgesic activity of indoprofen and pentazocine was evaluated in 60 patients with severe pain due to fractures. Twenty patients received indoprofen 400 mg intravenous (IV), 20 patients received pentazocine 30 mg IV, and 20 patients received placebo. The intensity of pain was assessed prior to medication administration and at 0.5 hour, 1 hour, 2 hours, 4 hours, and 6 hours following administration. The evaluation of efficacy was based on the visual analogue scale (VAS). The analgesic effects of indoprofen were found to be significantly superior to those of pentazocine, and both drugs had good tolerability[8].
Chang et al[9] investigated, in an RCT, the effectiveness of 4 oral analgesics in patients who had acute, moderate-to-severe extremity pain and were admitted to the ED. Patients received one of the following regimens: ibuprofen (IBP) (400 mg)/acetaminophen (APAP) (1000 mg); oxycodone (OXY) (5 mg)/APAP (325 mg); hydrocodone (HYCOD) (5 mg)/APAP (300 mg); or codeine (COD) (30 mg)/APAP (300 mg). The pain intensity was assessed using an 11-point numerical rating scale (NRS), ranging from the number 0, which indicates no pain, to the number 10, which indicates the most severe pain. The primary outcome was the difference in pain reduction between the groups at 2 hours after administration of medication. According to the NRS, the MCID was 1.3. There were no significant or clinical differences in pain reduction at 2 hours observed between IBP and APAP treatment or 3 different opioid and APAP combination analgesics. The use of opioids resulted in a higher prevalence of nausea and vomiting in patients[9].
Bijur et al[10] compared the efficacy and adverse effects of five oral analgesics in an RCT comprising 600 patients pre
In a cross-sectional, observational, prospective, cohort study (pseudo-randomized), Buccelletti et al[11] evaluated two oral analgesics in 134 patients presenting to the ED with acute traumatic musculoskeletal pain. The oral analgesics provided were APAP/COD at the dosage of 1000 mg/60 mg and ketorolac administered at the dosage of 15 mg. Seventy-six of the patients received ketorolac and 58 received APAP/COD. The NRS was recorded at 30 minutes and at 2 hours after the administration of the analgesic therapy. Patients with fractures and muscular pain were found to experience significantly higher analgesic relief when given the combination of APAP and COD compared with those who received ketorolac[11].
Craig et al[12] evaluated the effectiveness of 1 g IV APAP compared to 10 mg IV morphine (MORPH) in an RCT of 55 patients with moderate-to-severe traumatic limb pain. Using a VAS, the pain score was assessed at 0 minute, 5 minutes, 15 minutes, 30 minutes, and 60 minutes following administration of medication as the primary outcome measure. The frequency of adverse reactions and the need for rescue analgesia were also documented. There were no significant differences in analgesic effect of APAP and MORPH at any time interval. The rescue analgesia administered did not significantly differ between the two groups. However, the MORPH group experienced significantly more adverse reactions compared with the non-opioid group[12].
Jalili et al[13] evaluated, in an RCT, the effectiveness of APAP (1000 mg) relative to MORPH (0.1 mg/kg) in patients with acute limb trauma and a reported NRS of > 3/10. The primary outcome measure was the change in pain score of the NRS at 0 minute, 15 minutes and 30 minutes after medication administration. The frequency of adverse reactions and the need for rescue analgesia were also documented at 0 minute and 30 minutes after administration of medication. There were no significant differences in analgesic effect for APAP and MORPH at any time interval. The rescue analgesia administered did not differ significantly between the two groups. The MORPH group experienced significantly more adverse reactions. The authors found that the APAP group experienced significantly more pain relief than the MORPH group. The difference in the use of rescue analgesia between the APAP and MORPH groups was significant: The APAP group had 4 patients who need rescue analgesia, while the MORPH group had 15 patients who need rescue analgesia. There was no significant difference in the number of patients who experienced adverse effects between the two groups[13].
Farahmand et al[14] enrolled 50 patients with acute limb trauma in an RCT designed to compare intravenous lidocaine and MORPH for superiority in pain management (25 in each group). Lidocaine (1.5 mg/kg) was administered via IV to one group and MORPH (0.1 mg/kg) was administered via IV to the other group. At 15 minutes, 30 minutes, 45 minutes, and 60 minutes, the patients’ reported pain scores and adverse effects were documented, with their satisfaction with the pain control being assessed 2 hours later. According to the NRS, the MCID was 1.3. There were no clinically or statistically significant differences between two groups, although the pain score decreased significantly in both groups. In the MORPH group, compared with the group receiving lidocaine, the heart rate and the respiratory rate exhibited a statistically significant decrease. In both groups, only one subject reported adverse effects, i.e., nausea and vomiting[14].
Gurnani et al[15] compared the effectiveness of pain control between ketamine (KET) and MORPH in a pilot study of 40 ASA-I adults after acute musculoskeletal trauma. In 20 patients, an initial loading dose of KET (0.25 mg/kg) was given slowly via IV followed by low-dose KET via Subcutaneous (0.1 mg/kg/hour). In the control group, 20 patients received MORPH (0.1 mg/kg intravenously, every four hours). The VAS was used to assess pain at 0 minute, 1 minute, 2 minutes, 4 minutes, 8 minutes, 12 minutes, 15 minutes, and 24 hours. Vital parameters and patient acceptability for supplementary analgesia, drowsiness score, and early mobilization were also assessed. The authors found that KET infusion provided better pain relief than intermittent MORPH, with no need for additional analgesia for the patients in the KET group. Compared with patients in the MORPH group, it was easier to physically move the patients receiving KET. The drow
Shimonovich et al[16] evaluated the efficacy and adverse effects of intranasal (IN) KET compared with those of IV and IM MORPH in an RCT. Ninety patients with moderate-to-severe acute traumatic pain (> 80 mm on 100 mm VAS) were randomly assigned either 1.0 mg/kg IN KET, 0.1 mg/kg IV MORPH, or 0.15 mg/kg IM MORPH. The drug was given, and pain relief and adverse events were assessed for an hour. The 'time-to-onset' was used to determine effective
Tongbua et al[17] evaluated the pain-relieving effectiveness and safety of IN KET relative to IV MORPH in patients aged 65 years or older attending the ED with acute moderate-to-severe pain (score higher than 5 on an 11-point NRS). A decrease in NRS pain scores was the primary outcome, after 30 minutes of treatment; both rescue medication and the incidence of adverse effects were included in the secondary outcomes. The number of patients enrolled was 72, with 37 in the IN-KET group and 37 in the IV-MORPH group. At 30 minutes, the mean pain score for both groups did not differ significantly. Nausea and vomiting were present in one patient in the IN- KET group and in two patients of the IV-MORPH group, and only one patient in the IV-MORPH group subsequently received treatment with an anti-emetic drug[17].
Kampan et al[18] investigated the analgesic efficacy of nebulized KET relative to IV MORPH in older patients (aged 65 years and older) admitted to the ED with acute moderate-to-severe musculoskeletal pain (defined as a pain score of 5 or more on NRS). The outcomes were a decrease in NRS 30 minutes after treatment with nebulized KET or IV MORPH, as well as in the frequency of adverse events and the need for rescue therapy. The authors enrolled 92 patients, divided equally into each group. The nebulized KET and IV MORPH groups showed no significant difference in mean NRS at 30 minutes. The groups did not exhibit a difference in their rates of rescue therapy. The incidence of nausea in the MORPH group was significantly higher than in the KET group. None of the patients in the KET group reported nausea, whereas in the MORPH group, 8 patients experienced nausea[18].
An RCT conducted by Esfahani et al[19] compared the efficacy and safety of KET relative to MORPH in the reduction of pain associated with isolated traumatic limb injuries in patients referred to the ED. The number of patients enrolled was 73, with the KET group receiving 0.1 mg/kg of KET and the MORPH group receiving 0.05 mg/kg of MORPH. NRS and adverse drug reactions (ADRs) were recorded at baseline and every 5 minutes, for 30 minutes in total. At each assessed timepoint, the KET group had a significantly lower mean pain score than the MORPH group. Additionally, the KET group had significantly higher overall ADRs than the MORPH group[19].
The Intravenous Sub-dissociative-Dose Ketamine Versus Morphine for Prehospital Analgesia study is a recently published multicenter RCT assessing the non-inferiority of KET (dosed initially at 20 mg and followed by 10 mg every 5 minutes) compared with MORPH sulfate (2 mg or 3 mg administered every 5 minutes) to alleviate pain in adults with out-of-hospital traumatic pain (NRS > 5). A total of 251 patients were enrolled (KET being administered to 128 patients and MORPH administered to 123 patients). The primary outcome was the difference in NRS measured before medication administration (defined as the patient’s baseline pain level) and after 30 minutes, with an MCID of 1.3. KET and MORPH showed no difference in pain reduction in patients with out-of-hospital traumatic pain. More adverse events were observed in the KET group, although no patient was required to withdraw from the study and no intervention was needed to manage ADRs[20].
Another study regarding the treatment of out-of-hospital traumatic pain (NRS > 3) conducted by Lim et al[21] com
Most studies on the use of non-opioids have concluded that these drugs are non-inferior to opioids for the control of acute pain associated with acute musculoskeletal injury. Therefore, the use of non-opioids can be considered as an alternative to opioids for pain management in acute musculoskeletal injuries. Only two studies[8,11] (those that repre
The major limitation of our systematic review is the heterogeneity of the studies regarding the measured outcomes and the opioids and non-opioids that were utilized. This heterogeneity made it infeasible to conduct a meta-analysis. Only the studies of Tongbua et al[17] and Kampan et al[18] are homogeneous in regard to the type of study conducted, the opioid used (IV MORPH), its comparator (IN KET), and the outcome explored. A meta-analysis of the two studies, that together comprised 166 patients (Tongbua 74, Kampan 92), was not conducted because the studies’ results were similar in terms of drug efficacy, and we concluded that it did not provide any additional information.
Another limitation of our systematic review is that the outcomes of the included studies were focused on the imme
Of all the discussed non-opioid drugs, intravenous KET and methoxyflurane were associated with higher adverse events than opioids. Specifically, only intravenous KET was linked with more adverse events than MORPH[19,22], while inhaled or subcutaneous KET did not elicit more adverse events than MORPH. KET is commonly used as an analgesic in emergency medicine[20], and, according to the literature, intravenous administration of KET could cause more adverse events than other routes of administration (ROA).
In the latest 2022 Clinical Practice Guideline for Prescribing Opioids for Pain of the CDC, non-opioid therapies are recommended for many common minor orthopedic acute pain conditions such as sprains, strains, tendonitis, and bursitis[4]. Our systematic review shows that treatment with non-opioid medications can be effective even in major trauma.
The findings of this systematic review should be treated with discretion, owing to the heterogeneity of the studies inc
| 1. | Kharasch ED, Clark JD, Adams JM. Opioids and Public Health: The Prescription Opioid Ecosystem and Need for Improved Management. Anesthesiology. 2022;136:10-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Moran L, Ondocsin J, Outram S, Ciccarone D, Werb D, Holm N, Arnold EA. How do we understand the value of drug checking as a component of harm reduction services? A qualitative exploration of client and provider perspectives. Harm Reduct J. 2024;21:92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 3. | Gomes T, Ledlie S, Tadrous M, Mamdani M, Paterson JM, Juurlink DN. Trends in Opioid Toxicity-Related Deaths in the US Before and After the Start of the COVID-19 Pandemic, 2011-2021. JAMA Netw Open. 2023;6:e2322303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 4. | Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain - United States, 2022. MMWR Recomm Rep. 2022;71:1-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 865] [Article Influence: 288.3] [Reference Citation Analysis (0)] |
| 5. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40131] [Article Influence: 10032.8] [Reference Citation Analysis (2)] |
| 6. | Bramer WM, Giustini D, Kramer BM. Comparing the coverage, recall, and precision of searches for 120 systematic reviews in Embase, MEDLINE, and Google Scholar: a prospective study. Syst Rev. 2016;5:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15131] [Article Influence: 2521.8] [Reference Citation Analysis (0)] |
| 8. | Soave G, Lavezzari M, Ferrati G, Sacchetti G. Indoprofen and pentazocine in post-traumatic pain. A double-blind, parallel-group comparative trial. J Int Med Res. 1983;11:354-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 9. | Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a Single Dose of Oral Opioid and Nonopioid Analgesics on Acute Extremity Pain in the Emergency Department: A Randomized Clinical Trial. JAMA. 2017;318:1661-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 10. | Bijur PE, Friedman BW, Irizarry E, Chang AK, Gallagher EJ. A Randomized Trial Comparing the Efficacy of Five Oral Analgesics for Treatment of Acute Musculoskeletal Extremity Pain in the Emergency Department. Ann Emerg Med. 2021;77:345-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Buccelletti F, Marsiliani D, Zuccalà G, Iacomini P, Proietti L, Pola E, Zirio G, Genitiempo M, Marrocco R, Conti C, Brunetti C, Rocchi L, Merendi G, D'Aurizio G, Gilardi E, Franceschi F. Paracetamol-codeine compared to ketorolac for pain control in the Emergency Department. Eur Rev Med Pharmacol Sci. 2014;18:3139-3143. [PubMed] |
| 12. | Craig M, Jeavons R, Probert J, Benger J. Randomised comparison of intravenous paracetamol and intravenous morphine for acute traumatic limb pain in the emergency department. Emerg Med J. 2012;29:37-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Jalili M, Mozaffarpour Noori A, Sedaghat M, Safaie A. Efficacy of Intravenous Paracetamol Versus Intravenous Morphine in Acute Limb Trauma. Trauma Mon. 2016;21:e19649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Farahmand S, Hamrah H, Arbab M, Sedaghat M, Basir Ghafouri H, Bagheri-Hariri S. Pain management of acute limb trauma patients with intravenous lidocaine in emergency department. Am J Emerg Med. 2018;36:1231-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Gurnani A, Sharma PK, Rautela RS, Bhattacharya A. Analgesia for acute musculoskeletal trauma: low-dose subcutaneous infusion of ketamine. Anaesth Intensive Care. 1996;24:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Shimonovich S, Gigi R, Shapira A, Sarig-Meth T, Nadav D, Rozenek M, West D, Halpern P. Intranasal ketamine for acute traumatic pain in the Emergency Department: a prospective, randomized clinical trial of efficacy and safety. BMC Emerg Med. 2016;16:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Tongbua S, Sri-On J, Thong-On K, Paksophis T. Non-inferiority of intranasal ketamine compared to intravenous morphine for musculoskeletal pain relief among older adults in an emergency department: a randomised controlled trial. Age Ageing. 2022;51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Kampan S, Thong-On K, Sri-On J. A non-inferiority randomized controlled trial comparing nebulized ketamine to intravenous morphine for older adults in the emergency department with acute musculoskeletal pain. Age Ageing. 2024;53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Esfahani H, Khazaeipour Z, Safaie A, Aghili SM. Ketamine Sub-Dissociative Dose Vs. Morphine Sulfate for Acute Pain Control in Patients with Isolated Limb Injuries in the Emergency Department: A Randomized, Double-blind, Clinical Trial. Bull Emerg Trauma. 2021;9:73-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Le Cornec C, Le Pottier M, Broch H, Marguinaud Tixier A, Rousseau E, Laribi S, Janière C, Brenckmann V, Guillerm A, Deciron F, Kabbaj A, Jenvrin J, Péré M, Montassier E. Ketamine Compared With Morphine for Out-of-Hospital Analgesia for Patients With Traumatic Pain: A Randomized Clinical Trial. JAMA Netw Open. 2024;7:e2352844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 21. | Lim KJ, Koh ZX, Ng YY, Fook-Chong S, Ho AFW, Doctor NE, Said NAZM, Ong MEH. Comparison of inhalational methoxyflurane (Penthrox®) and intramuscular tramadol for prehospital analgesia. Singapore Med J. 2021;62:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Karlow N, Schlaepfer CH, Stoll CRT, Doering M, Carpenter CR, Colditz GA, Motov S, Miller J, Schwarz ES. A Systematic Review and Meta-analysis of Ketamine as an Alternative to Opioids for Acute Pain in the Emergency Department. Acad Emerg Med. 2018;25:1086-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |