Published online Nov 18, 2024. doi: 10.5312/wjo.v15.i11.1015
Revised: September 22, 2024
Accepted: October 10, 2024
Published online: November 18, 2024
Processing time: 156 Days and 12.7 Hours
Necrotizing fasciitis (NF) of the upper extremities is a severe surgical pathology, and the incidence of this disease has been steadily increasing in recent decades. Surgical treatment is accompanied by the formation of extensive wounds, which can be treated with significant difficulties. In recent years, negative pressure wound therapy (NPWT) has proven to be highly effective. It is also promising for the treatment of NF.
To explore the effectiveness of NPWT in the treatment of NF of the upper ex
The results of the treatment of 36 patients with NF of the upper extremities in two groups (NPWT group and control group; 2022−2023) were retrospectively an
Most patients experienced a significant treatment delay [4 d, interquartile range (IQR): 2–7 d], which led to the spread of the pathological process to the forearm and shoulder. The most common pathogens were Staphylococcus aureus (14; 38.9%) and Streptococcus pyogenes (22; 61.1%). The average number of debridements per patient was 5 (IQR: 3–7), with no difference between groups. The average time to prepare wounds for surgical closure was 11 ±
The predominant isolation of Staphylococcus aureus and/or Streptococcus pyogenes from the lesions allowed us to classify these patients as NF type II. Multiple debridement procedures have become a feature of this disease treatment. The use of NPWT has significantly reduced the time required to prepare wounds for surgical closure. Early closure of wounds allows for more frequent use of local tissue repair, which ensures better results. NPWT is a highly effective way to prepare wounds for early surgical closure in patients with upper extremity NF.
Core Tip: Negative pressure wound therapy, performed after full surgical debridement for necrotizing fasciitis of the upper extremities, significantly reduces the duration of postnecrectomy wound preparation for surgical closure, thereby improving patient treatment outcomes.
- Citation: Lipatov KV, Asatryan A, Melkonyan G, Kazantcev AD, Solov’eva EI, Krivikhin DV, Gorbacheva IV, Cherkasov UE. Effectiveness of negative pressure wound therapy in complex surgical treatment of necrotizing fasciitis of the upper limb. World J Orthop 2024; 15(11): 1015-1022
- URL: https://www.wjgnet.com/2218-5836/full/v15/i11/1015.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i11.1015
Necrotizing fasciitis (NF) was considered a fairly rare disease, and publications devoted to its diagnosis and treatment have rarely included more than 10 clinical cases. In recent years, the situation has changed significantly. The frequency of this pathology has increased, and the severity of its course, high mortality rate, and significant material costs make the search for effective treatment methods urgent[1,2].
Among all NF involvements, those in the upper limb account for 6%−27%, and the average in-hospital mortality/30-d mortality may range between 20% and 40%[3]. According to the classification of Choueka and De Tolla (2020), NF type II is the most common when it affects the extremities[4]. The causative agents of infection are Staphylococcus aureus and Streptococcus pyogenes, both as monoinfections and in combination with each other[5]. The disease usually develops after minor skin injuries or even after blunt trauma to soft tissues[6]. NF type II is more common in healthy people of working age, but cases of its occurrence are not uncommon in patients suffering from diabetes mellitus, drug addiction, or who is taking corticosteroid hormones[7]. Certain difficulties in diagnosing the disease at the initial stage can lead to untimely surgical intervention and extension of inflammation to surrounding tissues[8,9]. In these cases, as a result of delayed surgical treatment, extensive wound defects are formed, the further treatment of which can present significant difficulties[10]. This disease is characterized by the formation of secondary necrosis, which determines the need for repeated de
Negative pressure wound therapy (NPWT) has proven to be highly effective and is widely used for treating purulent wounds of various origins and locations. NPWT (syn. vacuum-assisted closure–VAC) offers many benefits: Wound isolation, reduced edema, increased tissue perfusion, and reduced bacterial load[13,14]. All of these factors contribute to subsequent reconstructive surgery. Using VAC reduces the absorption of toxins and mitigates pain. Less frequent dressings not only have a positive effect on the patient’s psycho-emotional state, but also have a significant economic effect, reducing the amount of material used in dressings and the labor costs of medical personnel. This is especially important for extensive wounds accompanying NF[15]. The effective use of NPWT requires strict adherence to a number of requirements and restrictions. Preliminary removal of the main mass of necrotic and nonviable tissue is considered a mandatory condition for the utilization of NPWT. It is also necessary to take into account that a vacuum dressing applied immediately after surgical treatment can provoke bleeding[14].
The choice of plastic wound closure method for the treatment of NF of the upper extremity is influenced by a number of factors. The most significant factors are: The size and location of the defect, the plastic reserves of the surrounding soft tissues, and the age and condition of the patient[11,13]. Additionally, the professional skills of surgeons should not be underestimated. When closing wound defects in the area of the upper limb, full-thickness skin grafting (with local tissue or displaced flaps–free or pedicled) is a priority[13]. Unfortunately, with significant skin loss, split-thickness skin grafting often becomes the only possible method[15].
The study was carried out in a single clinical center. The results of the examination and treatment of 36 patients with NF of the upper extremities were retrospectively analyzed.
Type of sampling: Non-repetitive subjects were consecutively recruited.
Inclusion criteria: NF of the upper limb.
Exclusion criteria: Extension of inflammation to the trunk, diabetes mellitus, intake of corticosteroid hormones, pre
All patients were divided into two groups depending on the characteristics of the local treatment of the wounds after surgical treatment: NPWT group and control group. In the NPWT group (18 patients), NPWT (120 mmHg; constant mode) was used. The number of vacuum-assisted dressings used in patients ranged from 1 to 3, depending on the dynamics of the wound process. The duration of fixation of one bandage was up to 2−3 d. In the control group (18 pa
The following indicators were analyzed and compared in the groups: The treatment delay, the prevalence of inflammation, the microbial landscape, the number of debridements, the duration of wound preparation for surgical closure, and the nature of skin plastic surgery.
The choice of method for surgical closure of wounds was determined by the size, location, and plastic reserves of the surrounding skin. To assess the severity of the general inflammatory reaction of the body, the level of leukocytes in the peripheral blood and the concentrations of C-reactive protein and procalcitonin in the plasma were studied.
The local institutional review board approved the use of patient data for research purposes prior to reviewing the data, and this study was deemed exempt from continued review.
Informed consent was not required due to the retrospective nature of this study. All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment. All procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statistical processing was carried out by collecting and grouping factual material, with the median and interquartile range (IQR) calculated. The arithmetic mean and standard deviation (SD) were calculated to estimate the average duration of wound preparation for surgical closure. The normality of the distribution was determined using the Shapiro-Wilk normality test. The significance of differences in nonparametric indicators was determined using the Mann-Whitney U test. Differences in indicators at P < 0.05 were considered significant. The study was reviewed by our biostatistical expert.
The age of the patients did not significantly differ between the two groups. The median age was 50 years (IQR: 39−67 years). Men predominated. The spread of the pathological process to the elbow joint was observed in 11 (61.1%) patients in the NPWT group and in 10 (55.6%) in the control group. The majority of patients in both groups were characterized by delays in seeking medical help. The associated treatment delay was not significantly different between groups (P = 0.6) and was 4 d (IQR: 3–7 d).
The average number of debridements per patient was 5 (IQR: 3–7), and there was no significant difference between the groups (P = 0.94). The average time to prepare wounds for surgical closure in the NPWT group was 11 ± 4 d, which was significantly less than that of the control group (29 ± 10 d) (P = 0.00001).
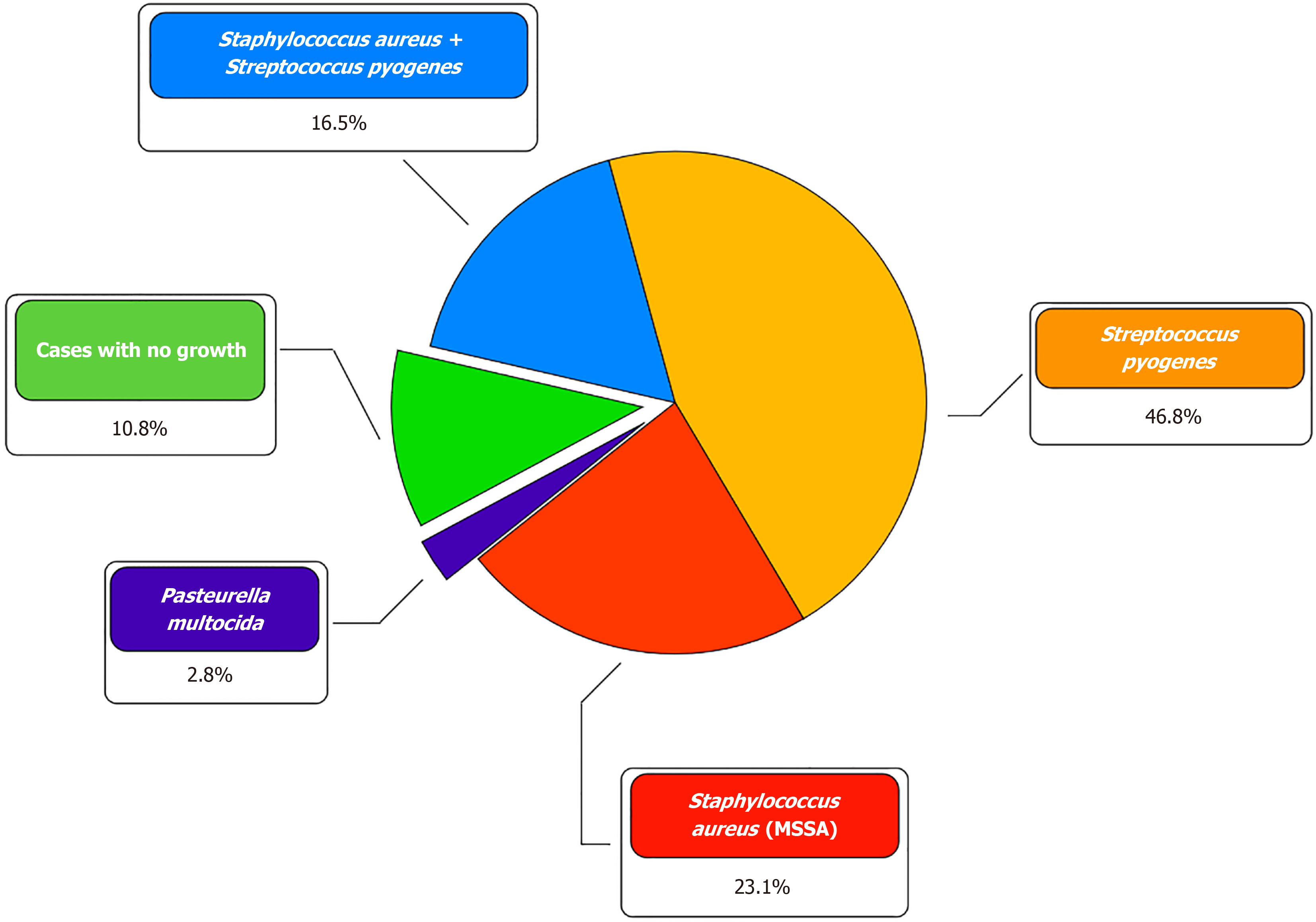
During microbiological examination, in the vast majority of cases, Staphylococcus aureus (14; 38.9%) and Streptococcus pyogenes (22; 61.1%) were isolated, both in the form of a monoculture (24; 66.7%) and in association with each other (6; 16.7%). In 1 (2.7%) patient, the causative agent of NF was Pasteurella multocida. In 4 (11.1%) patients, it was not possible to identify the pathogen (Figure 1). Initial empirical antibiotic therapy did not differ between the two groups and included intravenous administration of broad-spectrum drugs, mainly penicillins and cephalosporins. Based on the results of a microbiological study of wound exudate, antibacterial therapy was adjusted considering the sensitivity of the bacterial flora. The overwhelming majority of patients with NF of the upper limb had pronounced leukocytosis [NPWT group: 21 × 109/L (IQR: 18−27 × 109/L); control group: 22 × 109/L (IQR: 17−27 × 109)] and an increase in the concentration of C-reactive protein [NPWT group: 103 mg/L (IQR: 49−231 mg/L); control group: 222 mg/L (IQR: 180−334). Procalcitonin levels were moderately increased in the two groups [NPWT group: 0.69 ng/mL (IQR: 0.5−1.1 ng/mL); control group:
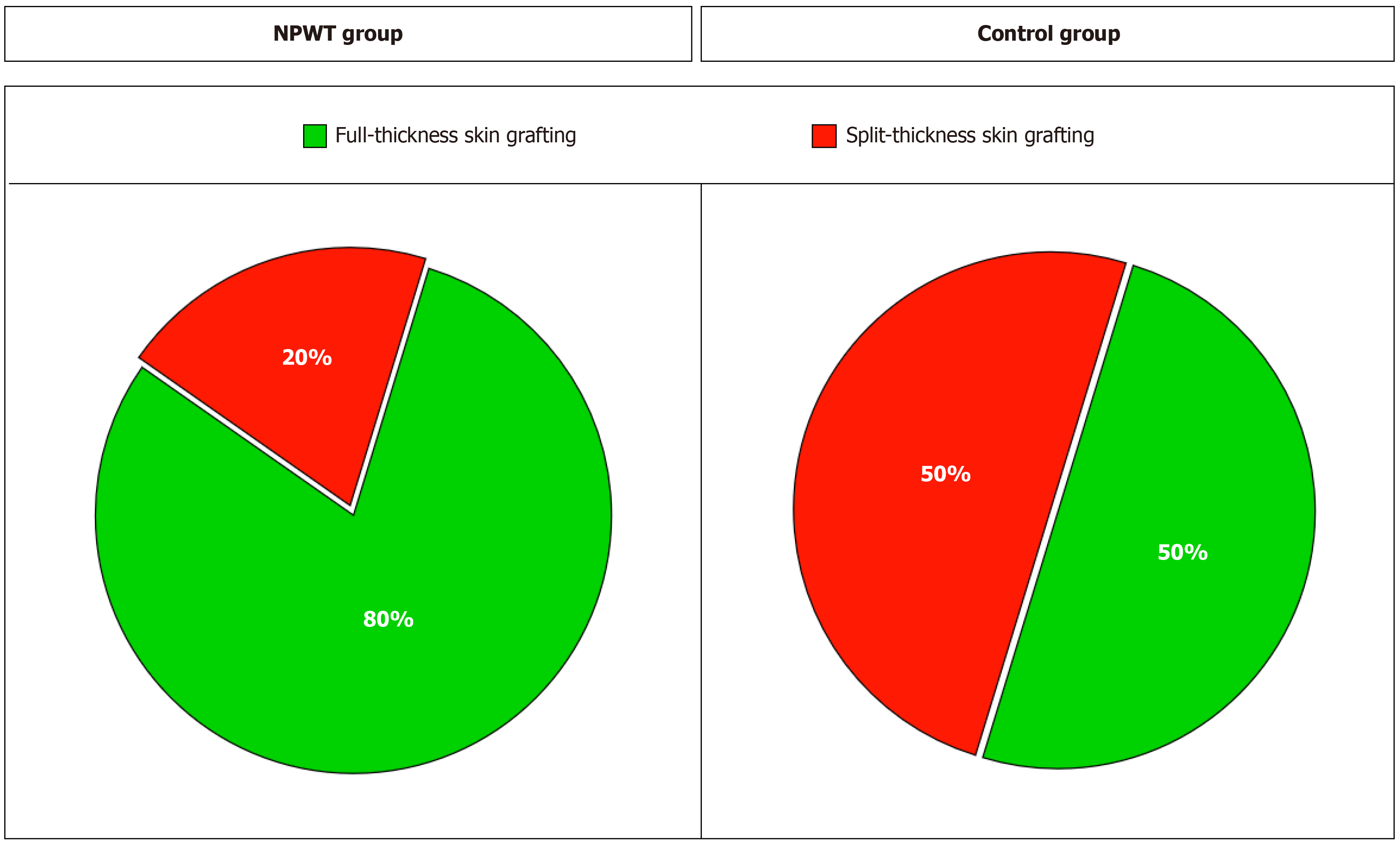
Surgical wound closure was performed in 15 (83.3%) patients in the NPWT group and in 8 (44.4%) in the control group. In the NPWT group, wounds were closed with local tissue in 12 (80%) patients and in 3 (20%) patients when a split-thickness skin graft was used. In the control group, full-thickness skin was used in 4 (50%) patients (Figure 2). In 2 patients, plastic surgery was performed with local tissues, and in 2 patients, plastic surgery was performed with a free vascularized fasciocutaneous flap from the groin area. Split-thickness grafting was performed in 4 (50%) patients (Supplementary Table 1).
In-hospital mortality rate of the patients with NF of the upper extremities was 11.1% (4 patients), and NF was not associated with the method of wound treatment.
A significant delay in treatment in most patients led to the spread of the pathological process. Moreover, in more than one third of patients, inflammation involved the entire upper limb. The severity of the disease and systemic inflammatory response syndrome was indicated by a sharp increase in the levels of leukocytes in the blood and C-reactive protein in the plasma.
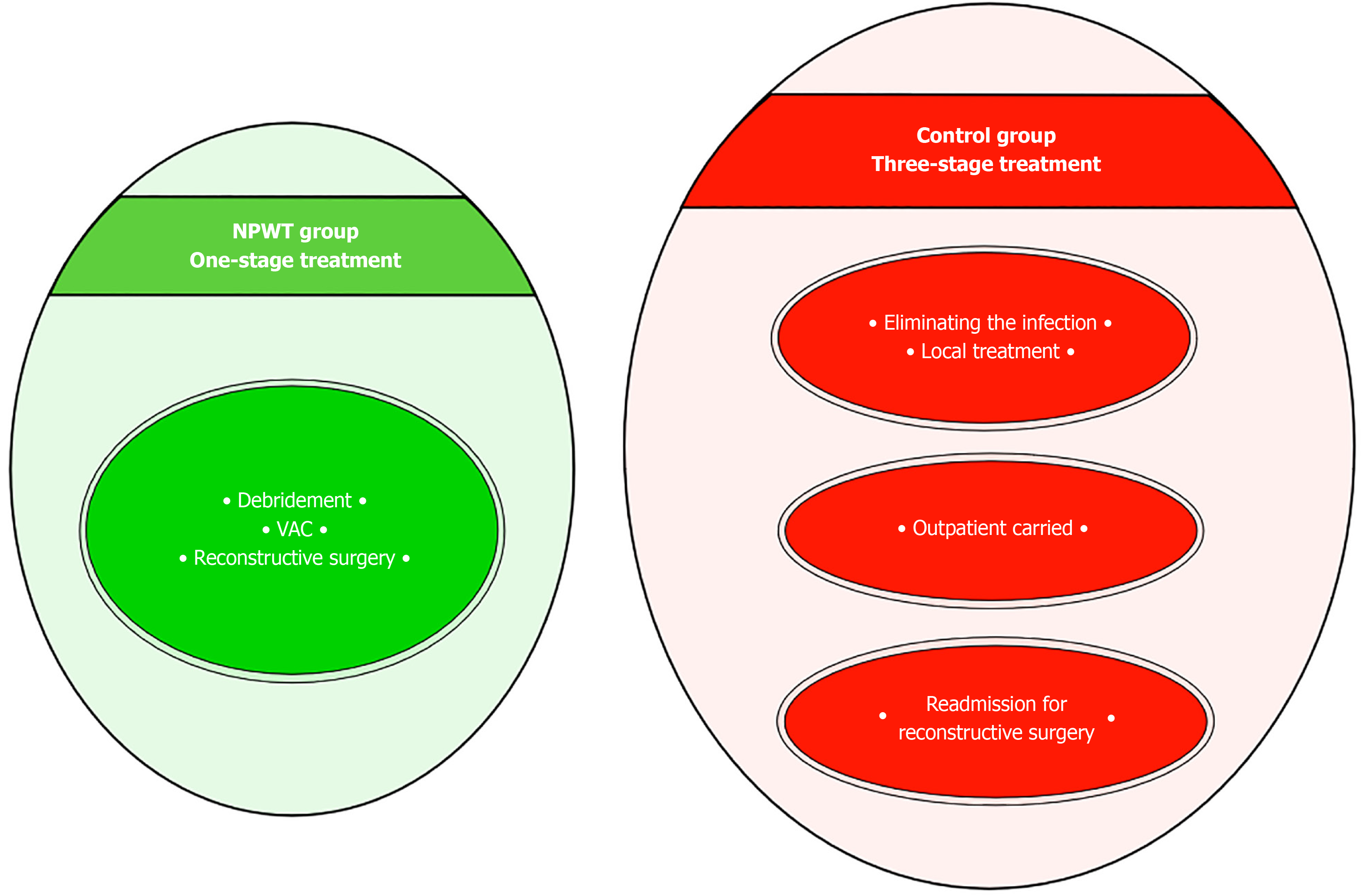
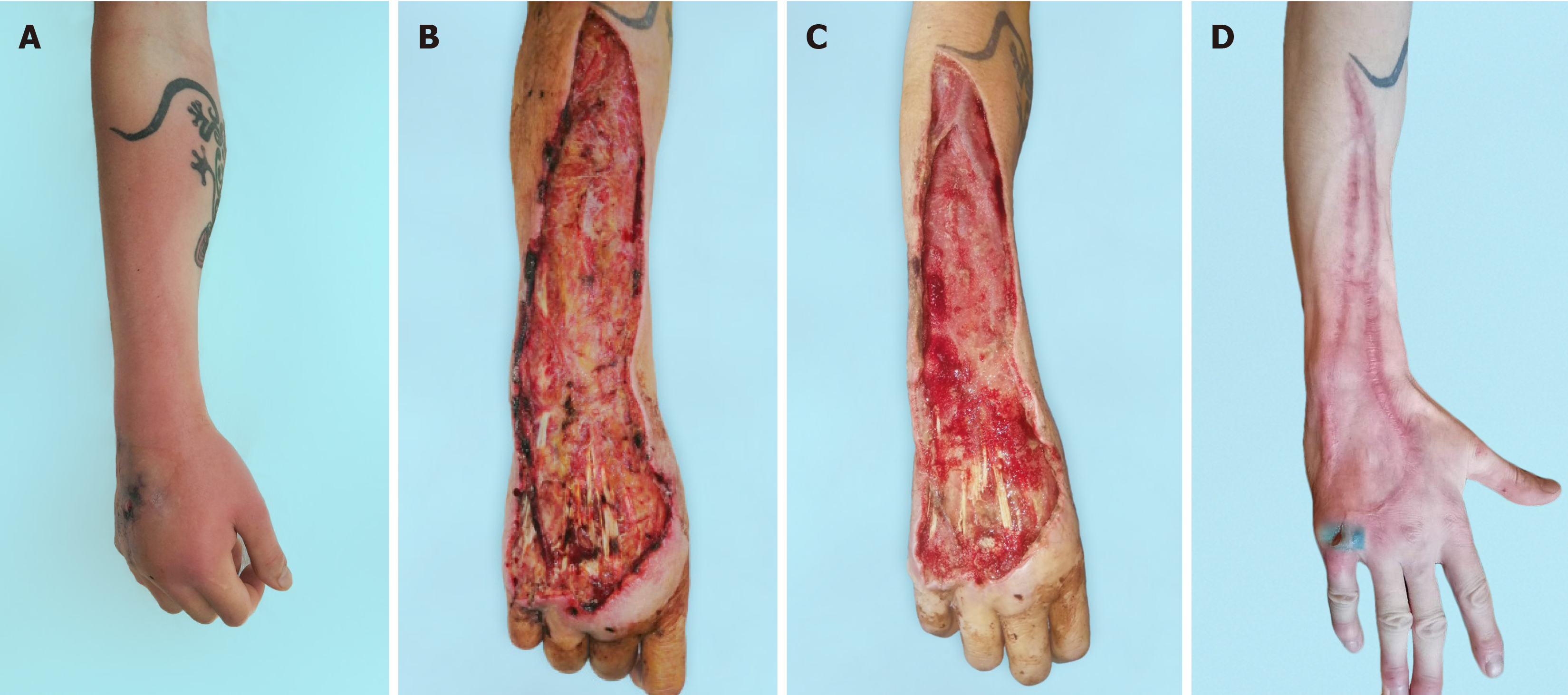
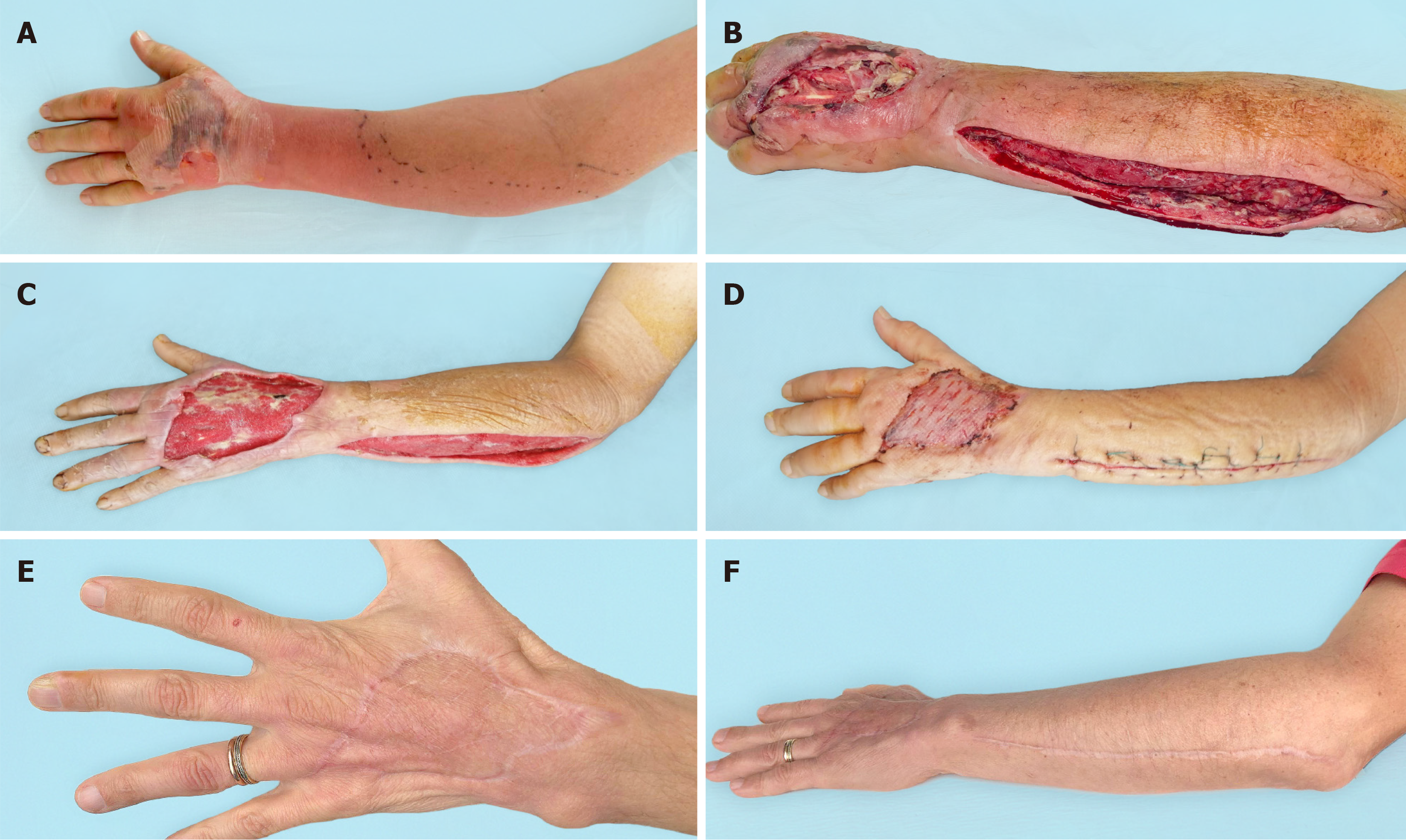
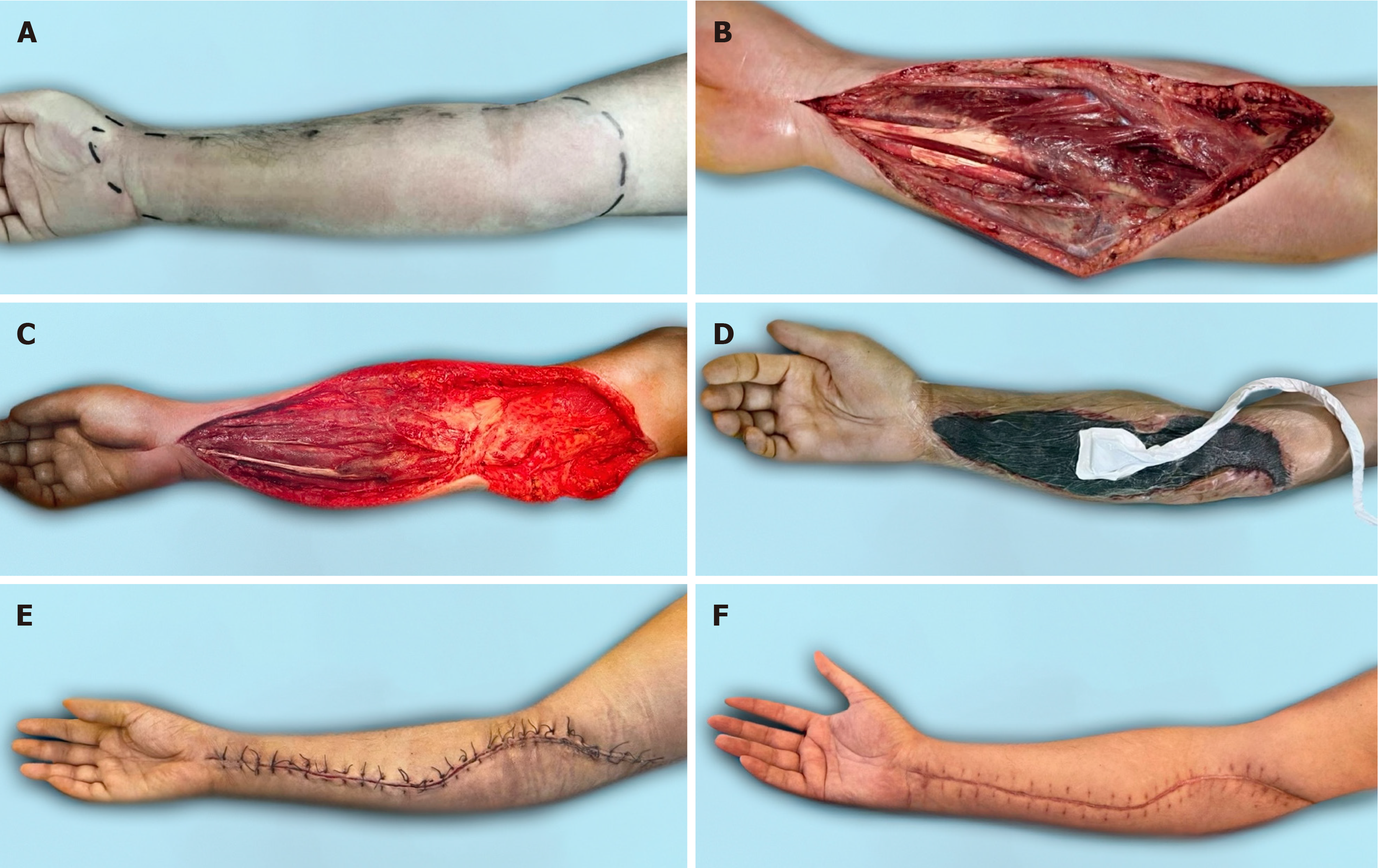
The vast majority of microflora species identified were associated with Staphylococcus aureus and Streptococcus pyogenes, which allowed these patients to be classified as NF type II[4]. The typical approach to surgical treatment of this disease has become multiple rounds of debridement, which creates conditions not only for eliminating the infection but also for the effective use of the NPWT method. The use of vacuum-assisted dressings has made it possible to significantly reduce the time required to prepare wounds for surgical closure[3]. As a result, there was a change in treatment tactics. In the NPWT group, one-stage inpatient treatment was used, which made it possible to eliminate the infection and close the wounds. After this, the patient was sent for rehabilitation. In the control group, a three-stage treatment option had to be used. At the first inpatient stage, the infection was eliminated. In the second stage, outpatient, local treatment of the wound was carried out until it was ready for surgical closure. In the third stage, stationary, skin grafting was performed (Figure 3). In addition to the difference in the timing of preparing wounds for plastic surgery, the difference in the range of surgical interventions also attracts attention. Early wound closure in the NPWT group allowed for more frequent use of local tissue, which provided better functional and cosmetic results. Delayed wound closure in the control group occurred against the background of a decrease in the plastic reserves of the surrounding skin and the scarring process[7]. The result has been an increase in the number of split skin grafts with poorer results (Figure 4, Figure 5 and Figure 6). The use of NPWT had no effect on the level of hospital mortality, which complies with data from other researchers[14].
NF of the upper extremities is a serious disease, for which timely and complete debridement is important. NPWT is an effective method for the local treatment of postnecrectomy wounds and can significantly reduce the duration of their preparation for surgical closure. Reaching a better cosmetic result is extremely important for the aesthetic appearance of the upper limb.
| 1. | Chen LL, Fasolka B, Treacy C. Necrotizing fasciitis: A comprehensive review. Nursing. 2020;50:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 2. | Nawijn F, Verhiel SHWL, Lunn KN, Eberlin KR, Hietbrink F, Chen NC. Factors Associated with Mortality and Amputation Caused by Necrotizing Soft Tissue Infections of the Upper Extremity: A Retrospective Cohort Study. World J Surg. 2020;44:730-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014;51:344-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 4. | Choueka J, De Tolla JE. Necrotizing Infections of the Hand and Wrist: Diagnosis and Treatment Options. J Am Acad Orthop Surg. 2020;28:e55-e63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Stevens DL, Bryant AE. Necrotizing Soft-Tissue Infections. N Engl J Med. 2017;377:2253-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 435] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 6. | Tessier JM, Sanders J, Sartelli M, Ulrych J, De Simone B, Grabowski J, Buckman S, Duane TM. Necrotizing Soft Tissue Infections: A Focused Review of Pathophysiology, Diagnosis, Operative Management, Antimicrobial Therapy, and Pediatrics. Surg Infect (Larchmt). 2020;21:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Melillo A, Addagatla K, Jarrett NJ. Necrotizing Soft Tissue Infections of the Upper Extremity. Hand Clin. 2020;36:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | El-Menyar A, Asim M, Mudali IN, Mekkodathil A, Latifi R, Al-Thani H. The laboratory risk indicator for necrotizing fasciitis (LRINEC) scoring: the diagnostic and potential prognostic role. Scand J Trauma Resusc Emerg Med. 2017;25:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Ditsios K, Chitas K, Christidis P, Charatsis K, Katsimentzas T, Papadopoulos P. Necrotizing Fasciitis of the Upper Extremity - A Review. Orthop Rev (Pavia). 2022;14:35320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Gawaziuk JP, Liu T, Sigurdson L, Buchel E, Hayakawa TEJ, Shiga S, Logsetty S. Free tissue transfer for necrotizing fasciitis reconstruction: A case series. Burns. 2017;43:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 959] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 12. | Corona PS, Erimeiku F, Reverté-Vinaixa MM, Soldado F, Amat C, Carrera L. Necrotising fasciitis of the extremities: implementation of new management technologies. Injury. 2016;47 Suppl 3:S66-S71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Lee JY, Jung H, Kwon H, Jung SN. Extended negative pressure wound therapy-assisted dermatotraction for the closure of large open fasciotomy wounds in necrotizing fasciitis patients. World J Emerg Surg. 2014;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Zhang R, Zhang Y, Hou L, Yan C. Vacuum-assisted closure versus conventional dressing in necrotizing fasciitis: a systematic review and meta-analysis. J Orthop Surg Res. 2023;18:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Reference Citation Analysis (0)] |
| 15. | Yin Y, Zhang R, Li S, Guo J, Hou Z, Zhang Y. Negative-pressure therapy versus conventional therapy on split-thickness skin graft: A systematic review and meta-analysis. Int J Surg. 2018;50:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |