Published online Dec 18, 2024. doi: 10.5312/wjo.v15.i12.1214
Revised: October 24, 2024
Accepted: November 14, 2024
Published online: December 18, 2024
Processing time: 112 Days and 7.1 Hours
Cervical spine pyogenic infection (CSPI) is a rare and challenging form of spinal infection that is typically caused by pyogenic bacteria and primarily affects the cervical vertebral bodies and surrounding tissues. Given its nonspecific sym
The presented case involved a 15-year-old man with CSPI caused by Staphylococcus aureus, which led to complications including bacteremia and a paronychia-associated abscess. Acute pyogenic infection was initially diagnosed by typical symptoms and blood culture. Fever improved after antibiotic treatment while developing progressive limbs dysfunction. Six days after admission, the patient underwent anterior cervical debridement + autogenous iliac bone graft fusion + plate internal fixation and received 12 weeks of antibiotic treatment after the operation. Re-examination 3 years postoperatively showed that the patient had stable cervical fixation, no significant neck pain or upper limb abnormalities, and normal urinary function.
Early imaging findings, laboratory markers, and timely antibiotic treatment are crucial for CSPI management, preventing complications and facilitating recovery.
Core Tip: Cervical spine pyogenic infection can be derived from unexpected sources, such as chronic paronychia. Imaging examination findings and laboratory markers are crucial for timely identification of the infection source. Multidisciplinary collaboration can enhance diagnostic and treatment efficiency. Prompt antibiotic therapy and surgical intervention can prevent severe complications and promote patient recovery. Regular post-operative follow-up is imperative for evaluating the recurrence of infection.
- Citation: Zhang D, Gan LY, Zhang WJ, Shi M, Zhang L, Zhang Y, Liu MW. Cervical spine infection arising from chronic paronychia: A case report and review of literature. World J Orthop 2024; 15(12): 1214-1225
- URL: https://www.wjgnet.com/2218-5836/full/v15/i12/1214.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i12.1214
Cervical spine pyogenic infection (CSPI) is a nonspecific spondylitis caused by pyogenic bacteria, mainly involving the vertebral bodies, paraspinal soft tissues, and intervertebral discs[1-3]. It can be clinically divided into intervertebral discitis, vertebral osteomyelitis, and epidural abscess[4]. Patients often experience fever, shiver, neck pain, limb sensory and movement abnormalities, and other clinical symptoms. The clinical symptoms associated with CSPI exhibit a lack of specificity, rendering early diagnosis a complex and challenging task[5]. This can potentially result in suboptimal treatment outcomes, prolonged illness duration, and even the development of sequelae such as spinal cord injury and spinal deformity. We previously treated a patient with acute pyogenic infection of cervical spine secondary to chronic paronychia, and the diagnosis and treatment process is reported below.
A 15-year-old male student at vocational high school, height 174 cm, weight 67 kg presented with low fever, neck and shoulder pain, limited neck movement, and tremors in both hands for 2 weeks.
The body temperature was 38 °C on admission and rose to 40 °C 6 hours later, accompanied by cold shiver, worsening neck pain, progressive numbness of the limbs and dysuria.
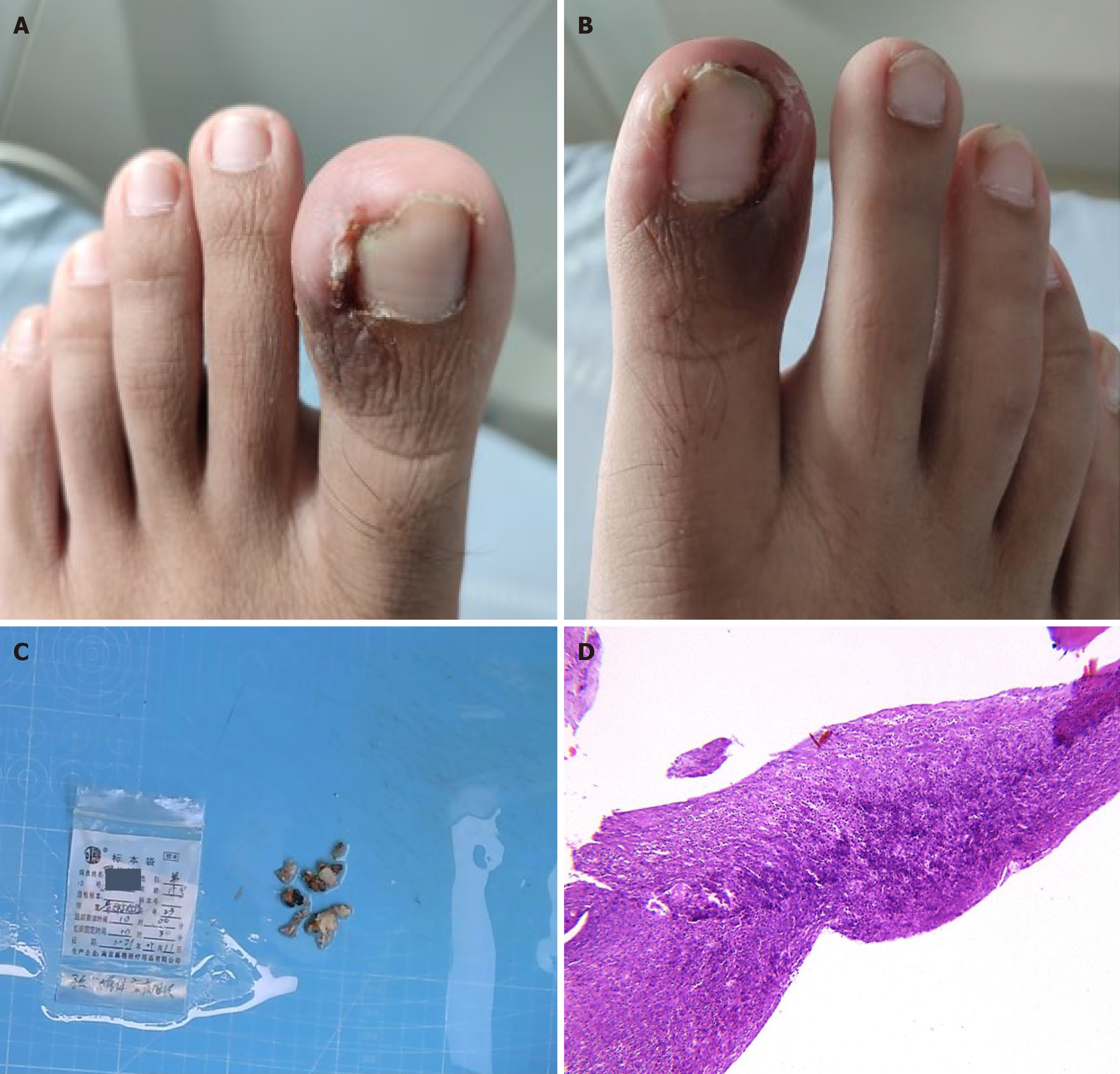
The patient suffered from soft tissue injury in both feet during exercise 3 years ago and underwent surgery for paronychia of bilateral big toes 1 year later.
No special personal history, no familial genetic disease.
Mild cervical lordosis, slight tension of cervical and shoulder muscles, tenderness over the spinous processes of C3-6, limited range of motion in neck flexion, extension, and rotation, involuntary trembling of both hands, normal biceps and triceps reflexes in both upper limbs, normal radial and ulnar reflexes, normal sensation, muscle strength, and tone in all four limbs, mild swelling and tenderness over the edge of the toenails of both big toes, normal knee and ankle reflexes, Hoffman’s sign (-), and Babinski’s sign (-).
Laboratory examination revealed no increase in white blood cell (WBC) count (7.7 × 103/μL) and procalcitonin (< 0.1 ng/L) at admission, while the C-reactive protein (CRP) concentration (118.4 mg/L), and erythrocyte sedimentation rate (ESR) (42 mm/hour) all increased. On the first night of admission, Staphylococcus aureus (S. aureus) was detected in blood by bacterial culture. On day 2 of admission, no WBCs or pyocytes were found in routine urine and stool tests. On day 9 of admission, S. aureus was found in the microbial culture of the right toenail paronychia tissue. On day 12 of admission, no bacteria were found in the blood culture. ESR (10 mm/hour) and CRP (< 5 mg/L) returned to normal after 1 week of discharge.
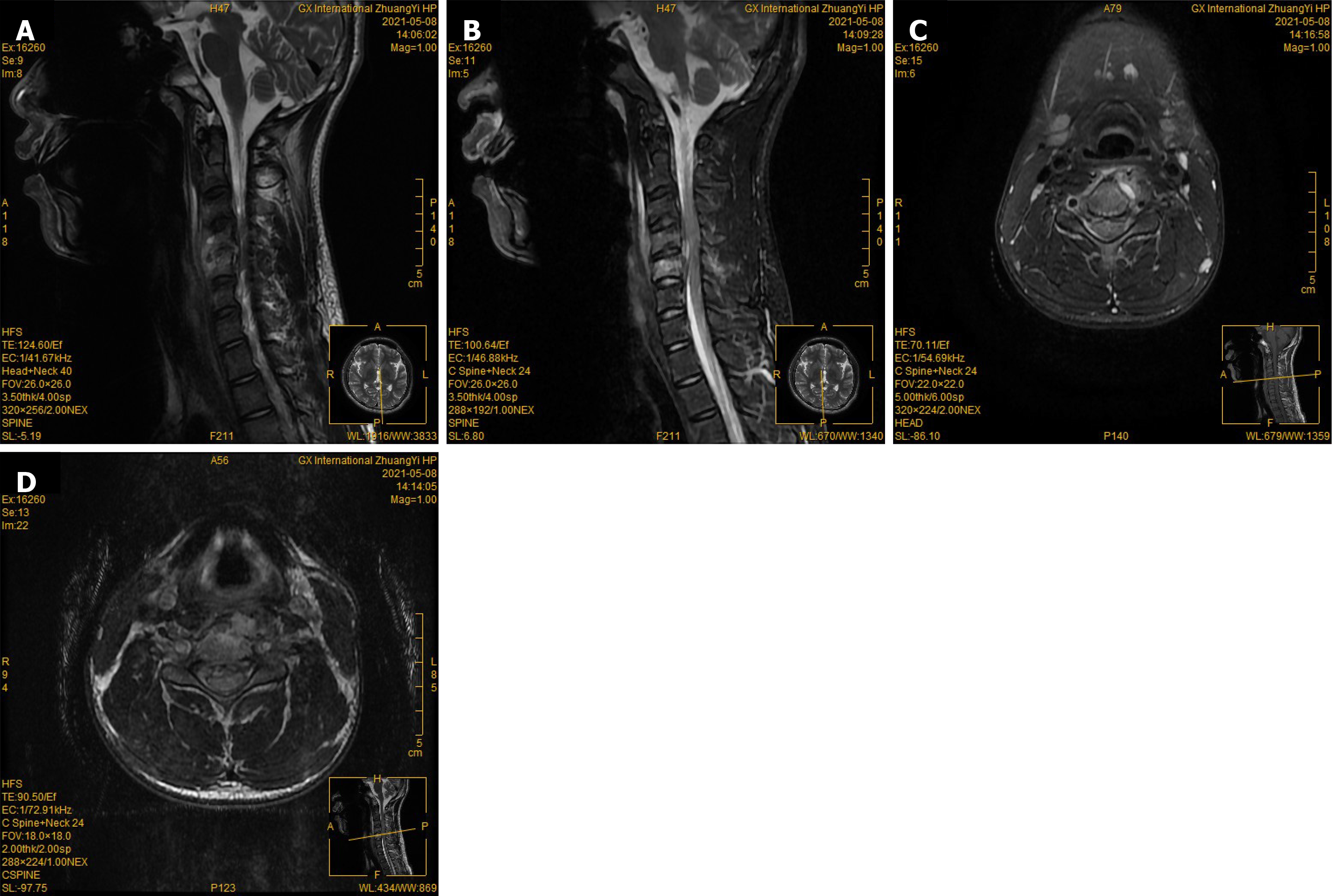
The cervical X-ray film showed that the physiological curvature of the cervical became straight, and no significant narrowing of the intervertebral space (Figure 1). Cervical spine computed tomography (CT) showed local bone defects at the upper margin of the C5 vertebral body (Figure 1). Cervical magnetic resonance imaging (MRI) indicated patchy hypointense signals in the C4 and C5 vertebral bodies in T2-weighted images. T2 signal shadows were seen in the soft tissues inside and around the vertebral canal, indicating abscess formation and compression of the C4-5 horizontal spinal cord (Figure 2).
The infectious origin was identified by microbial culturing of the paronychial tissue surrounding the great toe. The transmission pathway was ascertained through microbial blood culture, and the site of pathological damage was confirmed via histopathological analysis of the affected vertebral body (Figure 3). Integrating this information with the patient’s trauma history, clinical presentation, physical findings, and supplementary diagnostic tests, the diagnosis of acute CSPI secondary to chronic paronychia was established.
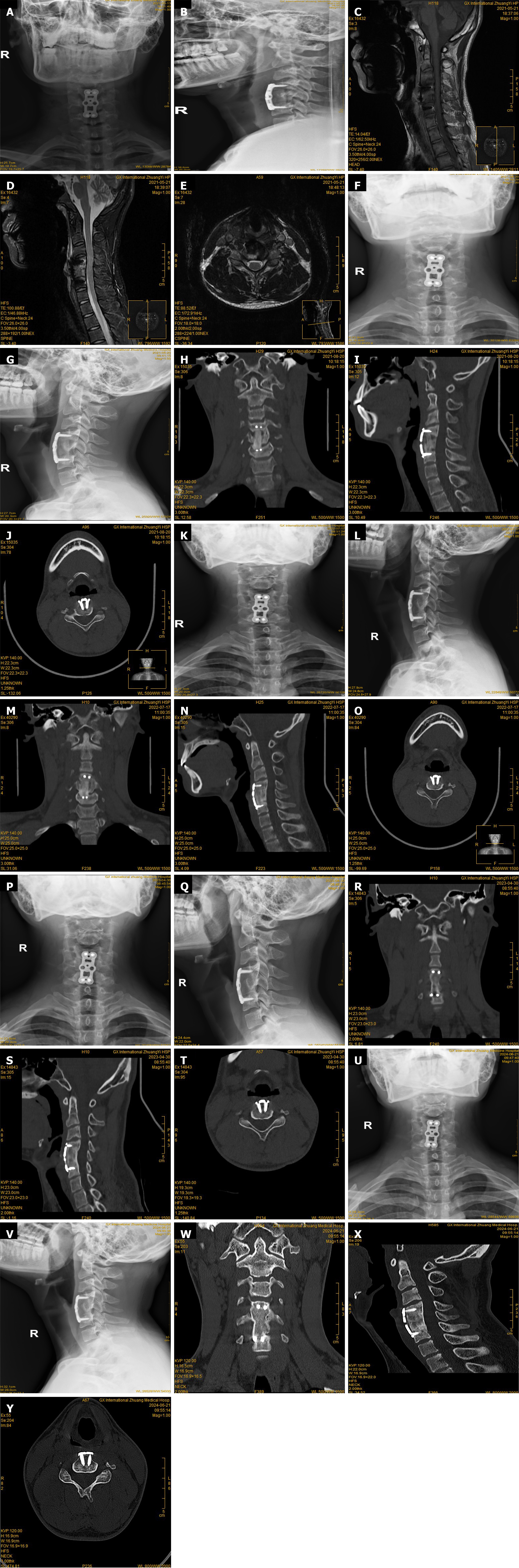
Six hours after admission, the patient’s temperature rose to 40 °C. Routine blood tests, biochemistry tests and blood cultures were immediately conducted. A portion of the patient’s right hallux nail para-canalicular tissue was excised and sent for microbiological culture. At the same time, intravenous rehydration and antipyretic treatment were provided. The next day, the patient’s neck pain worsened and his limbs progressively became numb, and he had difficulty urinating. Routine blood test results indicated WBC count 8.97 × 103/μL and ESR 42 mm/hour. The patient was treated with cefoperazone sodium and sulbactam sodium (2 g) once every 8 hours for infection, and catheterization for urination. Fever improved after treatment. Cervical CT and MRI showed abnormal C5 vertebral body, tissue edema of the paracervical region, and cervical cord compression at C4-C5 level. On day 6 of admission, the patient received anterior cervical debridement, autogenous iliac bone graft fusion and plate fixation. During the operation, the C5 vertebral lesion and surrounding necrotic tissue were removed for microbial culture and pathological examination. Autologous iliac bone of the patient was removed for structural bone grafting after trimming. Cefoperazone sodium and sulbactam sodium (2 g) once every 8 hours was given by intravenous drip for anti-infective treatment postoperatively. After discharge, the patient continued to wear a cervical collar and received oral antibiotics for 12 weeks.
Fever, numbness, neck pain, and trembling of both hands were significantly alleviated, and self-control urination significantly recovered. Postoperative cervical lateral X-ray at 2 days showed that the internal fixation was in place (Figure 4). Postoperative cervical MRI at 12 days showed that the range of paravertebral soft tissue edema had decreased, and the abnormal signal at the C4-5 cervical spinal cord was alleviated (Figure 4). ESR and CRP both returned to normal levels after 1 week of discharge. Serial postoperative imaging studies at 3 months, 12 months, 24 months, and 36 months consistently demonstrated stable cervical internal fixation of the bone graft, indicating successful and sustained integration and fusion (Figure 4). Additionally, the patient the patient had no prominent neck pain or abnormal sensation of upper limbs, and urinated well.
Spinal infections are increasingly prevalent, with an overall incidence rate of 2.4 per 100000 individuals per year, peaking at 6.5 per 100000 in the 50-70 years age group[6]. However, adolescent-specific incidence rates remain undocumented. These infections constitute 1%-7% of all osseous infections, with the cervical spine being the least commonly affected region, accounting for only 3%-10% of cases[3,7]. However, specific incidence rates for adolescents remain undocumented. S. aureus is the predominant pathogen in CSPI, although there is a noted rise in infections caused by Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa, partly due to the widespread use of antibiotics and the increase in intravenous drug abuse[8,9]. Additionally, polymicrobial infections or negative culture results are observed in approximately 20% of cases, and immunocompromised patients are at a higher risk of infections from low-virulence microorganisms[10], with approximately 30% of clinical cases lacking an identifiable pathogen[7].
The unique anatomical features of the cervical spine, particularly its intricate blood supply, facilitate the occurrence of infections through diverse routes[11]. These include arterial dissemination, direct inoculation, contiguous spread from neighboring infection sites, and retrograde spread via the vertebral venous system[3]. Hematogenous spread, where microorganisms enter the spine through the nutrient arteries or the paravertebral venous plexus, is the most prevalent mode of transmission[12]. This arterial dissemination is more common than venous spread due to the comprehensive arterial blood supply encompassing the lower portion of the superior vertebrae, the upper portion of the inferior vertebrae, and the intervertebral discs[13]. Consequently, infections can potentially damage not just the adjacent vertebrae but also the intervertebral discs and surrounding paravertebral soft tissues. CSPI is classified based on the affected anatomical structures, encompassing discitis, vertebral osteomyelitis, and epidural abscesses[4]. Common sources of these infections include urinary tract infections, skin and mucosal infections, and respiratory infections[3]. Postoperative pyogenic infections following cervical spine surgery must be vigilantly monitored, as S. aureus is frequently implicated in over half of deep surgical site infections[14]. Specific situations, such as tracheostomy, pharyngeal surgery, or cervical spine trauma, increase the vulnerability of the cervical spine to infection[2]. Additionally, CSPIs have been reported following radiotherapy and chemotherapy in patients with nasopharyngeal and tongue cancer[15,16]. The extensive venous plexus surrounding the odontoid process in the upper cervical spine, which communicates with veins superior to the nasopharynx, poses a significant risk for infections in individuals undergoing invasive procedures in the oropharyngeal region[17]. The clinical manifestations of CSPI are diverse, influenced by both the virulence of the causative pathogen and the immune status of the patient. These manifestations can range from acute to chronic presentations, often posing diagnostic challenges due to nonspecific early symptoms[12]. Neck pain is a frequent complaint, and in severe cases, sepsis symptoms may emerge. Notably, cervical spondylitis complicated by epidural abscesses is a significant concern in this anatomical region, carrying a high risk of paralysis[18]. The diagnosis of CSPI requires a multifaceted approach, integrating clinical symptoms, laboratory markers, and imaging studies. Laboratory investigations, specifically the measurement of CRP and ESR, serve as vital inflammatory markers for initial screening, particularly when imaging findings are equivocal[19]. CRP and ESR are crucial inflammatory markers for initial screening, with CRP being particularly useful for assessing the presence and severity of infection, as well as monitoring the effectiveness of treatment[8,20].
Imaging techniques play a pivotal role in the diagnostic armamentarium[21]. Although X-rays are often used as a preliminary screening tool, their sensitivity and specificity are limited, often revealing changes only after a significant delay following the onset of infection[4]. MRI is the preferred modality for diagnosing CSPI due to its exceptional sensitivity and specificity in detecting early bone marrow edema and surrounding soft tissue inflammation[22]. It is recommended to perform a full spine MRI to screen for multifocal infections, which are more frequently encountered in cervical infections, and to avoid diagnostic oversights[23]. CT is the diagnostic modality of choice for patients with contraindications to MRI, providing superior visualization of bone structures, and dual-energy CT, in particular, offers significant advantages in differentiating cervical crystal diseases such as tophi and pyrophosphate deposits[24,25]. When MRI results remain inconclusive in diagnosing CSPI, radionuclide bone scans using Ga-67 and Tc-99m diphosphonate, as well as labeled WBC imaging, provide valuable insights[26]. However, it is crucial to be mindful of the potential for false-positive results in patients with spinal tumors or post-traumatic conditions. 18F-fluorodeoxyglucose positron emission tomography/CT has garnered significant attention in the field of spinal infections due to its high sensitivity and specificity in detecting occult infection processes, surpassing even MRI in some instances[27,28]. This has led some experts to recommend its consideration as a primary imaging modality for postoperative spinal infection diagnosis[29].
During differential diagnosis, it is crucial to distinguish cervical tuberculosis[30], brucellosis[31], fungal infections[32], calcium pyrophosphate deposition disease[33], and tumors[34]. For CSPI confirmation, isolation of pathogens through blood culture and biopsy is essential[8]. S. aureus remains the pre-eminent single pathogen in CSPI, particularly notable for its elevated colonization rate in the cervical spine among methicillin-sensitive strains[35]. Prior to initiating antibiotic therapy, the prompt collection of microbial samples is paramount to enhancing detection accuracy. However, it is worth noting that blood cultures may not always yield positive results, necessitating the consideration of alternative diagnostic methods. Image-guided percutaneous biopsies, while generally safe, carry the risk of false negatives and should be interpreted cautiously[36]. In more intricate cases where a definitive diagnosis remains elusive, surgical biopsy may be the deciding factor. For treatment of CSPI, nonsurgical options are viable for early-stage disease without neurological impairment and minimal bone destruction[2]. These treatments typically consist of antimicrobial therapy and the use of external orthotic devices. For patients exhibiting signs of sepsis, prompt initiation of broad-spectrum antimicrobials is crucial. Initially, when the pathogen is unknown, empirical use of antimicrobials such as cephalosporins is beneficial for patients with mild symptoms, surgical intolerance, or negative blood cultures but positive biopsies[4,37]. Once the pathogen is identified, therapy is tailored based on culture and sensitivity results. For Gram-positive bacteria, semisynthetic penicillins and cephalosporins are preferred. In methicillin-resistant cases, vancomycin, teicoplanin, or linezolid are considered. For Gram-negative bacteria, antibiotics targeting Pseudomonas species, such as beta-lactam antibiotics, are selected, while other infections may respond to cephalosporins, quinolones, or aminoglycosides[5].
Intravenous antimicrobial therapy, administered for a duration of 6-12 weeks[38], followed by a 6-week course of oral antibiotics, is crucial for the complete eradication of CSPI[39]. Shorter treatment durations, lasting < 4 weeks, have been associated with a significant 25% relapse rate[2]. External orthosis of the cervical spine serves to alleviate pain, prevent the development of deformity, and safeguard spinal cord neural functions. In instances where virulent pathogens cause erosion of the vertebrae and their attachments, particularly involving the upper cervical vertebrae and the atlantoaxial joint, rapid progression of cervical deformity and instability may occur, leading to the impairment of motion and instability. In such scenarios, the use of a halo-vest is recommended to provide stable immobilization, typically required for a period of 3-4 months[40]. Although the combination of external orthosis and antimicrobial drug treatment is ef
For patients with CSPI who present with spinal cord compression, spinal instability and/or deformity, and show no clinical improvement within 2-3 weeks of antimicrobial therapy, early surgical decompression is recommended[42]. Early surgical intervention has consistently shown superior outcomes compared to conservative treatment in terms of recurrence or failure rates, mortality, and length of hospital stay[42]. Studies indicate that surgery during the acute phase of hematogenous pyogenic spondylitis is both safe and effective, while delayed surgery can lead to poor prognosis[43,44]. The quality of life for patients who undergo surgery combined with sensitive antimicrobial therapy is significantly better than for those managed nonsurgically[45]. The surgical management of CSPI often involves a two-stage approach: Initial anterior debridement followed by a second stage of anterior bone grafting and internal fixation, or standalone bone grafting[46]. However, recent evidence increasingly favors a single-stage procedure combining anterior debridement, bone grafting, and internal fixation[47]. In cases where the anterior approach fails, such as when there is destruction of the atlantoaxial joint[48], dorsal epidural abscesses[49], multisegmental cervical involvement with instability or deformity[50], a combined anterior and posterior surgical approach may be warranted[5]. The anterior cervical debridement and fusion technique allows direct visualization and thorough removal of necrotic and infected tissues, as the infection foci are commonly located in the anterior and middle columns of the spine. The utilization of metal plates in anterior cervical debridement and fusion offers superior biomechanical stability and higher fusion rates compared to nonmetallic alternatives[47]. Given the severe bone destruction often encountered, bone graft fusion is essential for spinal stabilization and prevention of kyphotic deformity. Traditional methods involve autologous bone grafting from the iliac crest, ribs, or fibula, which exhibit high fusion rates[51]. Alternatively, polyetheretherketone cages have demonstrated comparable outcomes without increasing the risk of infection recurrence compared to autologous bone grafting[52]. Bone morphogenetic protein represents an effective and safe adjunct in the surgical treatment of CSPI, without increasing the risk of infection recurrence, revision surgery, or radiculitis[53]. In recent years, minimally invasive surgical techniques have been explored for CSPI, including anterior percutaneous endoscopic ventral epidural abscess drainage[54,55] and posterior percutaneous endoscopic dorsal epidural abscess drainage[56]. However, due to the limited operative field associated with minimally invasive surgery, complete vertebral body resection and intervertebral fusion remain challenging and are currently not feasible. Regarding the prognosis of patients with CSPI, those presenting with neurological impairment and undergoing prompt surgical decompression often exhibit notable neural function recovery. Conversely, patients with complete neurological damage who undergo delayed treatment generally face a poorer prognosis[57]. Elderly individuals and those with sepsis are more likely to experience a less favorable outcome[58,59]. Additionally, the prognosis for patients managed nonsurgically is influenced by multiple factors, including age and immune status[60].
We have presented a unique case of neglected hematogenous cervical spondylitis in an adolescent, which was suc
Our thanks go out to all involved staff at the Guangxi University of Traditional Chinese Medicine Affiliated International Zhuang Hospital.
| 1. | Barnes B, Alexander JT, Branch CL Jr. Cervical osteomyelitis: a brief review. Neurosurg Focus. 2004;17:E11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Acosta FL Jr, Chin CT, Quiñones-Hinojosa A, Ames CP, Weinstein PR, Chou D. Diagnosis and management of adult pyogenic osteomyelitis of the cervical spine. Neurosurg Focus. 2004;17:E2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Sato K, Yamada K, Yokosuka K, Yoshida T, Goto M, Matsubara T, Iwahashi S, Shimazaki T, Nagata K, Shiba N; RESEARCH GROUP FOR SPINE AND SPINAL CORD DISORDERS (HONNEKAI). Pyogenic Spondylitis: Clinical Features, Diagnosis and Treatment. Kurume Med J. 2019;65:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Aljawadi A, Jahangir N, Jeelani A, Ferguson Z, Niazi N, Arnall F, Pillai A. Management of Pyogenic Spinal Infection, review of literature. J Orthop. 2019;16:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Treffy RW, Laing B, Eraky AM, Shabani S. Cervical spine spondylodiscitis: Review of literature on current treatment strategies. Heliyon. 2023;9:e17875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Saleh ES, Vasileff CC, Omari AM, Khalil JG. The Diagnosis and Management of Pediatric Spine Infections. Cureus. 2021;13:e16748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Lener S, Hartmann S, Barbagallo GMV, Certo F, Thomé C, Tschugg A. Management of spinal infection: a review of the literature. Acta Neurochir (Wien). 2018;160:487-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 8. | Kim NJ. Microbiologic Diagnosis of Pyogenic Spondylitis. Infect Chemother. 2021;53:238-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Ziarko TP, Walter N, Schindler M, Alt V, Rupp M, Lang S. Risk Factors for the In-Hospital Mortality in Pyogenic Vertebral Osteomyelitis: A Cross-Sectional Study on 9753 Patients. J Clin Med. 2023;12:4805. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | George AJ, Santhanagopal S, Mohan MM, Lal JV, Basappa M, Thomas JC, Jeevo J. Spondylodiscitis: A Diagnostic and Management Dilemma. Cureus. 2024;16:e58284. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Morales H. Infectious Spondylitis Mimics: Mechanisms of Disease and Imaging Findings. Semin Ultrasound CT MR. 2018;39:587-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Babic M, Simpfendorfer CS. Infections of the Spine. Infect Dis Clin North Am. 2017;31:279-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Fournier DE, Kiser PK, Shoemaker JK, Battié MC, Séguin CA. Vascularization of the human intervertebral disc: A scoping review. JOR Spine. 2020;3:e1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Ogihara S, Yamazaki T, Shiibashi M, Chikuda H, Maruyama T, Miyoshi K, Inanami H, Oshima Y, Azuma S, Kawamura N, Yamakawa K, Hara N, Morii J, Okazaki R, Takeshita Y, Nishimoto J, Tanaka S, Saita K. Risk factors for deep surgical site infection after posterior cervical spine surgery in adults: a multicentre observational cohort study. Sci Rep. 2021;11:7519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Fujii K, Imai T, Morita S, Saijyo S, Yamazaki T, Asada Y. Pyogenic spondylitis caused by Streptococcus dysgalactiae subspecies equisimilis in a patient with nasopharyngeal cancer. J Infect Chemother. 2022;28:1332-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Karakida K, Uchibori M, Nakanishi Y, Tamura M, Takahashi M, Hoshimoto Y, Hamada Y, Aoki J. Pyogenic Spondylitis with Rapid Bone Destruction After Chemoradiotherapy for Tongue Cancer: A Case Report and Literature Review. Tokai J Exp Clin Med. 2020;45:182-188. [PubMed] |
| 17. | Hashimoto E, Miyazaki K, Hirose K, Maeno T. A Case of Odontoid Osteomyelitis. Cureus. 2024;16:e52012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976). 2000;25:1668-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 437] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 19. | Kugimiya F, Muraki S, Nagakura D, Umekoji H, Oda H, Takahashi K. Predictors of conservative treatment for pyogenic spondylitis. Spine Surg Relat Res. 2017;1:135-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Takahashi T, Inose H, Hirai T, Matsukura Y, Morishita S, Egawa S, Hashimoto J, Takahashi K, Yoshii T. Factors associated with the time required for CRP normalization in pyogenic spondylitis: A retrospective observational study. N Am Spine Soc J. 2024;17:100301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | Wangaryattawanich P, Condos AM, Rath TJ. Bacterial and Viral Infectious Disease of the Spine. Magn Reson Imaging Clin N Am. 2024;32:313-333. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Rotzinger R, Omidi R, Gebhard H, Shariat K, Ahlhelm F. [Spondylodiscitis and epidural abscesses]. Radiologe. 2021;61:275-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Balcescu C, Odeh K, Rosinski A, Nudelman B, Schlauch A, Shah I, Ungurean V Jr, Prasad P, Leasure J, Stepansky F, Piple A, Kondrashov D. Pyogenic spinal infections warrant a total spine MRI. J Bone Jt Infect. 2023;8:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Boudabbous S, Paulin EN, Delattre BMA, Hamard M, Vargas MI. Spinal disorders mimicking infection. Insights Imaging. 2021;12:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Shroyer S, Boys G, April MD, Long B, Mehta S, Davis WT. Imaging characteristics and CT sensitivity for pyogenic spinal infections. Am J Emerg Med. 2022;58:148-153. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Love C, Palestro CJ. Nuclear medicine imaging of bone infections. Clin Radiol. 2016;71:632-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Zhang Q, Feng H, Li J, Feng R. Diagnostic accuracy of fluorine-18 fluorodeoxyglucose positron emission tomography for suspected primary and postoperative pyogenic spondylitis. J Orthop Surg Res. 2023;18:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Kim SJ, Pak K, Kim K, Lee JS. Comparing the Diagnostic Accuracies of F-18 Fluorodeoxyglucose Positron Emission Tomography and Magnetic Resonance Imaging for the Detection of Spondylodiscitis: A Meta-analysis. Spine (Phila Pa 1976). 2019;44:E414-E422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Kloiber R, Lafford H, Koslowsky IL, Tchajkov I, Rabin HR. A practical approach to interpretation of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for postoperative spine infection. Skeletal Radiol. 2024;53:741-752. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Li Y, Wang Y, Ding H, Zhang N, Ma A, Shi J, Niu N. Pathologic characteristics of spinal tuberculosis: analysis of 181 cases. Int J Clin Exp Pathol. 2020;13:1253-1261. [PubMed] |
| 31. | Spernovasilis N, Karantanas A, Markaki I, Konsoula A, Ntontis Z, Koutserimpas C, Alpantaki K. Brucella Spondylitis: Current Knowledge and Recent Advances. J Clin Med. 2024;13:595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Reference Citation Analysis (0)] |
| 32. | Adelhoefer SJ, Gonzalez MR, Bedi A, Kienzle A, Bäcker HC, Andronic O, Karczewski D. Candida spondylodiscitis: a systematic review and meta-analysis of seventy two studies. Int Orthop. 2024;48:5-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Moon AS, Mabry S, Pittman JL. Calcium pyrophosphate deposition disease of the cervical and thoracolumbar spine: A report of two cases. N Am Spine Soc J. 2020;3:100026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Compagnone D, Cecchinato R, Pezzi A, Langella F, Damilano M, Redaelli A, Vanni D, Lamartina C, Berjano P, Boriani S. Diagnostic Approach and Differences between Spinal Infections and Tumors. Diagnostics (Basel). 2023;13:2737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Stangenberg M, Mende KC, Mohme M, Krätzig T, Viezens L, Both A, Rohde H, Dreimann M. Influence of microbiological diagnosis on the clinical course of spondylodiscitis. Infection. 2021;49:1017-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Kim BJ, Lee JW, Kim SJ, Lee GY, Kang HS. Diagnostic yield of fluoroscopy-guided biopsy for infectious spondylitis. AJNR Am J Neuroradiol. 2013;34:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Mohamad G, Amritanand R, David KS, Krishnan V, Arockiaraj J. Treatment Strategy and Outcomes in Patients with Hematogenous Culture-Negative Pyogenic Vertebral Osteomyelitis. Asian Spine J. 2019;13:61-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Bernard L, Dinh A, Ghout I, Simo D, Zeller V, Issartel B, Le Moing V, Belmatoug N, Lesprit P, Bru JP, Therby A, Bouhour D, Dénes E, Debard A, Chirouze C, Fèvre K, Dupon M, Aegerter P, Mulleman D; Duration of Treatment for Spondylodiscitis (DTS) study group. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 314] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 39. | Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 411] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 40. | Ujigo S, Kishi K, Imada H, Shibuya H, Nakanishi K, Adachi N. Upper Cervical Osteomyelitis with Odontoid Process Destruction Treated with a Halo Vest in a Child: A Case Report. Spine Surg Relat Res. 2020;4:287-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 41. | Onen MR, Yuvruk E, Karagoz G, Naderi S. Efficiency of Hyperbaric Oxygen Therapy in Iatrogenic Spinal Infections. Spine (Phila Pa 1976). 2015;40:1743-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Thavarajasingam SG, Vemulapalli KV, Vishnu K S, Ponniah HS, Vogel AS, Vardanyan R, Neuhoff J, Kramer A, Shiban E, Ringel F, Demetriades AK, Davies BM. Conservative versus early surgical treatment in the management of pyogenic spondylodiscitis: a systematic review and meta-analysis. Sci Rep. 2023;13:15647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 43. | Canouï E, Zarrouk V, Canouï-Poitrine F, Desmoulin U, Leflon V, Allaham W, de Lastours V, Guigui P, Fantin B. Surgery is safe and effective when indicated in the acute phase of hematogenous pyogenic vertebral osteomyelitis. Infect Dis (Lond). 2019;51:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Pluemer J, Freyvert Y, Pratt N, Robinson JE, Cooke JA, Tataryn ZL, Godolias P, Daher ZA, Oskouian RJ, Chapman JR. An Assessment of the Safety of Surgery and Hardware Placement in de-novo Spinal Infections. A Systematic Review and Meta-Analysis of the Literature. Global Spine J. 2023;13:1418-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Behmanesh B, Gessler F, Duetzmann S, Seifert V, Weise L, Setzer M. Quality of Life Following Surgical and Conservative Therapy of Pyogenic Spinal Infection: A Study of Long-term Outcome in 210 Patients. J Neurol Surg A Cent Eur Neurosurg. 2023;84:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Nakase H, Matsuda R, Tamaki R, Tei R, Park YS, Sakaki T. Two-stage management for vertebral osteomyelitis and epidural abscess: technical note. Neurosurgery. 2006;58:E1219; discussion E1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Kim SK, Park JB, Chung JY, Lee DH, Kim YY, Park YJ, Lee NH. Anterior Cervical Debridement and Fusion for Cervical Pyogenic Spondylodiscitis: Use of Anterior Cervical Plating or Not? Spine (Phila Pa 1976). 2020;45:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Reid PJ, Holman PJ. Iatrogenic pyogenic osteomyelitis of C-1 and C-2 treated with transoral decompression and delayed posterior occipitocervical arthrodesis. Case report. J Neurosurg Spine. 2007;7:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Hijazi MM, Siepmann T, El-Battrawy I, Aweimer A, Engellandt K, Podlesek D, Schackert G, Juratli TA, Eyüpoglu IY, Filis A. Diagnostics, Management, and Outcomes in Patients with Pyogenic Spinal Intra- or Epidural Abscess. J Clin Med. 2023;12:7691. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 50. | Deshmukh VR. Midline trough corpectomies for the evacuation of an extensive ventral cervical and upper thoracic spinal epidural abscess. J Neurosurg Spine. 2010;13:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Miyazaki M, Abe T, Ishihara T, Kanezaki S, Notani N, Kataoka M, Tsumura H. Cervical alignment after single-level anterior cervical corpectomy and fusion using autologous bone graft without spinal instrumentation for cervical pyogenic spondylitis. Eur J Orthop Surg Traumatol. 2020;30:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Novak I, Košak R, Travnik L, Gorenšek M, Bošnjak K, Vengust R, Zupanc O. Polyetheretherketone (PEEK) cages for anterior column reconstruction in pyogenic vertebral osteomyelitis. J Orthop Surg (Hong Kong). 2019;27:2309499019842490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 53. | Yaw Tee LY, Hunter S, Baker JF. BMP use in the surgical treatment of pyogenic spondylodiscitis: Is it safe? J Clin Neurosci. 2022;95:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Muzii VF, Mariottini A, Zalaffi A, Carangelo BR, Palma L. Cervical spine epidural abscess: experience with microsurgical treatment in eight cases. J Neurosurg Spine. 2006;5:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Kotheeranurak V, Jitpakdee K, Singhatanadgige W, Limthongkul W, Yingsakmongkol W, Kim JS. Anterior transcorporeal full-endoscopic drainage of a long-span ventral cervical epidural abscess: A novel surgical technique. N Am Spine Soc J. 2021;5:100052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Chang KS, Sun LW, Cheng CY, Chang SW, Chen CM. Full Endoscopic Removal of Cervical Spinal Epidural Abscess: Case Report and Technical Note. Neurospine. 2020;17:S160-S165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Jung N, Ernst A, Joost I, Yagdiran A, Peyerl-Hoffmann G, Grau S, Breuninger M, Hellmich M, Kubosch DC, Klingler JH, Seifert H, Kern WV, Kaasch AJ, Rieg S. Vertebral osteomyelitis in patients with Staphylococcus aureus bloodstream infection: Evaluation of risk factors for treatment failure. J Infect. 2021;83:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Zarrouk V, Gras J, Dubée V, de Lastours V, Lopes A, Leflon V, Allaham W, Guigui P, Fantin B. Increased mortality in patients aged 75 years or over with pyogenic vertebral osteomyelitis. Infect Dis (Lond). 2018;50:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 59. | Kinamon T, Dagher M, Park L, Ruffin F, Fowler VG Jr, Maskarinec SA. Risk Factors and Outcomes of Hematogenous Vertebral Osteomyelitis in Patients With Staphylococcus aureus Bacteremia. Clin Infect Dis. 2023;77:1226-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Bhattacharyya S, Bradshaw MJ. Infections of the Spine and Spinal Cord. Continuum (Minneap Minn). 2021;27:887-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |