Published online Jun 18, 2024. doi: 10.5312/wjo.v15.i6.593
Revised: May 14, 2024
Accepted: May 24, 2024
Published online: June 18, 2024
Processing time: 133 Days and 21.7 Hours
Mazabraud’s syndrome (MS) is a rare and slowly progressive benign disease cha
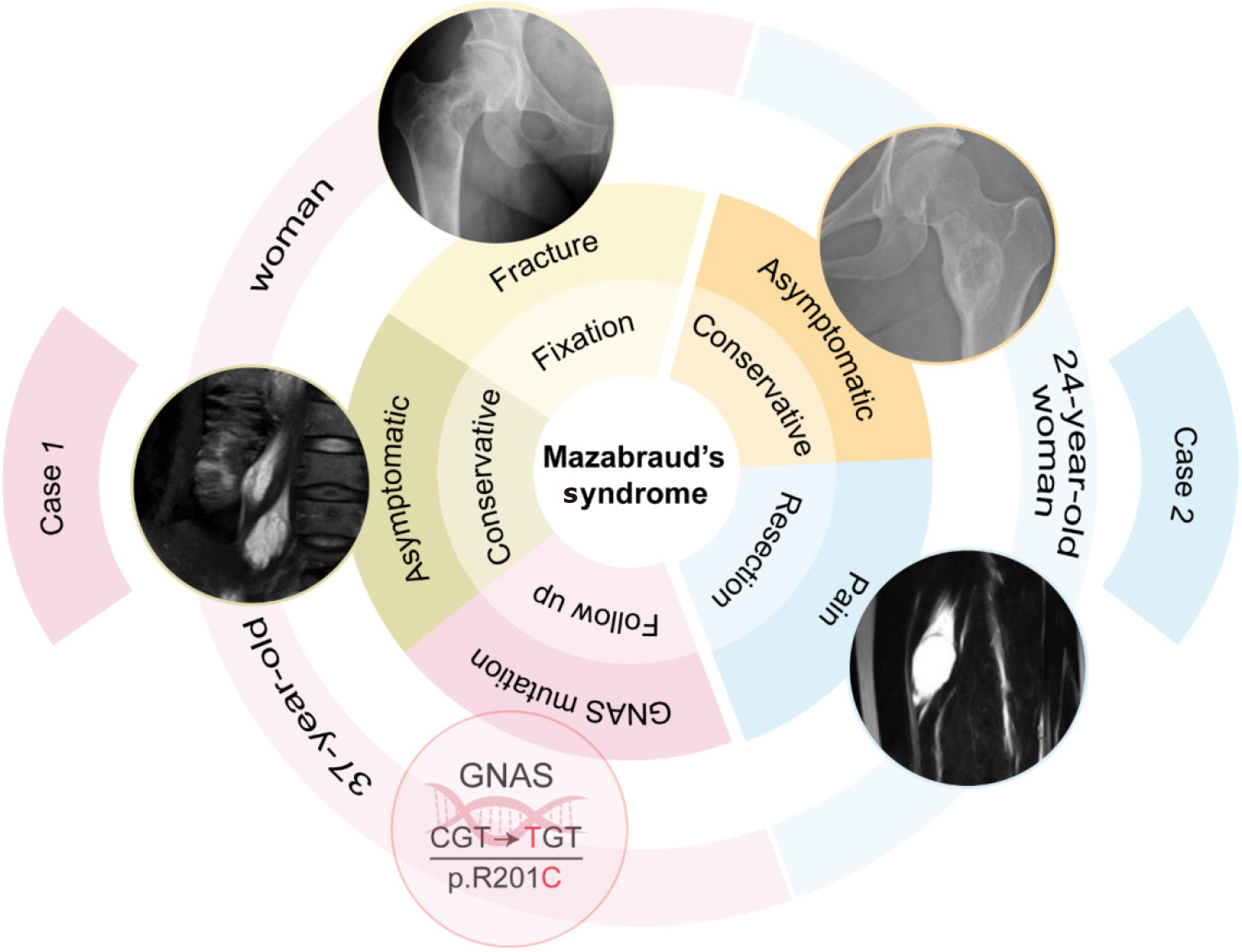
We report two cases of MS in young women who underwent different treatments based on their symptoms and disease manifestations. The first patient, aged 37, received internal fixation and intravenous bisphosphonate for a pathological fracture of the right femoral neck, excision of a right vastus medialis myxoma was subsequently performed for pain control, and asymptomatic psoas myxomas were monitored without surgery. Genetic testing confirmed a GNAS gene mutation in this patient. The second patient, aged 24, underwent right vastus intermedius muscle myxoma resection, and conservative treatment for fibrous dysplasia of the ilium. These patients were followed-up for 17 months and 3 years, respectively, and are now in a stable condition.
Various treatments have been selected for MS patients who suffer different symptoms. The main treatment for myxomas is surgical resection, while fibrous dysplasia is selectively treated if the patient experiences pathological fracture or severe pain. However, given the documented instances of malignant transformation of fibrous dysplasia in individuals with MS, close follow-up is necessary.
Core Tip: Mazabraud’s syndrome (MS) is a rare, slowly progressive disorder characterized by the coexistence of fibrous dysplasia and intramuscular myxoma. Here, we present two rare young female patients with MS, for whom different treatment strategies such as internal fixation, tumor resection and intravenous bisphosphonate were chosen based on their respective clinical manifestations. To date, only about 100 cases of MS have been reported in the literature, and their standard treatment strategy remains unclear. In view of the fact that this disorder is not well understood, we also systematically reviewed the literature on the managements of this disease.
- Citation: Li XM, Chen ZH, Wang KY, Chen JN, Yao ZN, Yao YH, Zhou XW, Lin N. Mazabraud’s syndrome in female patients: Two case reports. World J Orthop 2024; 15(6): 593-601
- URL: https://www.wjgnet.com/2218-5836/full/v15/i6/593.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i6.593
Mazabraud‘s syndrome (MS) is a rare and slowly progressive benign disease characterized by the concurrent presence of fibrous dysplasia (FD) of bone and intramuscular myxoma (IM)[1]. First described by Henschen in 1926, the disease was further elaborated by Mazabraud in 1957 in relation to FD and IM, and was named MS[2]. The global incidence of MS is approximately one in a million, and only about 100 cases been documented in the literature to date[3]. Among indi
The primary clinical manifestation of MS is typically pain, followed by swelling, pathological fracture, and neuro
At present, there is a lack of research on the epidemiology, clinical features, disease development, diagnosis and treatment outcomes of MS. Here we report two cases of MS to illustrate their clinical features, radiologic presentations, and treatment strategies associated with this rare disease. We discussed our management of this challenging disease in order to provide guidance for future treatments of MS.
Case 1: A 37-year-old woman presented to our hospital complaining of pain in her right hip.
Case 2: A 24-year-old woman presented with a two-week history of mild pain and discomfort in her left thigh.
Case 1: The symptom of hip pain was intermittent and aggravated after walking. The patient had no history of trauma, and there were no discomfort symptoms such as redness, swelling and fever.
Case 2: Although she denied any history of injury, she noted the presence of a mass on her left thigh, approximately the size of a pigeon egg. There was no obvious numbness or limited activity in the lower limbs of the patient.
Both patients denied any relevant past illness.
Case 1: She married at the age of 23 and gave birth to two sons, both natural births. Her menstrual cycle was regular without long-term oral contraceptives.
Case 2: Her menstrual cycles were also regular. She had never been pregnant and there was no record of contraceptive pill use.
Cases 1 and 2: Neither of the patient had a special family history.
Case 1: Physical examination showed percussion pain of the right hip with limited mobility.
Case 2: Physical examination confirmed that there was a mass on her left thigh, about the size of a pigeon egg, acco
Case 1: The patient's routine blood tests, blood biochemical tests (including alkaline phosphatase, calcium ions, magnesium ions, etc.), parathyroid hormone, and conventional tumor markers (including carcinoembryonic antigen, alpha-fetoprotein, squamous cell carcinoma antigen, neuron-specific enolase, carbohydrate antigen 199, carbohydrate antigen 125, etc.) were all within the normal range.
Case 2: The patient's routine blood and biochemical tests, such as alkaline phosphatase, were approximately normal, while tumor markers were not measured.
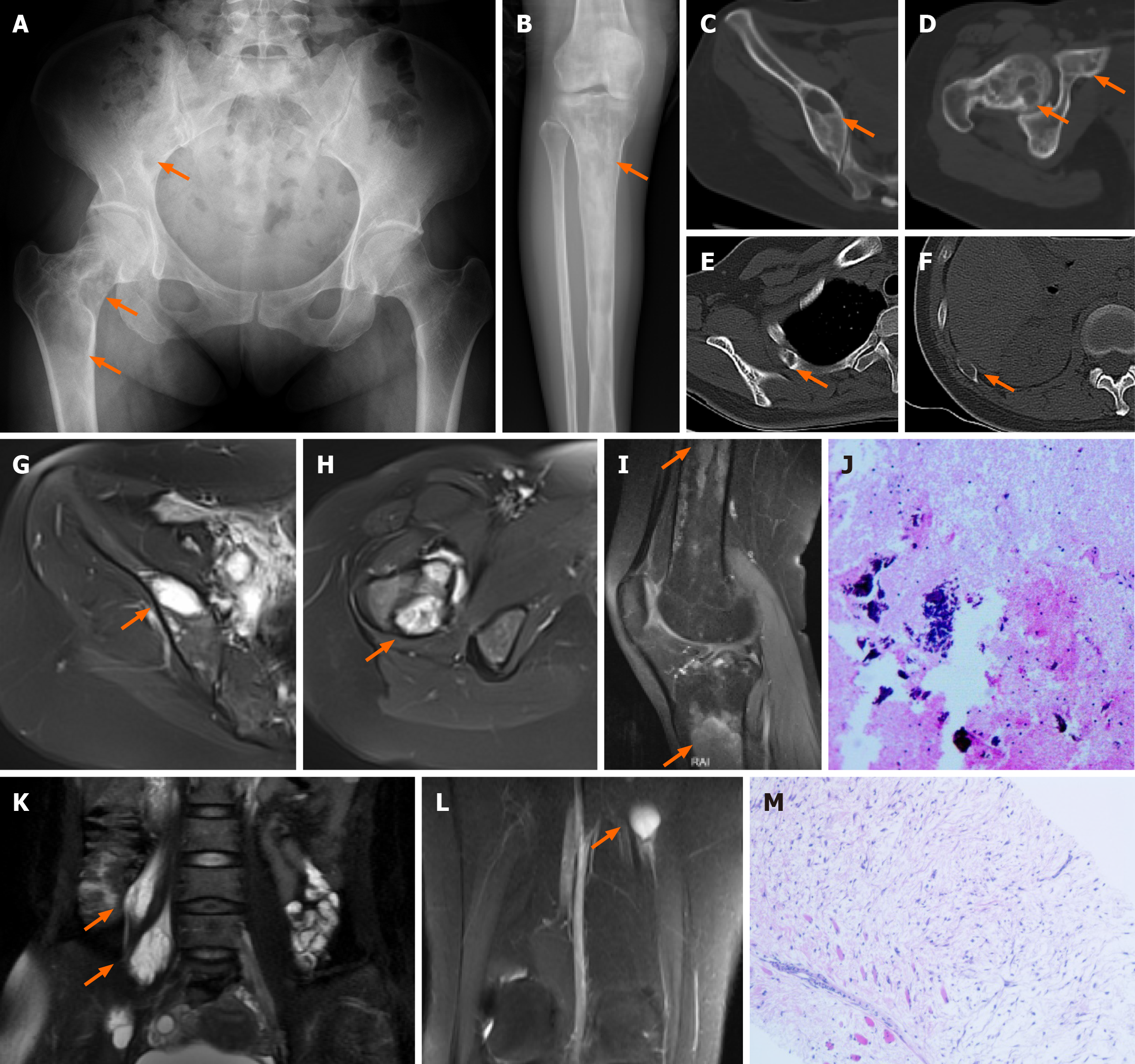
Case 1: The X-ray image and CT scan showed multiple bone abnormalities in the pelvis, femur, tibia and ribs, all of which were located on the right side (Figure 1A-F). An MRI scan then indicated long T1 and long T2 signals from these lesions with irregular shapes, and no obvious enhancement was found on contrast-enhanced examination (Figure 1G-I). In addition, two intramuscular lesions with T1 and T2 relaxation characteristics were identified in the right psoas major muscle according to MRI, and one similar lesion in the right vastus medialis muscle (Figure 1K and L).
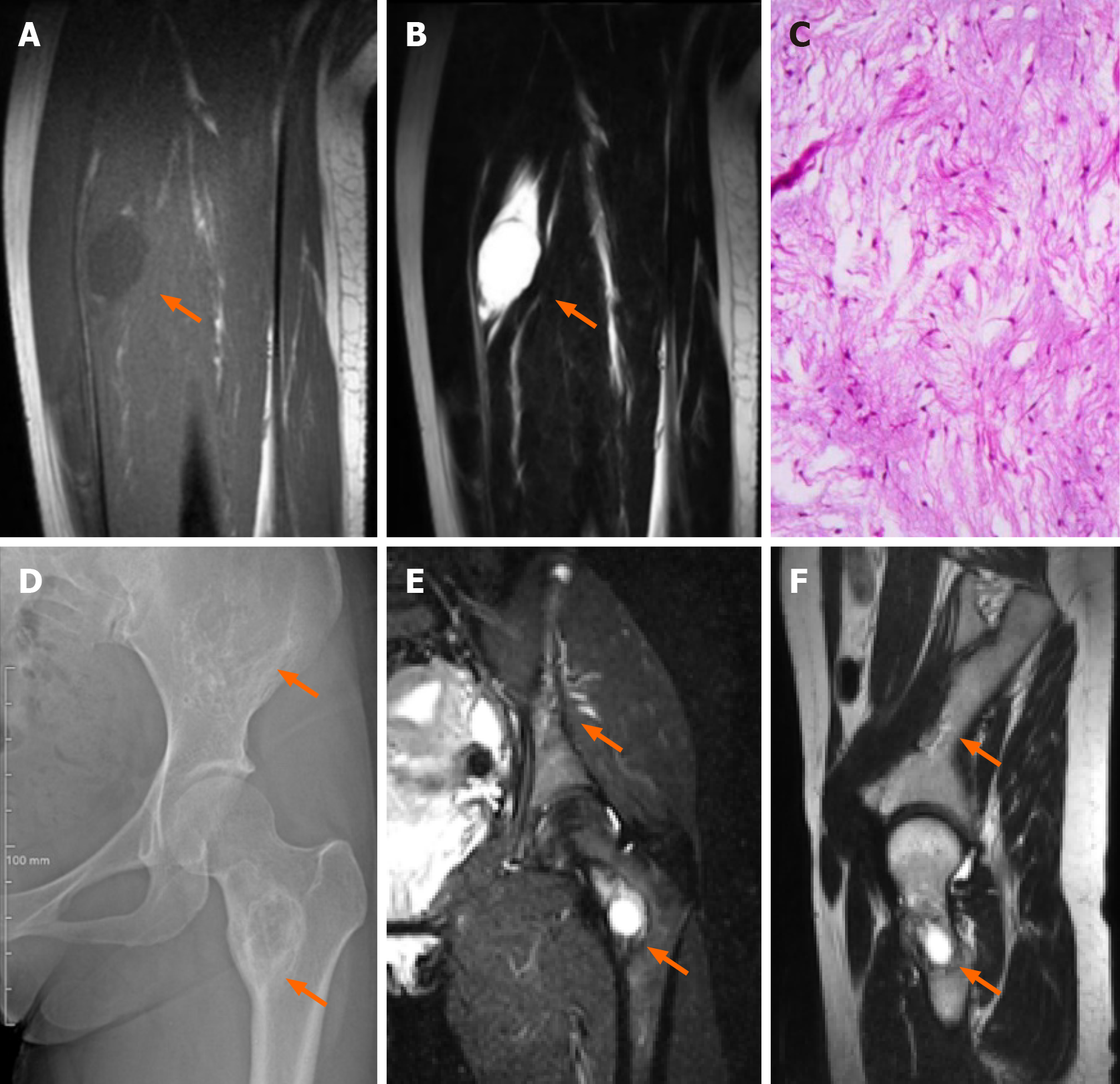
Case 2: B ultrasound examination revealed that the tumor had a regular shape, low echo, and was located in the vastus intermedius muscle. An MRI scan then confirmed a circular abnormal signal in the vastus intermedius muscle of her left thigh, with a slightly lower signal on T1WI and higher signal on both T2WI and fat suppression images. The lesion showed uneven enhancement according to an enhanced scan, and was 22.8 mm × 22.3 mm × 34.1 mm in size (Figure 2A and B). No signal abnormalities were detected in the femur adjacent to the tumor. Of note, her pelvic X-ray and MRI revealed destructive lesions with unclear borders in the left ilium and femoral neck, within which striated bony ridges were observed but not surrounding sclerosis (Figure 2D-F).
Both patients were diagnosed with MS.
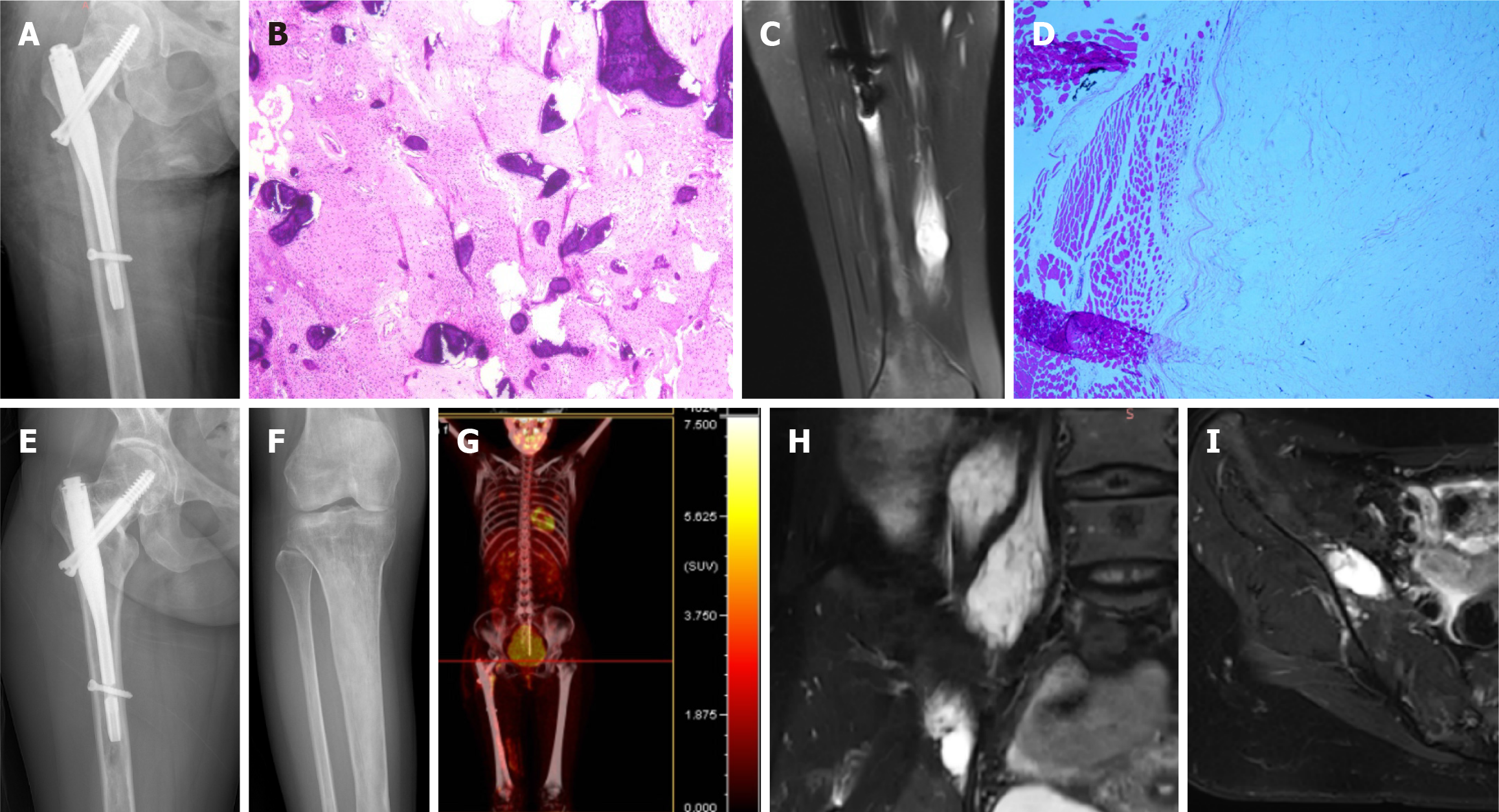
After admission, CT-guided puncture biopsy examinations of both the femoral lesion and the psoas major muscle lesion were arranged for this patient, with pathological results indicating FD and myxoma, respectively (Figure 1J and M). Following a multidisciplinary team (MDT) discussion, it was decided that the patient had multiple FD complicated by IM, which was consistent with the diagnosis of MS. Considering the bone destruction and pathological fracture of her right femoral neck, the patient was treated with surgical curettage, bone graft and internal fixation (Figure 3A). The postoperative pathological report on the femur lesion confirmed the diagnosis of FD (Figure 3B). One month later, due to pain, the patient opted for surgical removal of the myxoma (Figure 3C and D). The patient declined surgical treatment of the iliopsoas IM and requested conservative treatment and regular follow-up due to unremarkable symptoms of low back pain and the high risks associated with surgery, including nerve injury. Genetic testing results from multiple foci revealed mutation in exon 8 of GNAS gene (CGT->TGT), resulting in a p.R201C mutant.
As the patient had multifocal FD in her right lower extremity, some of which showed thinning of the bone cortex and were at risk of pathologic fracture, she was given once-yearly intravenous zoledronic acid 5 mg for fracture prevention[6,7].
After MDT discussion, it was concluded that the patient had FD accompanied by IM, which is consistent with MS. Due to the patient's pain and enlargement of the mass, tumor excision was performed under general anesthesia. Postoperative pathological and immunohistochemistry results revealed the following characteristics (Figure 2C): glial fibrillary acidic protein (GFAP) (-), B-catenin cytoplasmic/nuclear (+), mucin (MUC) 4 (+), Desmin (+), spinal muscular atrophy (SMA) in blood vessel walls (+), cluster of differentiation 68 (CD68) scattered (+), signal transducers and activators of transcription-6 (STAT-6) (-), SRY-related HMG-box 10 (SOX-10) (-), transducin-like enhancer protein 1 (TLE-1) (uncertain), creatine kinase (CK) (-), cluster of differentiation 34 (CD34) in blood vessels (+), S-100 (-), alcian blue/periodic acid-Schiff (AB/PAS) (+/-), Masson (-), reticulin (-), and proliferation marker Ki-67 approximately 3%. Combined with hematoxylin and eosin staining, these findings were consistent with IM.
The patient was regularly followed-up every 6 months. X-rays, MRI and positron emission tomography-CT at the 1-year postoperative follow-up, showed that the FD lesions were relatively stable, and the iliopsoas myxoma was slightly enlarged compared to that at her first visit (Figure 3E-I). Her symptoms in the left thigh were relieved and there were no obvious gait abnormalities at the most recent follow-up, 17 months postoperatively.
The patient was followed up every 6 months for the first year after surgery and then annually thereafter. At present, the patient has no obvious pain and no signs of tumor recurrence at the latest follow-up more than 3 years after surgery.
MS is generally considered benign, and characterized by the coexistence of bone FD and IM[1]. Some cases of MS are associated with McCune-Albright syndrome (MAS), which is characterized by café-au-lait spots on the skin and endocrine abnormalities[8]. Previous studies have reported that although FD is usually a benign lesion, malignant transformation can occur over time[9]. The possible outcomes of disease deterioration include osteosarcoma, fibr
Studies over the past decade have revealed that the pathogenesis of MS may be associated with mutations in the GNAS gene. The GNAS gene, located on the long arm of chromosome 20, region 1, band 3, encodes the alpha subunit of g protein (GSα), which plays a role in the regulation of cell proliferation. GSα contains a GTPase domain that inactivates its downstream receptor signaling pathway by binding to and hydrolyzing GTP to GDP[14]. Missense mutations at the R201 and Q221 sites disrupt the intrinsic GTPase enzyme activity of GSα, leading to sustained activation of downstream signaling. As a result, adenylate cyclase is in a continuous-activated state, producing excessive cyclic AMP. The affected bone progenitor cells generate abnormally woven bony trabeculae within the fibrous stroma. Such structural inadequacy contributes to fractures, ultimately manifesting as characteristic osteolytic lesions seen in imaging studies[15]. Mutations in the GNAS1 gene have been found in FD cases and cause abnormal proliferation of precursor cells. Since bone, skeletal muscle, cartilage and adipocytes are derived from common precursor mesenchymal cells, mutations in this gene can give rise to both FD and IM phenotypes[16].
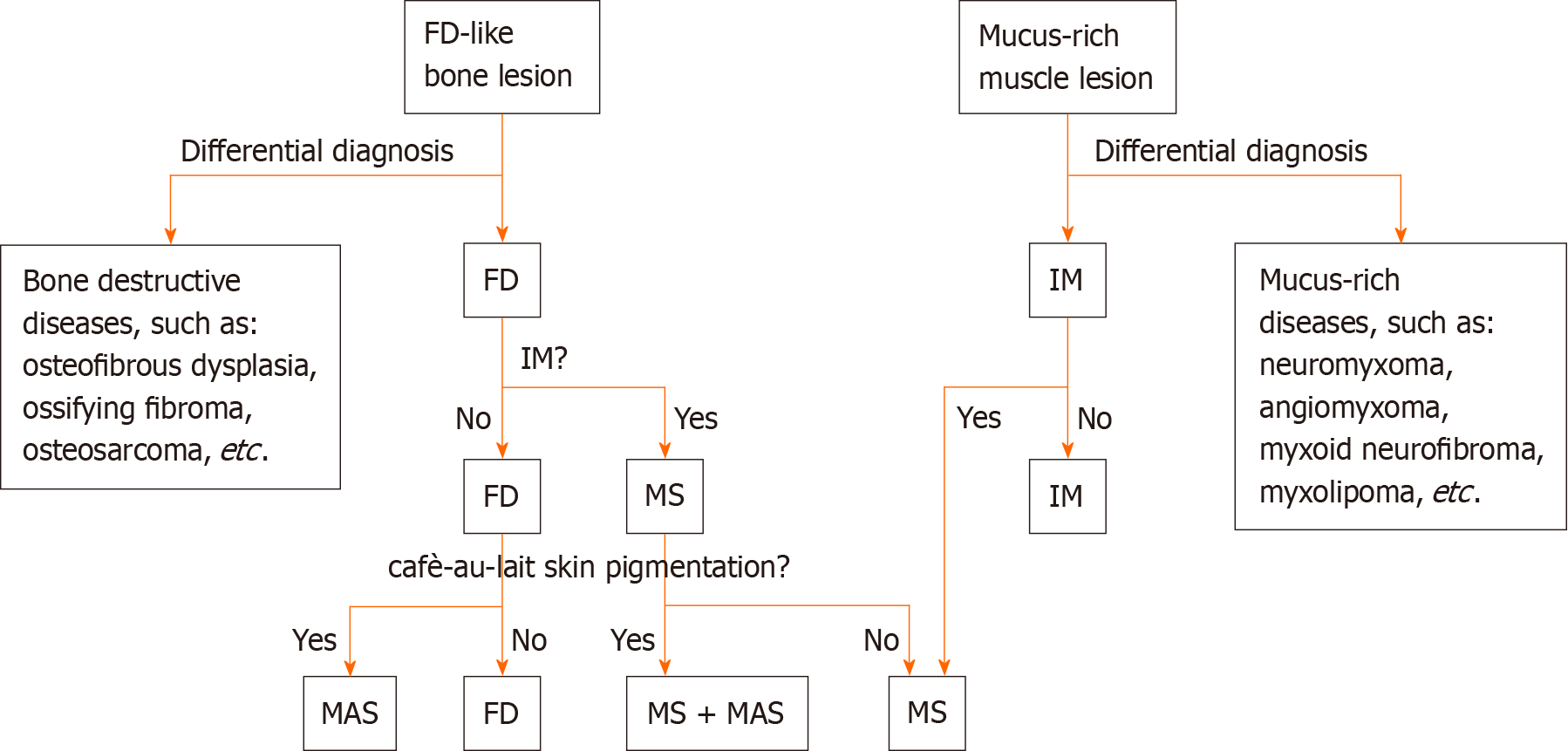
Clinically, the diagnosis of MS is established when FD is identified in conjunction with IM, irrespective of whether the lesions are isolated (monostotic) or multiple (polyostotic). When possible, genetic testing is recommended to enhance diagnostic precision by detecting mutations in the GNAS gene. It is crucial to differentiate MS from MASs, a rare benign disorder characterized by polyostotic FD, cafè-au-lait skin pigmentation, and associated endocrine dysfunctions such as precocious puberty, diabetes mellitus, goiter and breast fibroadenomatosis[17]. As previously mentioned, some MS patients may have concomitant MAS, and according to a literature review, there appears to be an elevated risk of malignant transformation of FD into osteosarcoma in these patients[11]. In addition, as MS often initially presents with either FD or IM, FD needs to be differentiated from other diseases that cause abnormal bone signaling or bone destruction, such as osteofibrous dysplasia, ossifying fibroma, and osteosarcoma[18]. Similarly IM needs to be distinguished from mucus-rich tumors, such as neuromyxoma, angiomyxoma, myxoid neurofibroma, myxolipoma, and low-grade myxoid fibrosarcoma[19].
MS is characterized by a benign lesion with slow development, therefore conservative treatment and regular follow-up are appropriate for most cases, unless patients develop symptoms such as pain that require medication. With the progression of FD, patients may encounter symptoms such as pain, deformity and pathological fractures, prompting consideration of surgical interventions such as internal fixation. In addition, it has been reported that a small subset of FD cases are at risk of progressing to malignant osteosarcoma, so close follow-up is required and surgical treatment may be considered if deemed necessary[20]. In cases of symptomatic myxomas, surgical removal is an effective way of alleviating pain. However, when compared to solitary IM, myxomas in MS are prone to recurrence, with a median recurrence time of 8.5 years[4]. It has also been reported that zoledronic acid can effectively relieve bone pain and reduce intramuscular mucoid volume in MS but does not affect the extension of FD[3]. Studies on FD-related diseases, such as MAS, have also indicated the success of denosumab therapy and the potential predictive value of interleukin 6 and the nuclear factor-kappa B ligand/ osteoprotegerin (RANKL/OPG) ratio for clinical response[21,22]. The safety and efficacy of this treatment regimen remains to be validated by further studies.
Our study describes two young female patients with MS who were followed up for 17 months and 3 years, respectively (Figure 4). Patient 1 was treated with internal fixation surgery for pathological fracture of the right femoral neck, followed by once-yearly intravenous zoledronic acid for fracture prevention. Both patients underwent resection of myxoma due to pain caused by the lesion invading the muscle. No further examinations were performed to confirm the presence of other lesions in Patient 2, as she did not report discomforts elsewhere. Genetic testing confirmed a GNAS gene mutation in both the FD and the IM in Patient 1[23]. MS is a rare musculoskeletal disease with a very low incidence and it may be underestimated and prone to misdiagnosis in clinical practice, as FD and myxoma are often identified independently without considering a possible association between them[24]. Most cases are asymptomatic and are typically discovered incidentally during examinations for unrelated reasons, while a minority of patients may present with pathologic fra
Various treatments are selected for MS patients who suffer different symptoms. We report two cases of MS to illustrate the clinical features, radiologic presentations, and treatment strategies associated with this rare disease. We discuss our management of this challenging disease in order to provide guidance for future treatments of MS. Furthermore, given the documented cases of malignant transformation of FD in MS patients, close follow-up is necessary[25].
The authors gratefully acknowledge the patients who agreed to participate in this study, as well as pathologist Yan-Biao Fu for his assistance in pathological diagnosis of these challenging cases, and Jun-Yan Xie and Jia-Dan Wu for their assistance during the manuscript preparation.
| 1. | Hagelstein-Rotman M, Appelman-Dijkstra NM, Boyce AM, Chapurlat R, Dur NBJ, Gensburger D, Majoor BCJ, van de Sande MAJ, Dijkstra PDS. Extent of Extraskeletal Manifestations of Fibrous Dysplasia/McCune-Albright Syndrome in Patients with Mazabraud's Syndrome. Calcif Tissue Int. 2022;110:334-340. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Hasan F, Dhingra V, Singh A, Misra V. Cytological Diagnosis of Mazabraud's Syndrome. J Cytol. 2018;35:128-129. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Vescini F, Falchetti A, Tonelli V, Carpentieri M, Cipri C, Cosso R, Kara E, Triggiani V, Grimaldi F. Mazabraud's Syndrome: A Case Report and Up-To-Date Literature Review. Endocr Metab Immune Disord Drug Targets. 2019;19:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Majoor BCJ, van de Sande MAJ, Appelman-Dijkstra NM, Leithner A, Jutte PC, Vélez R, Perlaky T, Staals EL, Bovée JVMG, Hamdy NAT, Dijkstra SPD. Prevalence and Clinical Features of Mazabraud Syndrome: A Multicenter European Study. J Bone Joint Surg Am. 2019;101:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Sunitsch S, Gilg MM, Kashofer K, Gollowitsch F, Leithner A, Liegl-Atzwanger B. Detection of GNAS mutations in intramuscular / cellular myxomas as diagnostic tool in the classification of myxoid soft tissue tumors. Diagn Pathol. 2018;13:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Wang Y, Wang O, Jiang Y, Li M, Xia W, Meng X, Xing X. Efficacy and safety of bisphosphonate therapy in mccune-albright syndrome-related polyostotic fibrous dysplasia: a single-center experience. Endocr Pract. 2019;25:23-30. [PubMed] [DOI] [Full Text] |
| 7. | Valadares LP, de Araújo Ferreira BS, da Cunha BM, Moreira LA, Batista FGA, da Fonseca Hottz C, Magalhães GGR. Effects of zoledronic acid therapy in fibrous dysplasia of bone: a single-center experience. Arch Endocrinol Metab. 2022;66:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Lung H, Hsiao EC, Wentworth KL. Advances in Models of Fibrous Dysplasia/McCune-Albright Syndrome. Front Endocrinol (Lausanne). 2019;10:925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Oh SH, Kang JH, Seo YK, Kim JH, Choi YS, Hwang EH. Malignant transformation of fibrous dysplasia into angiosarcoma. Oral Radiol. 2020;36:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Hartley I, Zhadina M, Collins MT, Boyce AM. Fibrous Dysplasia of Bone and McCune-Albright Syndrome: A Bench to Bedside Review. Calcif Tissue Int. 2019;104:517-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Boudin L, De Luca V, Mescam-Mancini L, Niziers V, Perrot D, Guiramand J, Monneur A, Chagnaud C, Bertucci F. Mazabraud Syndrome Associated with McCune-Albright Syndrome: A Case Report and Literature Review. Case Rep Oncol. 2023;16:294-301. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Riddle ND, Bui MM. Fibrous dysplasia. Arch Pathol Lab Med. 2013;137:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Reiter A, Trumm K, Ballhause TM, Weiss S, Frosch KH, Korthaus A, Bechler U, Duprée A, Luebke A, Bannas P, Schlickewei CW, Priemel MH. Diagnostic and Therapeutic Pathways of Intramuscular Myxoma. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Boyce AM, Collins MT. Fibrous Dysplasia/McCune-Albright Syndrome: A Rare, Mosaic Disease of Gα s Activation. Endocr Rev. 2020;41:345-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 15. | Cox JL, Cushman-Vokoun AM, McGarry SV, Kozel JA. Two cases of Mazabraud syndrome and identification of a GNAS R201H mutation by next-generation sequencing. Virchows Arch. 2017;470:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Javaid MK, Boyce A, Appelman-Dijkstra N, Ong J, Defabianis P, Offiah A, Arundel P, Shaw N, Pos VD, Underhil A, Portero D, Heral L, Heegaard AM, Masi L, Monsell F, Stanton R, Dijkstra PDS, Brandi ML, Chapurlat R, Hamdy NAT, Collins MT. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis. 2019;14:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 17. | Meier ME, Vágó E, Abrahamsen B, Dekkers OM, Horváth-Puhó E, Rejnmark L, Appelman-Dijkstra NM. Incidence and Prevalence of Fibrous Dysplasia/McCune-Albright Syndrome: A Nationwide Registry-Based Study in Denmark. J Clin Endocrinol Metab. 2024;109:1423-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Benhamou J, Gensburger D, Messiaen C, Chapurlat R. Prognostic Factors From an Epidemiologic Evaluation of Fibrous Dysplasia of Bone in a Modern Cohort: The FRANCEDYS Study. J Bone Miner Res. 2016;31:2167-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Kašpar L, Balko J, Strnadová M, Krsková L, Máška D, Zámečník J. Mazabraud's syndrome: A case report supported by molecular studies and review of the literature. Bone Rep. 2023;18:101685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Multani I, Popovic S, Parasu N, Ghert M. Osteosarcomatous Transformation in the Setting of Mazabraud's Syndrome: A Case Report and Review of the Literature. Case Rep Orthop. 2019;2019:2638478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Majoor BCJ, Papapoulos SE, Dijkstra PDS, Fiocco M, Hamdy NAT, Appelman-Dijkstra NM. Denosumab in Patients With Fibrous Dysplasia Previously Treated With Bisphosphonates. J Clin Endocrinol Metab. 2019;104:6069-6078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Meier ME, Hagelstein-Rotman M, Streefland TCM, Winter EM, Bravenboer N, Appelman-Dijkstra NM. Clinical value of RANKL, OPG, IL-6 and sclerostin as biomarkers for fibrous dysplasia/McCune-Albright syndrome. Bone. 2023;171:116744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Kushchayeva YS, Kushchayev SV, Glushko TY, Tella SH, Teytelboym OM, Collins MT, Boyce AM. Fibrous dysplasia for radiologists: beyond ground glass bone matrix. Insights Imaging. 2018;9:1035-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 24. | Jalan D, Jain P. Mazabraud's Syndrome - A Diagnosis Commonly Missed. J Orthop Case Rep. 2019;9:26-29. [PubMed] |
| 25. | Muthusamy S, Subhawong T, Conway SA, Temple HT. Locally aggressive fibrous dysplasia mimicking malignancy: a report of four cases and review of the literature. Clin Orthop Relat Res. 2015;473:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |