Published online Dec 18, 2024. doi: 10.5312/wjo.v15.i12.1175
Revised: November 12, 2024
Accepted: December 5, 2024
Published online: December 18, 2024
Processing time: 184 Days and 14.7 Hours
When patients with a failed hip arthroplasty are unsuitable for reimplantation, Girdlestone resection arthroplasty (GRA) is a viable treatment option. We report on a patient who was treated with a GRA due to a periprosthetic infection. We discovered partial paralysis of the quadriceps muscle in this patient. We investigated the femoral nerve anatomy, particularly the nerve entry points, to better understand this phenomenon.
To reveal the femoral nerve anatomy with respect to severe proximal migration after GRA.
Eight cadaveric hemipelves were investigated. The branches of the femoral nerve were dissected and traced distally. The GRA was performed by the direct anterior approach. Axial stress to the lower extremity was applied, and the relative movement of the femur was recorded. The femoral nerve and its entry points were assessed.
GRA led to a 3.8 cm shift of the femur in vertical direction, a 1.8 cm shift in the dorsal direction, and a 2.3 cm shift in the lateral direction. A 36.5° external shift was observed. This caused stress to the lateral division of the femoral nerve. We observed migration of the femoral nerve entry point at the following locations: (1) Vastus medialis (5.3 mm); (2) The medial part of the vastus intermedius (5.4 mm); (3) The lateral part of the vastus intermedius (16.3 mm); (4) Rectus femoris (23.1 mm); (5) Tensor vastus intermedius (30.8 mm); and (6) Vastus lateralis (28.8 mm).
Migration of the femur after GRA altered the anatomy of the femoral nerve. Stress occurred at the lateral nerve division leading to poor functional results.
Core Tip: Girdlestone resection arthroplasty (GRA) is a viable treatment option after a failed hip arthroplasty due to severe periprosthetic infections, poor bone stock, damaged pelvitrochanteric muscles, or comorbid disease. We initially treated a patient with bilateral GRA, who subsequently developed femoral nerve palsy. We investigated the femoral nerve anatomy after GRA in four cadavers (a total of eight hips). We detected migration of the femoral nerves, which caused stress at the lateral oblique femoral nerve division where the major psoas muscle acted as a hindrance. The partial femoral nerve palsy after GRA leads to poor functional outcomes.
- Citation: Spuehler D, Kuster L, Ullrich O, Grob K. Femoral nerve palsy following Girdlestone resection arthroplasty: An observational cadaveric study. World J Orthop 2024; 15(12): 1175-1182
- URL: https://www.wjgnet.com/2218-5836/full/v15/i12/1175.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i12.1175
Girdlestone resection arthroplasty (GRA) is a viable treatment option after failed total hip replacement[1] due to periprosthetic infections with difficult-to-treat microbes, poor bone stock, recurrent hip dislocations, damaged pelvitrochanteric muscles, or comorbid disease[2-4]. The hip resection arthroplasty procedure was first described in 1818, and in 1928 Gathorne Robert Girdlestone developed GRA to treat tuberculosis of the hip as well as severe hip-joint infections and arthritis[5,6]. The GRA procedure overcame the unsatisfactory results of earlier treatment methods[7].
Patients report satisfactory pain relief, a good range of motion, and high effectiveness in eradicating infection after GRA. The GRA procedure is able to eradicate 75%-93% of hip joint infections[5,8,9]. However, poor functional outcomes including limb shortening and quadriceps insufficiency with consequent severe limping are frequently observed[8]: 45% of patients are unable to walk, and only up to 29% are able to ambulate independently without walking aids[3,8]. Because of these unsatisfactory outcomes, GRA is typically the last option to control pain and the infection[2].
The femoral nerve, formerly known as the crural nerve, is the largest and longest nerve branch of the lumbar plexus that is formed from the posterior divisions of the ventral rami of the 2nd-4th lumbar nerves and unites within the psoas muscle[10-12]. The nerve then descends beneath the iliac fascia between the psoas and iliacus muscles. It emerges from the lateral border of the psoas 4 cm above the inguinal ligament (Poupart’s ligament)[10,11,13]. At this point it branches to the psoas and iliacus internus muscles[12,14]. From there it passes under the inguinal ligament and enters the thigh lateral to the vessels about 1-4 cm distal of the inguinal ligament. On the major psoas muscle, it divides into an anterior and posterior branch and is often separated by the lateral circumflex femoral artery[11,13,15]. The anterior branch passes superficially to the vessel, and the posterior branch passes beneath the vessel[16]. Often the posterior branch is shown as an outer and inner branch[12].
The femoral nerve motor branches innervate the pectineus and sartorius muscles and the quadriceps muscle group (vastus lateralis, tensor vastus intermedius, vastus intermedius, and vastus medialis)[17]. Its sensory branches arise from the anterior branch as the intermediate femoral cutaneous nerve and the medial femoral cutaneous nerve and from the inner branch of the posterior branch as the saphenous nerve, which is the largest and longest of the anterior cutaneous branches[11,12,15,16,18]. The saphenous nerve approaches the femoral artery where the vessel passes beneath the sartorius muscle and runs with the vasa femoralia to the adductor canal[16,19].
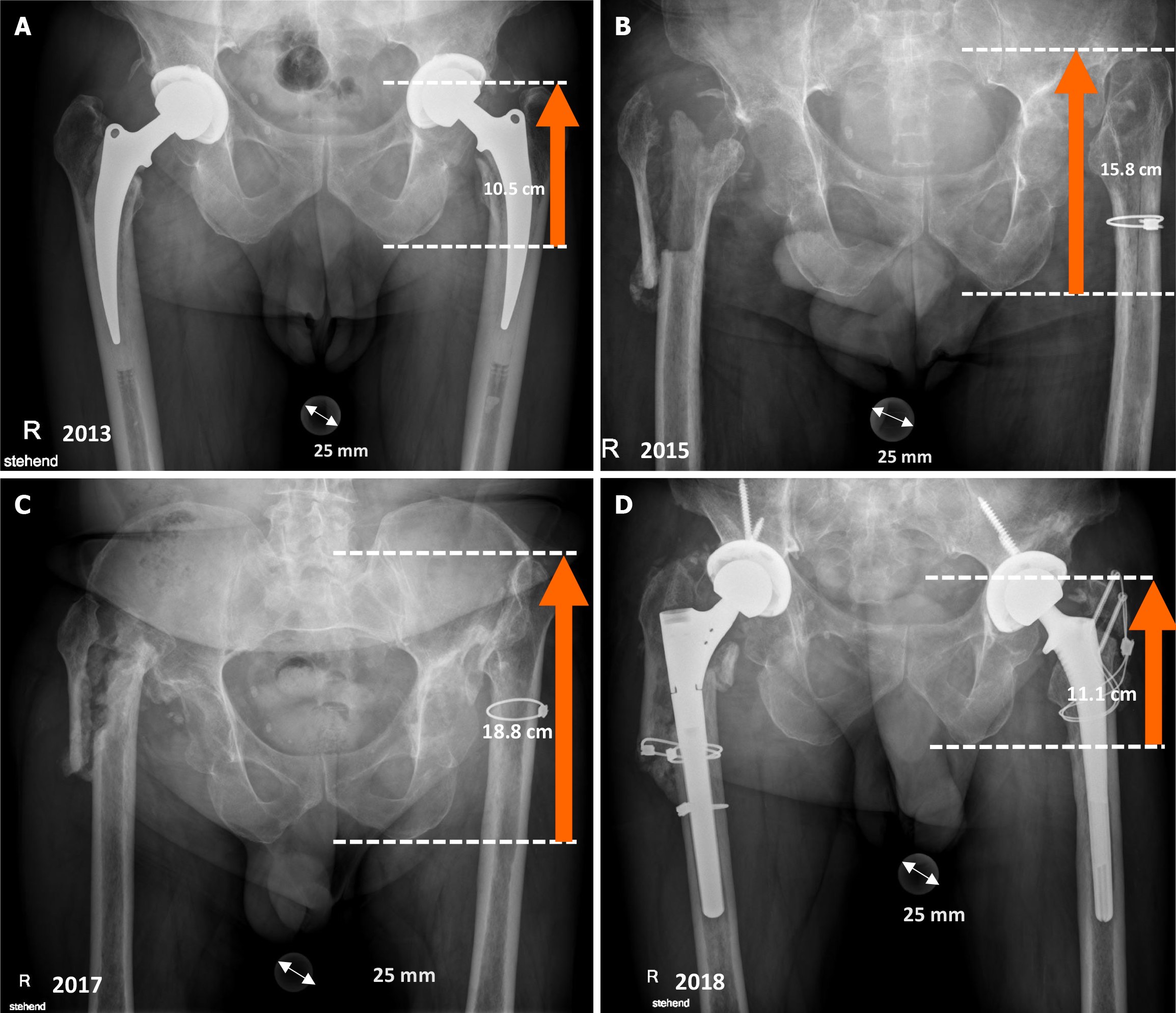
A comorbid 58-year-old patient presented to our clinic with paralysis of the lateral component of the quadriceps (vastus lateralis, vastus intermedius, and rectus femoris muscles) after bilateral GRA due to systemic and periprosthetic infection with Candida albicans. The patient developed pronounced leg shortening due to proximal migration of the femur after 4 years. We also observed subsequent neurological paralysis of the lateral quadriceps, including the rectus femoris, vastus lateralis, tensor vastus intermedius, and the lateral part of the vastus intermedius[17]. We hypothesized that the progressive leg shortening led to additional stress on the femoral nerve causing paralysis. Three years after reimplantation of a total hip, the paralysis disappeared completely (Figure 1).
Studies regarding the neurological complications due to GRA are scarce. Most studies discuss infection eradication and poor functional outcomes using the Harris hip or Merle d’Aubigné scores with respect to severe leg length discrepancy and altered biomechanics of the hip joint[9,20]. The purpose of the following study was to better understand the anatomy of the femoral nerve in the quadriceps after GRA.
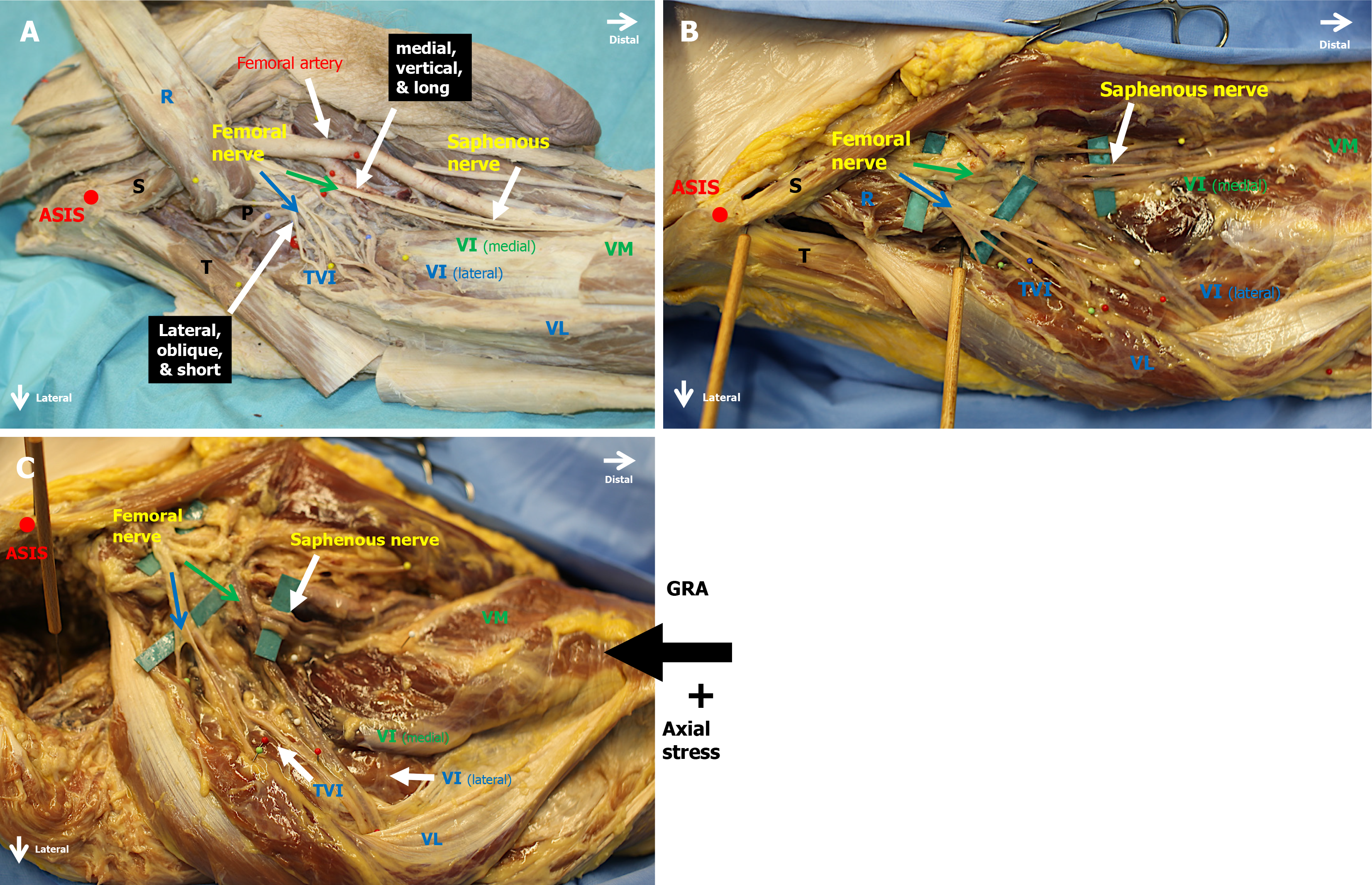
Eight cadaveric hemipelves from four specimens were investigated using microdissection techniques. All specimens were preserved using the Walter Thiel embalming method[18]. An investigation of the femoral nerve anatomy was also performed in four formalin-embalmed specimens (Figure 2A).
The specimens were placed supine on a dissection table. The femoral nerve was localized in the lacuna musculorum through an ilioinguinal approach. A long incision was made following the anterior part of the iliac crest to the anterior superior iliac spine (ASIS). From there the incision continued lateral of the sartorius muscle towards the lateral border of the patella. Skin and fatty tissue were removed. The femoral nerve and its branches were dissected, and their course was carefully traced distally to the entry points into each component of the quadriceps muscle group. The entry point of each component of the quadriceps was recorded and marked with pins. Finally, in the four Thiel embalmed specimens, two parallel Kirschner wires (K-wires) were applied first to the ASIS and second to the greater trochanter. The distances and angles between the K-wires were measured before and after performing GRA.
Resection of the femoral head was performed through a classic direct anterior approach to the hip joint[19], without disturbing any muscle components of the hip joint and quadriceps muscle group (Figure 2B and C). Then a body weight adapted axial stress was applied to the calcaneus tuber using a digital force gauge (Sauter FK, Swiss Waagen DC GmbH) with the torso lying on the metallic dissection table. Depending on bodyweight of the specimen, a mean force of 98 N (range: 88-107 N; SD: 5.09) was needed to start body movement, and the proximal advancement of the leg was measured (Table 1 and Figure 3).
The following parameters were measured before and after force application: (1) Distance between the ASIS and the major trochanter; (2) Distances between the ASIS to the various entry points into the different components to the quadriceps muscle group; (3) Vertical, dorsal, and lateral dislocation of the greater trochanter; and (4) External rotational angle between the two initially parallel oriented K-wires placed in the ASIS and major trochanter (Figure 2B and C).
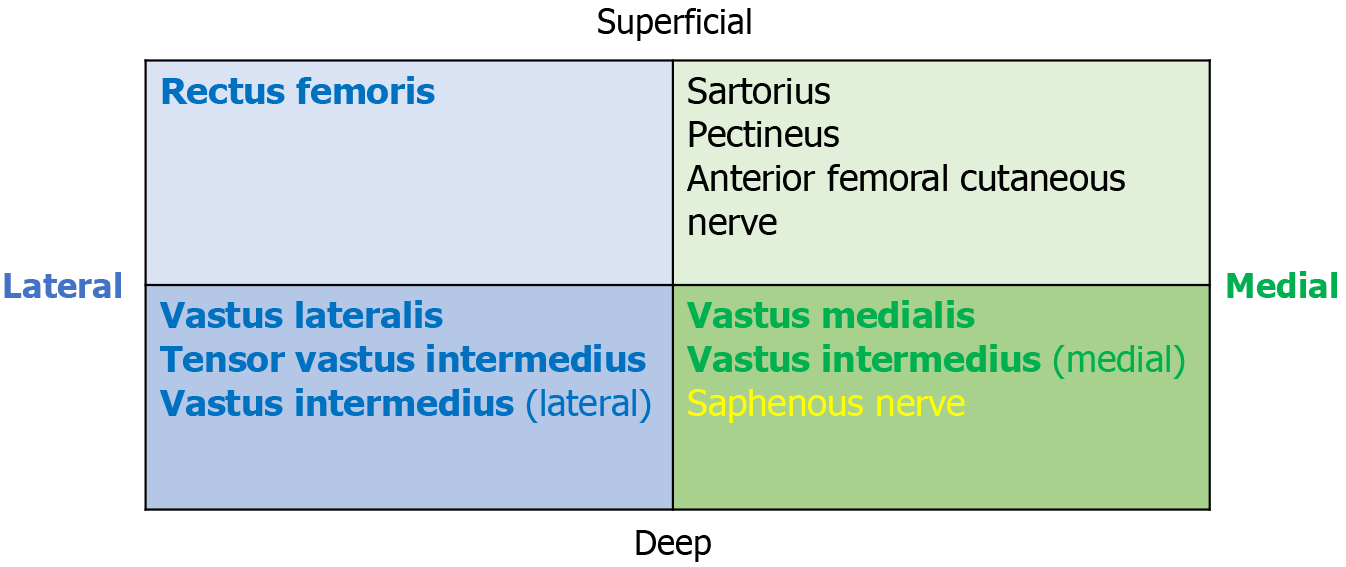
After GRA we observed that the femoral nerve distal to the inguinal ligament was divided into medial and lateral divisions supplying the four different components of the quadriceps muscle group. The medial and lateral divisions consisted of superficial and deep areas (Figure 4). The medial division is located vertically, and the superficial area branches into the sartorius and pectineus muscles. One of these branches develops into the anterior femoral cutaneous nerve. A deep medial muscle branch supplies the medial areas of the vastus intermedius and finally travels adjacent to the saphenous nerve to the vastus medialis muscle. The saphenous nerve is the most medial branch of the medial femoral division.
The lateral division consists of short and oblique nerve branches that are located over the iliopsoas muscle. The superficial area of the lateral division branches to the rectus femoris and sartorius muscles, while the deep nerve branches supply the lateral area of the vastus intermedius, the tensor vastus intermedius, and the vastus lateralis muscles (Figure 2A).
After GRA, the mean angle of external rotation was 36.5° (range: 28°-45°; SD: 5.59). We observed an average femoral displacement in vertical direction of 38 mm (range: 32-49 mm; SD: 4.92), in the dorsal direction of 18 mm (range: 13-22 mm; SD: 2.99), and in the lateral direction of 23 mm (range: 18-30 mm; SD: 4.01) (Table 1).
The proximal migration of the femoral nerve entry points after axial stress application was recorded for the following components of the quadriceps muscle group: (1) Vastus medialis of the medial vertical femoral nerve division (mean: 5.3 mm; range: 3-8 mm; SD: 1.64); and (2) The medial area of the vastus intermedius (mean: 5.4 mm; range: 2-8 mm; SD: 1.73).
We also measured the migration of the following nerve entry point for the lateral short oblique nerve division: (1) Lateral area of the vastus intermedius (mean: 16.3 mm; range: 12-21 mm; SD: 3.12); (2) Rectus femoris (mean: 23.1 mm; range: 17-30 mm; SD: 3.85); (3) Tensor vastus intermedius (mean: 30.8 mm; range: 24-40 mm; SD: 4.87); and (4) Vastus lateralis (mean: 28.8 mm; range: 19-37 mm; SD: 5.24) (Table 2). These observations indicated that the psoas muscle acted as a hindrance for muscle branches to the lateral components of the femoral nerve.
Most studies of anatomy describe a separation of the femoral nerve into an anterior and posterior division. However, GRA majorly impacts the anatomy of the thigh, which results in limb shortening, dorsal dislocation, and external rotation. GRA alters the course of the femoral nerve. Instead of anterior and posterior divisions, we found a separation into medial and lateral divisions. To our knowledge, no study has investigated femoral nerve palsy and soft tissue alterations after GRA that lead to these poor outcomes. Our observations indicated that the anatomy changes when the leg and joint position are altered. This information should be accounted for in revision procedures when GRA is converted to total hip arthroplasty.
This is the first study to document the migration of the femur in multiple directions after GRA. Our anatomical investigation demonstrated femoral migration in the vertical, dorsal, lateral, and external directions, thereby altering the anatomical course of the femoral nerve at its entry points into the quadriceps muscle. We observed that the nerve branches of the short lateral division were located obliquely around the prominent psoas muscle supplying the lateral part of the vastus intermedius, tensor vastus intermedius, and vastus lateralis muscles (Figure 2A). We concluded that the psoas muscle acted as an obstacle for the oblique lateral nerve branches causing more stress on the lateral femoral nerve division. Accordingly, we measured only short migration distances for the medial vertical division innervating the medial area of the vastus intermedius and the vastus medialis.
These observations explain the clinical symptoms of the 58-year-old patient treated with bilateral GRA who inspired this study. The lateral components of the quadriceps muscle group were the most severely affected. Enormous femoral dislocation due to GRA was observed in this patient. This dislocation led to extraordinary stress on the short lateral muscle branches that are oblique to the psoas muscle. Therefore, partial femoral nerve palsy is a potential cause of poor functional outcomes following GRA. In our cadaveric study we recorded a mean proximal femoral migration of 3.8 cm, which is less than that reported by other studies[1,5,20] or in our patient due to the four-fold higher forces acting on the hip joint during walking compared to the force applied during our dissection.
Patient satisfaction after GRA is a subjective parameter that leads to variable published data[8]. One explanation is the lack of standardization for which a patient compares their situation. A patient may compare their satisfaction based on their situation before the resection arthroplasty, or with a total hip prosthesis, or with a normal hip[21-23]. De Laat et al[20] conducted a retrospective study of 40 cases treated with GRA after removal of a total hip prosthesis. They measured residual hip function by scoring pain, walking distance, and the use of walking aids. The objective results were good in 40.0% of the patients, moderate in 42.5% of patients, and bad in 17.5% of patients. Subjectively, 62.5% were satisfied with the overall result.
Sharma et al[4,21] reported an overall satisfaction of 83.3% and 71.0% after GRA in their two studies. Basu et al[3] reported that 65% of their patients had adequate pain control, whereas 29.0% reported no pain. Another 29% of patients were dissatisfied with the GRA. Vincenten et al[22] observed that GRA had a negative impact on functional outcome, health status, and quality of life. Sixty-three patients completed the World Health Organization Quality of Life and the EuroQol 5-dimension 3 Level version questionnaires. The results from the questionnaires revealed that the participants’ health statuses were significantly lower compared to patients with myocardial infarction or with lower limb amputation[22].
GRA limits the ability to walk, and patients are frequently severely disabled[23]. Basu et al[3] and Castellanos et al[5] reported that all patients in their respective studies were restricted in walking and dependent on walking aids. Cordero-Ampuero[9] stated that in a geriatric population up to 45% of patients were unable to ambulate and 29% managed to walk with walking aids only (one or two crutches) after GRA. These patients also had severe functional impairment after GRA according to the Harris hip scores ranging from 25-64[9].
Grauer et al[24] described a strong correlation between four anatomical levels of femoral head resection and the final walking and functional scores. Patients who received a procedure where the resection was more proximal had a better clinical outcome. However, the difference was not statistically significant. After the most recent follow-up, all of their patients had a Trendelenburg gait and required a walking aid.
Schröder et al[1] compared a group of patients treated with GRA (n = 32) to a group of patients treated with reimplantation (n = 16). They observed a leg length discrepancy of 4.0 ± 1.5 cm in the GRA group, a positive Trendelenburg sign in all patients, and a Harris hip score of 58 (range: 31-85) (not significant). A similar limb shortening of 4.1 cm (1-8 cm) was described by Castellanos et al[5]. In a study from 1991, the mean leg-shortening was 4.5 cm[20]. These three studies reported an actual proximal dislocation that was higher than our cadaveric study (mean migration = 3.8 cm). However, these three papers reported less migration than our 58-year-old patient with a bilateral GRA (limb-shortening on weight bearing X-rays = 8.3 cm) (Figure 1). This massive limb-shortening may have been due to the inability to unload the lower extremity to the contralateral side. Therefore, the risk of partial femoral nerve palsy may be greater in patients with bilateral GRA where force is applied to both sides.
Migration of the femur after GRA altered the anatomical course of the femoral nerve into the quadriceps muscle group. Stress occurred primarily to the lateral division of the femoral nerve that supplies the vastus lateralis, tensor vastus intermedius, lateral area of the vastus intermedius, and rectus femoris. Partial femoral nerve palsy is likely an additional cause for poor functional results following GRA. The small study group size and its’ cadaveric nature are potential limitations to the applicability in vivo, and there is a lack of prior studies on femoral nerve palsies. Nevertheless, our hypothesis was confirmed in our patient with bilateral GRA. Future investigations in Girdlestone patients with long migration distances or high axial stress should include neurologic and radiological monitoring and follow-up to gain further knowledge about the altered anatomy in a GRA.
| 1. | Schröder J, Saris D, Besselaar PP, Marti RK. Comparison of the results of the Girdlestone pseudarthrosis with reimplantation of a total hip replacement. Int Orthop. 1998;22:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Malcolm TL, Gad BV, Elsharkawy KA, Higuera CA. Complication, Survival, and Reoperation Rates Following Girdlestone Resection Arthroplasty. J Arthroplasty. 2015;30:1183-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Basu I, Howes M, Jowett C, Levack B. Girdlestones excision arthroplasty: current update. Int J Surg. 2011;9:310-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Sharma H, De Leeuw J, Rowley DI. Girdlestone resection arthroplasty following failed surgical procedures. Int Orthop. 2005;29:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Castellanos J, Flores X, Llusà M, Chiriboga C, Navarro A. The Girdlestone pseudarthrosis in the treatment of infected hip replacements. Int Orthop. 1998;22:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Vincenten CM, Gosens T, van Susante JC, Somford MP. The Girdlestone situation: a historical essay. J Bone Jt Infect. 2019;4:203-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Esenwein SA, Robert K, Kollig E, Ambacher T, Kutscha-lissberg F, Muhr G. Langzeitresultate der Resektionsarthroplastik nach Girdlestone beim therapierefraktären Hüftgelenkinfekt. Chirurg. 2001;72:1336-1343. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Cordero-Ampuero J. Girdlestone procedure: when and why. Hip Int. 2012;22 Suppl 8:S36-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Wong TL, Kikuta S, Iwanaga J, Tubbs RS. A multiply split femoral nerve and psoas quartus muscle. Anat Cell Biol. 2019;52:208-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Moore AE, Stringer MD. Iatrogenic femoral nerve injury: a systematic review. Surg Radiol Anat. 2011;33:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Al-Ajmi A, Rousseff RT, Khuraibet AJ. Iatrogenic femoral neuropathy: two cases and literature update. J Clin Neuromuscul Dis. 2010;12:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Tubbs RS, Shoja MM, Loukas M. Bergman's Comprehensive Encyclopedia of Human Anatomic Variation. 1st ed. Wiley-Blackwell, 2016. [DOI] [Full Text] |
| 14. | Grob K, Ackland T, Kuster MS, Manestar M, Filgueira L. A newly discovered muscle: The tensor of the vastus intermedius. Clin Anat. 2016;29:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Refai NA, Black AC, Tadi P. Anatomy, Bony Pelvis and Lower Limb: Thigh Femoral Nerve. 2023 Aug 22. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 16. | Schünke M, Schulte E, Schumacher U, Voll M, Wesker KH. Prometheus Allgemeine Anatomie und Bewegungssystem. Stuttgart: Georg Thieme Verlag KG, 2018. [DOI] [Full Text] |
| 17. | Rittmeister ME, Manthei L, Hailer NP. Prosthetic replacement in secondary Girdlestone arthroplasty has an unpredictable outcome. Int Orthop. 2005;29:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Thiel W. Die Konservierung ganzer Leichen in natürlichen Farben. Ann Anat. 1992;174:185-195. [RCA] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 548] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 19. | Kennon R, Keggi J, Zatorski LE, Keggi KJ. Anterior approach for total hip arthroplasty: beyond the minimally invasive technique. J Bone Joint Surg Am. 2004;86-A Suppl 2:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | de Laat EA, van der List JJ, van Horn JR, Slooff TJ. Girdlestone's pseudarthrosis after removal of a total hip prosthesis; a retrospective study of 40 patients. Acta Orthop Belg. 1991;57:109-113. [PubMed] |
| 21. | Sharma H, Kakar R. Outcome of Girdlestone's resection arthroplasty following complications of proximal femoral fractures. Acta Orthop Belg. 2006;72:555-559. [PubMed] |
| 22. | Vincenten CM, Den Oudsten BL, Bos PK, Bolder SBT, Gosens T. Quality of life and health status after Girdlestone resection arthroplasty in patients with an infected total hip prosthesis. J Bone Jt Infect. 2019;4:10-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Kantor GS, Osterkamp JA, Dorr LD, Fischer D, Perry J, Conaty JP. Resection arthroplasty following infected total hip replacement arthroplasty. J Arthroplasty. 1986;1:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Grauer JD, Amstutz HC, Oʼcarroll PF, Dorey FJ. Resection arthroplasty of the hip. J Bone Jt Surg. 1989;71:669-678. [DOI] [Full Text] |