Published online Sep 18, 2023. doi: 10.5312/wjo.v14.i9.707
Peer-review started: April 13, 2023
First decision: July 4, 2023
Revised: August 3, 2023
Accepted: August 21, 2023
Article in press: August 21, 2023
Published online: September 18, 2023
Processing time: 153 Days and 20.2 Hours
Plantar fasciitis (PF) affects around 10% of the population. Prefabricated orthotics with arch support has been shown to provide symptom relief in PF by decreasing the repetitive stress sustained by the plantar fascia. However, prefabricated ortho
To compare the combined use of prefabricated orthotics and orthotic sandals vs the sole use of prefabricated orthotics in the treatment of PF.
98 participants with PF were randomised into two groups. The intervention group received the Aetrex L420 Compete orthotics and the Aetrex L3000 Maui Flips (orthotic sandals), whilst the control group received the Aetrex L420 Compete orthotics only. Foot pain was assessed both by the numerical rating scale (NRS) and the pain sub-scale of the foot health status questionnaire (FHSQ). Foot func
Foot pain scores measured both by NRS and FHSQ pain sub-scale showed statistically significant reductions in foot pain in both groups (P < 0.05) at six months. Both groups also reported statistically significant improvements (P < 0.05) in function as measured by the FHSQ function subscale and improvement of symptoms as measured by the GROC scale. Between-group analysis showed that the intervention group with the combined use of orthotics and orthotic sandals scored better on all four outcome measures as compared to the control group with the sole use of orthotics. However, the between-group analysis only reached statistical significance on the NRS pain score (P < 0.05).
Combined use of prefabricated orthotics and orthotic sandals provides a greater decrease in foot pain and im
Core Tip: Plantar fasciitis (PF) is a common cause of heel pain and affects 10% of the population. Prefabricated orthotics provides relief of symptoms by supporting the arch but can only be used with shoes. Using both prefabricated orthotics and orthotic sandals can extend the period of support. This study finds that the combined use of Aetrex L420 orthotics and Aetrex L3000 orthotic sandals and the sole use of Aetrex L420 orthotics provide statistically significant decreases in foot pain and improved foot function in PF. The effect was greater when both the orthotic and orthotic sandals were used in combination.
- Citation: Amoako-Tawiah P, Love H, Chacko Madathilethu J, LaCourse J, Fortune AE, Sims JMG, Ampat G. Use of orthotics with orthotic sandals versus the sole use of orthotics for plantar fasciitis: Randomised controlled trial. World J Orthop 2023; 14(9): 707-719
- URL: https://www.wjgnet.com/2218-5836/full/v14/i9/707.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i9.707
Plantar fasciitis (PF) is the most common cause of heel pain, affecting 10% of the population[1]. Despite the name “fasciitis”, which suggests inflammatory causes, the condition is indicated to result from degeneration of the plantar fascia. Histological findings typically reveal degenerative changes such as localised fibrosis, matrix calcification, collagen necrosis and angiofibroblastic hyperplasia. Hence, the term plantar fasciopathy may be preferred[2]. PF is most commonly found in individuals between 40 and 70 years old and is more frequent in women than in men[1,3]. Both physically active and sedentary populations can develop the condition, and risk factors consist of, among others, a recent increase in running, prolonged standing activities, tightness of the gastrocnemius, obesity, pes cavus or pes planus foot types, and the use of footwear that is either unsupportive or that alters foot kinematics[1]. Although the exact aetiology of the condition remains unclear, mechanical overload is thought to contribute to its development[4]. Studies have shown that excessive tensile forces on the fascia cause microscopic changes[5]. Disparity in leg length and tightness of the gastrocnemius contribute to increased tensile loading of the fascia, which heightens the pressure on the longitudinal arch[6]. This may result in microscopic tears and degeneration in the fascia. Symptoms include sharp pain following palpation of the medial plantar calcaneal region and heel pain during the first steps of the day or following extended periods of inactivity[4]. The resulting pain from PF can cause activity limitation and considerable disability, negatively impacting overall quality of life[7].
PF is a self-limiting condition, with most cases resolving within 6 to 18 mo[8]. However, some reports suggest that up to 49% of PF patients are symptomatic for 1.5 to 5 years following the onset of symptoms, and an estimated 2 million people worldwide will receive treatment for the condition annually[9,10]. However, there is currently no standardised treatment method for the condition, with very few studies providing high-quality analysis across different treatment modalities[11]. Furthermore, many studies use treatment methods in combination, making it difficult to determine which is the most beneficial as a stand-alone modality.
Gastrocnemius contracture is an evident contributor to PF[12], and so stretching techniques such as calf-stretching and plantar-fascia-specific-stretching are frequently prescribed. However, a systematic review of the literature found only very low-quality evidence to support their use over sham stretching[13]. Non-steroidal anti-inflammatory drugs (NSAIDs) are frequently used in the treatment of PF. Long-term use of NSAIDs, defined as consumption of three or more times a week for more than three months[14], is not without risk of complications, including gastrointestinal bleeding, nephrotoxicity, and dependency[15]. A 2015 systematic review evaluated the literature investigating the efficacy of various taping techniques, including calcaneal and low dye taping, in the treatment of PF. The review found that taping, in general, is beneficial in treating the condition. However, all studies included in the review only assessed short-term effects, so no evidence was available regarding long-term outcomes[16]. Probe et al[17] assessed the effects of the combined use of Achilles stretching exercises, shoe recommendations and anti-inflammatory medications, with and without the addition of night splints, on PF symptoms. They found that the addition of night splints provided no statistical difference in improvements.
Orthotics have been found to decrease foot pain and increase function in PF patients compared to other non-interventional methods[18]. Such benefits from orthotics result from evened weight distribution across the plantar area and arch, shock absorption, and enhanced proprioception[19,20]. The arches of the foot are maintained not just by bony contours but also by the soft tissues that surround the structure. During weight bearing, the arches are depressed and lengthened. The repetitive lengthening whilst weight bearing has been implicated as one of the causative mechanisms of PF[21]. A contoured orthotic which provides mechanical support to the foot prevents repetitive deformation whilst weight bearing and thereby can provide symptom relief and prevent relapse of PF[21]. However, a recent systematic review and meta-analyses of orthotics for plantar heel pain have reported low-quality evidence due to the high risk of bias in studies[22]. Another systematic review and meta-analyses suggest that conclusions have been drawn from low-quality trials and future high-quality trials are required[23].
Like orthotic insoles, orthotic sandals may offer improved arch support and even weight distribution. Research has shown that the use of orthotic sandals and flip-flops with moulded footbeds can have significant effects on the symptoms of PF, including foot pain, function, and foot health[24,25]. The combined use of orthotics and orthotic sandals may be appropriate, as they are both non-invasive and complement each other. Although PF is self-limiting, it is painful and disabling during periods of activity, and hence symptom relief is essential. Unfortunately, orthotics can only be used when donning shoes. Utilising orthotic sandals in conjunction with prefabricated orthotics may enhance symptom relief in PF, as orthotic sandals can be used when donning shoes may not be practical, as whilst at home and indoors. The pain and symptoms from PF is also most severe early in the morning on waking. It may be more feasible to get into orthotic sandals rather than into shoes and orthotics as one takes the first step in the morning. To our knowledge, no research has been conducted into the effects of the combined use of prefabricated orthotics and orthotic sandals in the treatment of PF.
Hence the aim of this study is to identify if the combined use of prefabricated orthotics and orthotic sandals is superior to the use of orthotics only in the treatment of PF. The clinical significance for health providers and patients is that though PF is a self-limiting disorder, symptom relief is required during the active phase of the disease. As most cases of PF only require symptomatic treatment, it is needless to burden the overstretched healthcare system to treat this disorder. This trial hopes to identify a method by which patients can safely and reliably choose a non-invasive treatment modality to address plantar heel pain.
This study is a non-blinded randomised control trial (RCT) and was conducted over a period of 17 mo between July 2021 and November 2022 through an independent musculoskeletal clinic. The participants were volunteers who responded to social media advertisements about the study. The primary objective is to investigate whether the combined use of prefabricated orthotics and orthotic sandals vs the sole use of prefabricated orthotics is more beneficial in decreasing foot pain and increasing foot functionality in PF. The Wales Research Ethics Committee (REC) 5 provided ethical approval on April 14, 2021 by REC reference: 21/WA/0099 and IRAS project ID: 297181. Reporting of the study conforms to the CONSORT statement (CONSORT 2010 statement)[26].
A sample size calculation was performed using a target of a one-point change in the numerical rating scale (NRS) pain score (SD 1.6) (the primary outcome), with 80% power and a significance level of 5%. A 20% drop-out rate was allowed. This led to a required sample size of 52 per group, resulting in a total sample size of 104 study participants.
Participants were recruited from mainland United Kingdom including England, Scotland, Wales and Northern Ireland on a voluntary basis through social media advertisements. Potential participants were provided with a participant information sheet by post or email.
PF whose ICD-10-CM diagnosis code is M72.2[27], is diagnosed both from history and examination findings. The study was designed during the coronavirus pandemic when non-urgent and non-acute cases were diverted to telephone or online consultations. In telephone and online consultations, a physical clinical examination is not possible. To ac
Inclusion criteria required participants to be aged between 18-75 years old and to have experienced symptoms of PF for at least two months. Participants were excluded if they had received any treatment other than analgesia within the last 12 mo, had any history of foot surgery, and had any congenital or acquired foot abnormalities that would prevent the use of normal footwear. Participants were informed that they could withdraw from the study at any time without needing to give a reason. Participants were then given the opportunity to raise any questions they had about the study. Subse
Following recruitment, participants were randomised into one of two groups using an allocation ratio of 1: 1, the intervention group, or the control group. Randomisation was achieved by opening sealed, opaque envelopes, which either contained labels stating, “O and F”, denoting orthotics and flips (sandals) and corresponding to the intervention group or “O Only”, denoting orthotics only and corresponding to the control group. An individual independent of the research team then randomly selected an envelope, the contents of which assigned the participant to their group. Blinding of participants or researchers was not possible due to the nature of the study.
Participants in the intervention group received both the Aetrex L420 Compete orthotics (Figure 1) and the Aetrex L3000 Maui Flips (Figure 2) by post according to their shoe size. Participants in the control group received the Aetrex L420 Compete orthotics only. Participants were instructed to use the devices where possible for a period of 6 mo.
Basic demographic information and baseline data were collected upon recruitment. The outcome measures used in this study were foot pain by NRS and foot health status questionnaire (FHSQ), foot functionality (by FHSQ) and change in PF symptoms [by global rating of change scale (GROC)]. Data for all outcomes were collected via questionnaires upon initial recruitment, then at three weeks, six weeks, three months, and six months. Participants completed this questionnaire online at smartsurvey.co.uk or as a paper copy provided via post, depending on their preference.
The primary outcome measure was to measure the change of foot pain between baseline and 6 mo follow-up using an 11-point NRS, which ranged from zero, denoting “no pain”, and ten, denoting “extremely severe pain”. The minimal clinically important difference (MCID) on the NRS scale is a 1.7 score change of the median[28].
Foot pain was also scored along with foot functionality using the “foot pain” and “foot functionality” sub-scales of the FHSQ. For these sub-scales, 5-point likert scales ranging from “no problems, pain or limitations” to “severe problems, pain or limitations” were provided. A dedicated FHSQ programme (https://www.fhsq.org/) was then utilised to calculate an overall score between 0 and 100, depending on the participants’ answers, with 0 representing “the worst foot health” and 100 representing “the best foot health”. The MCID for the FHSQ pain subscale is 13 points and for the FHSQ function subscale is 7 points[29]. Change in symptoms was assessed using the GROC, an 11-point scale from -5 to + 5, with “-5” representing “very much worse“, “0“ representing “no change” and “+5” representing “completely recovered“.
A data monitoring committee consisting of patients, an independent doctor not part of the research team, and a medical statistician was implemented during the study to ensure that the data collected was legitimate and reliable and to observe for any significant adverse outcomes.
NRS for pain: Wilcoxon signed-rank test was used to assess the within-group statistical significance of changes from baseline to 6-mo follow-up of the NRS pain scale. The Mann-Whitney U test was used to assess the between-group statistical differences for the two groups from baseline to six months.
FHSQ pain and function sub-scales: The paired T-test was used to assess the within-group statistical significance of changes from baseline to 6-mo follow-up of the FHSQ pain and function sub-scale. The independent sample t-test was used to compare the between-group differences.
GROC: Wilcoxon signed-rank test was used to assess the within-group statistical significance of changes from 3 wk to 6-mo follow-up on the GROC scale. The Mann-Whitney U test was used to assess the between-group statistical differences for the two groups from 3 wk to six months. The statistical methods of this study were reviewed by an independent statistician from the department of data health sciences, University of Liverpool.
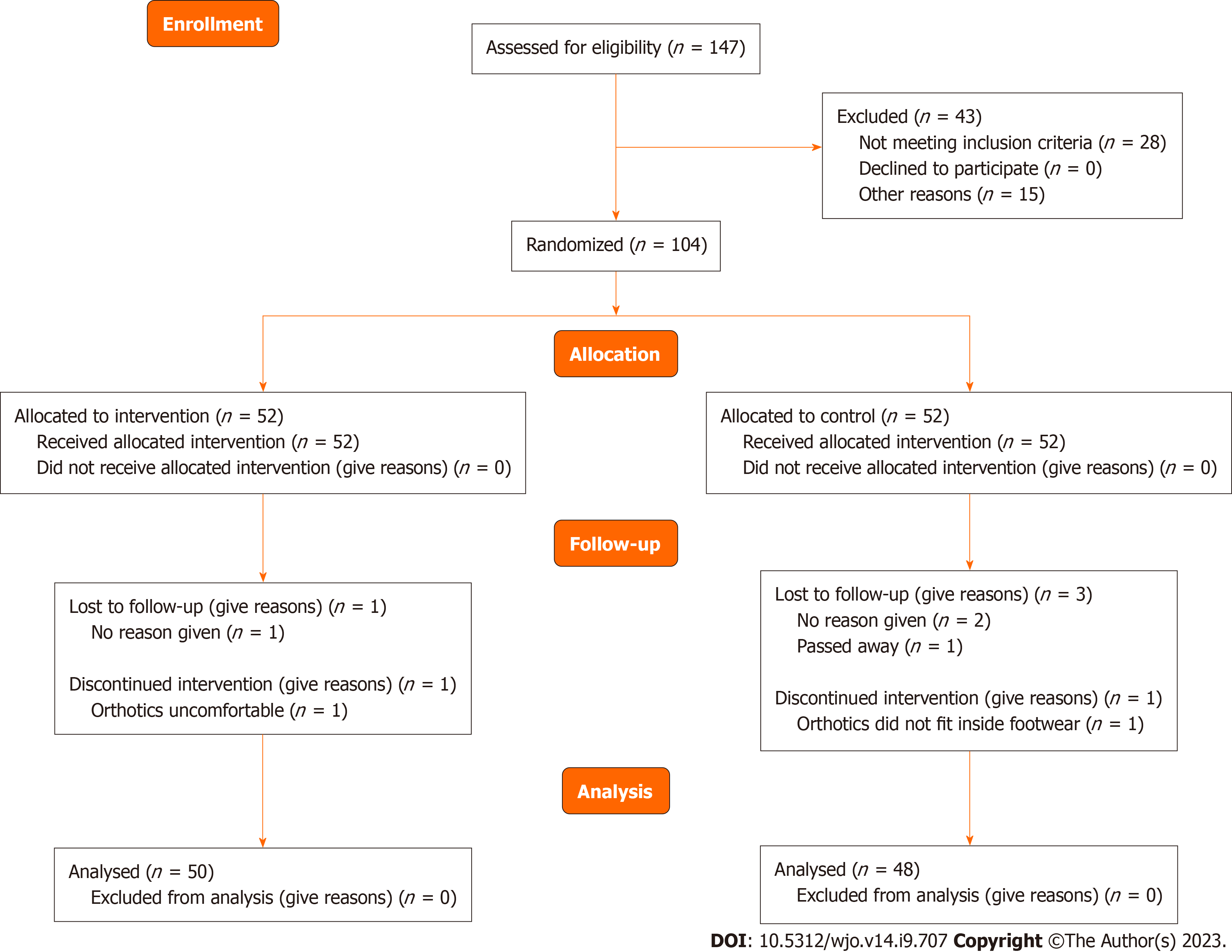
One hundred and four participants were recruited for this RCT. Of these 104 participants, six were not included in the final analysis (drop-out rate = 5.7%), leaving a total of 98 participants to be included in the analysis. In the intervention group (orthotics and orthotic sandals) 1 participant failed to respond and 1 discontinued as the orthotic was uncomfortable. In the control group 2 participants failed to respond, 1 passed away due to unrelated reasons and 1 discontinued as the orthotic did not fit in the shoe. Ninety-eight of the 104 complied with the treatment giving a compliance rate of 94.2%. Details of participant flow through the study, including the number of withdrawals and reasons, are provided in Figure 3. Fifty of the 98 participants included in the analysis were randomly allocated to the intervention group and 48 to the control group. Data collection began in July 2021 and ended in November 2022.
The baseline demographics of the participants in the intervention and control groups are shown in Table 1. The participants’ ages ranged from 29 to 71 years old (mean age = 48.81). Participants in the intervention group with the combined use of orthotics and the orthotic sandals were, on average, slightly younger (mean age = 48.44) than participants in the control group with the sole use of orthotics (mean age = 49.19). Participants in both the intervention group (94%) and the control group (91.6%) were predominantly female. Results for all outcome measures are provided in Table 2. All outcomes were collected at baseline, 3 wk, 6 wk, 3 mo and 6 mo, aside from the GROC, which was collected at all time points except baseline.
| Intervention group orthotics and orthotic sandals (%) | Control group orthotics only (%) | Both groups (%) | |
| Sex | |||
| Male | 3 (6.0%) | 4 (8.3%) | 7 (7.1%) |
| Female | 47 (94.0%) | 44 (91.7%) | 91 (92.9%) |
| Age (year) | |||
| 20-29 | 1 (2.0%) | 0 (0.0%) | 1 (1.0%) |
| 30-39 | 4 (8.0%) | 10 (20.8%) | 14 (14.3%) |
| 40-49 | 21 (42.0%) | 13 (27.1%) | 34 (34.7%) |
| 50-59 | 20 (40.0%) | 18 (37.5%) | 38 (38.8%) |
| 60-69 | 4 (8.0%) | 6 (12.5%) | 10 (10.2%) |
| 70-79 | 0 (0.0%) | 1 (2.1%) | 1 (1.0%) |
| Mean age | 48.44 | 49.19 | 48.81 |
| Total | 50 | 48 | 98 |
| Intervention group (orthotics and orthotic sandals) | Control group (orthotics only) | Between-group | |||||||||||||
| Baseline | 3 wk | 6 wk | 3 mo | 6 mo | Change | Sig.test | Baseline | 3 wk | 6 wk | 3 mo | 6 mo | Change | Sig.test | ||
| Foot pain NRS (IQR) | 7 (2) | 5 (4) | 4 (2) | 2 (3) | 1.5 (3) | -6 (3.25) | P < 0.001a | 8 (3) | 5 (3) | 4 (3) | 4 (4) | 3 (3) | -4 (4.75) | P < 0.001a | P = 0.003b |
| Foot pain FHSQ (SD) | 28.48 (16.32) | 56.50 (20.83) | 66.83 (17.23) | 74.98 (18.51) | 79.98 (15.80) | 51.49 (25.15) | P < 0.001c | 29.17 (19.70) | 49.57 (19.34) | 57.93 (20.23) | 62.79 (21.96) | 71.24 (18.98) | 42.07 (24.79) | P = 0.001c | P = 0.07d |
| Foot function FHSQ (SD) | 46.13 (21.47) | 71.5 (22.13) | 79.25 (17.83) | 82.63 (16.72) | 87.63 (14.86) | 41.50 (24.94) | P < 0.001c | 43.10 (23.68) | 65.48 (21.82) | 71.74 (23.31) | 75.56 (22.80) | 80.73 (21.95) | 37.64 (26.34) | P = | P = 0.46d |
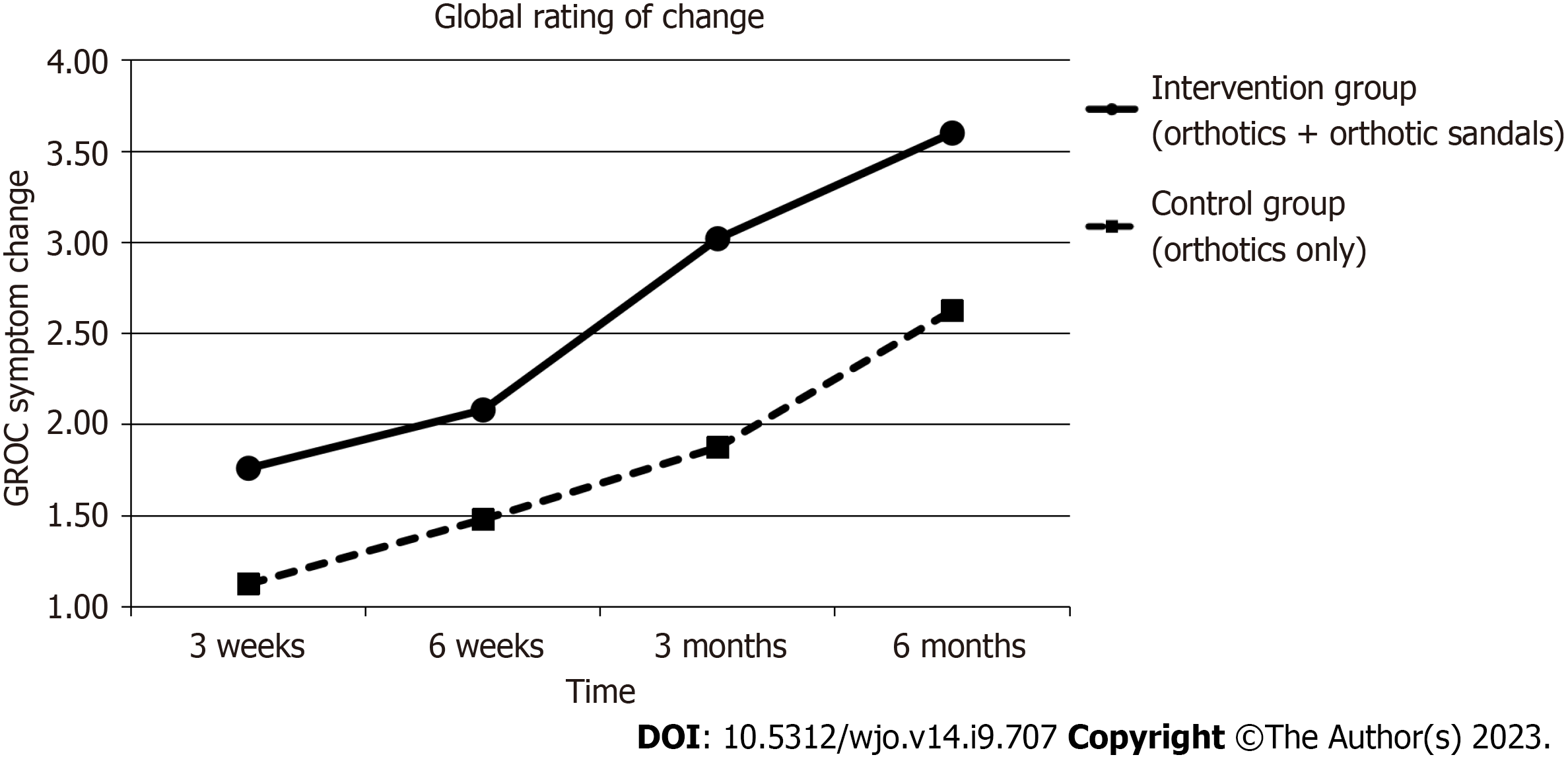
| Symptom change GROC (IQR) | 1 (2) | 2 (2) | 3 (2) | 4 (2) | 2(2) | P < 0.001a | 1 (2) | 1.5 (3) | 2 (2) | 3 (3) | 2 (3) | P < 0.001a | P = 0.93b | ||
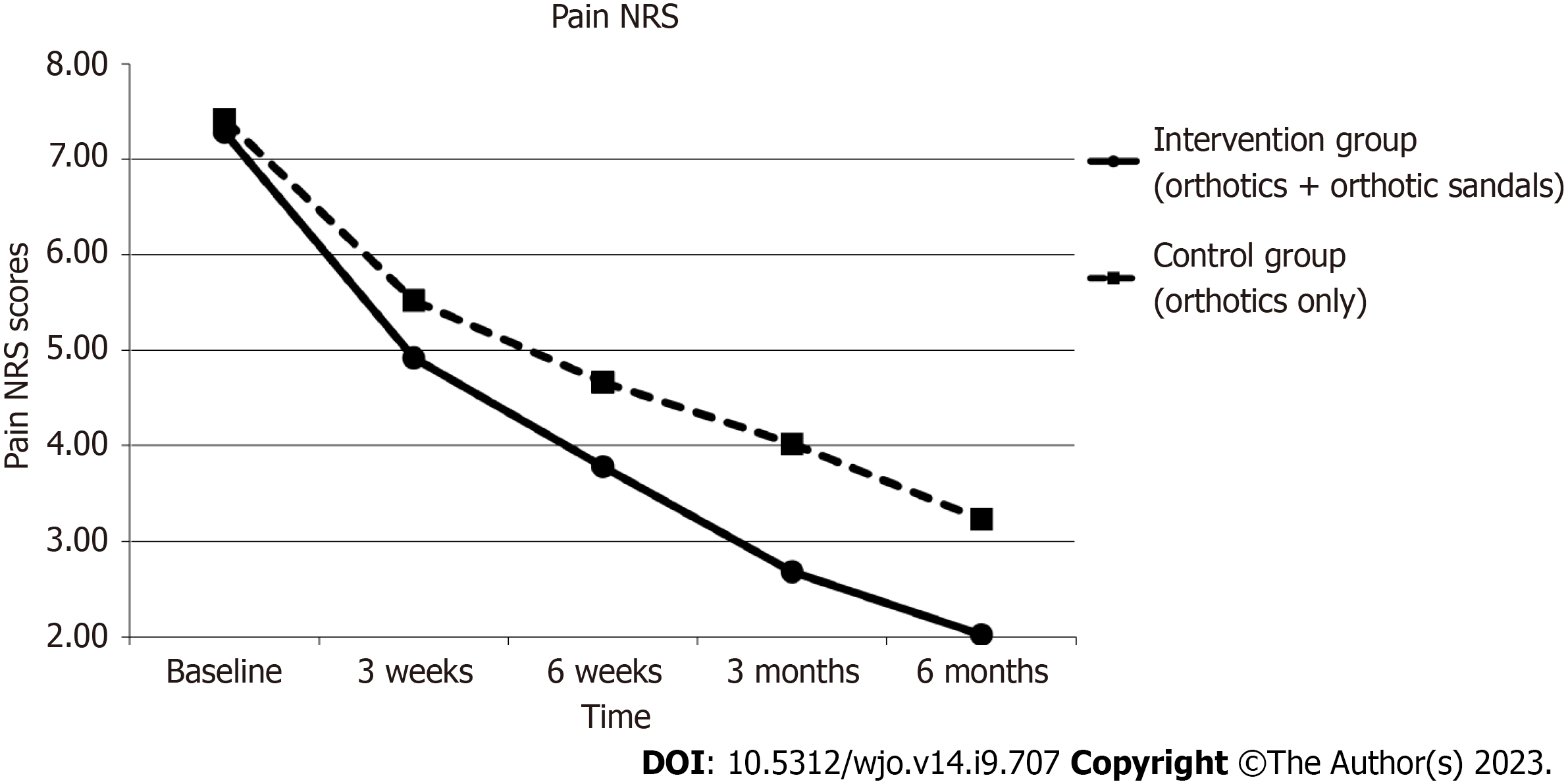
At 6 mo, there was a statistically significant improvement in foot pain by the NRS in both groups. Median change of pain was 6 [interquartile range (IQR) 3.25, P < 0.001] in the interventional group with the combined use of orthotics and orthotic sandals and 4 (IQR 4.75, P < 0.001) in the control group with the sole use of orthotics. Between-group analysis with the Mann-Whitney U test showed statistically significant improvement (P = 0.003) in the interventional group with the combined use of orthotics and orthotic sandals as compared to the sole use of orthotics.
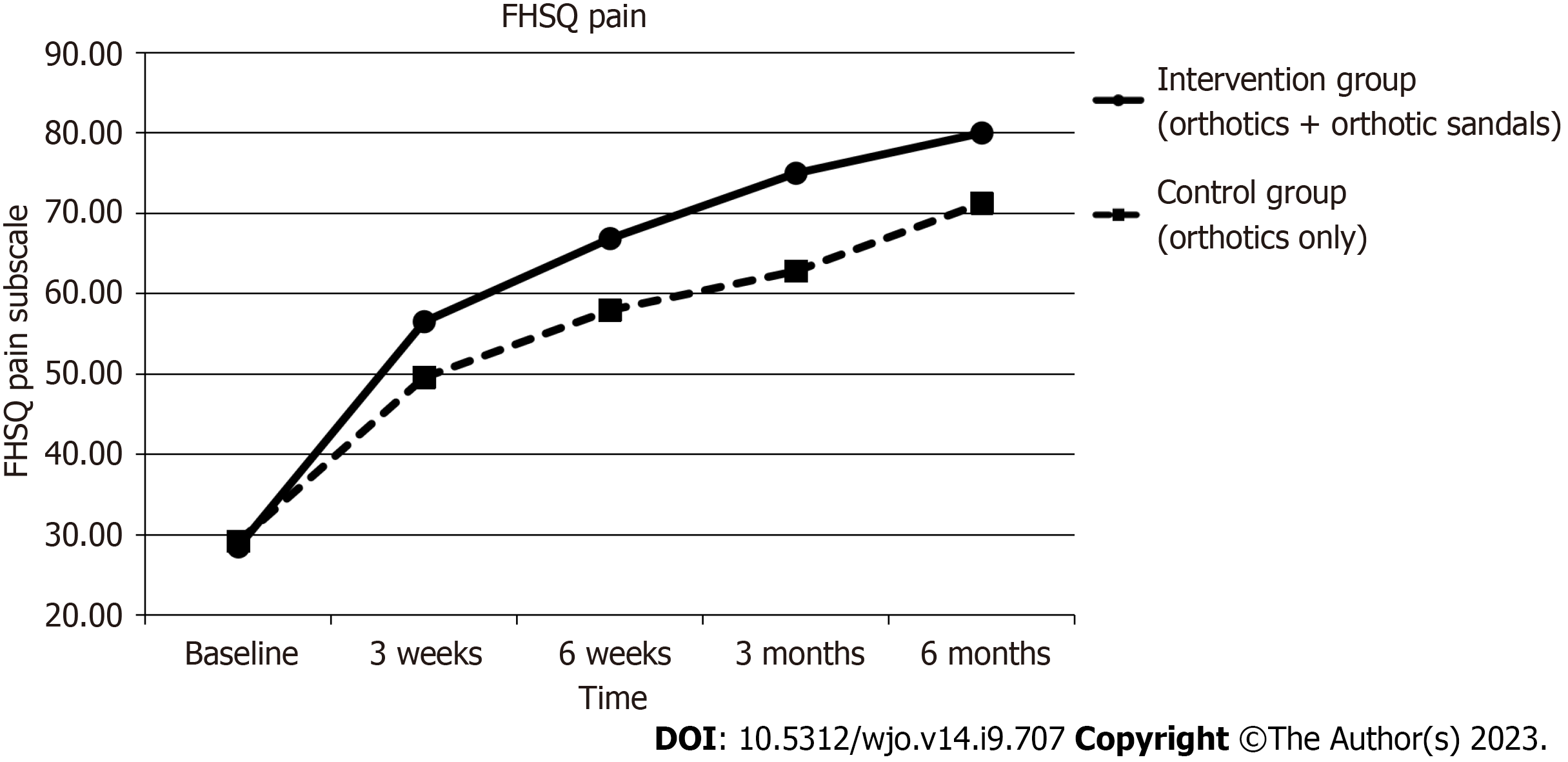
Mean pain scores on the FSHQ subscale improved significantly during the six-month period in the interventional group by 51.49 points [95% confidence interval (CI): 44.52 to 58.46, P < 0.001] and in the control group by 42.07 points (95% CI: 35.20 to 48.94, P < 0.001). Between-group analysis showed that though the mean pain score improved more in the intervention group with the combined use of orthotics and orthotic sandals as compared to the control group with the sole use of orthotics, it did not reach statistical significance (P = 0.07).
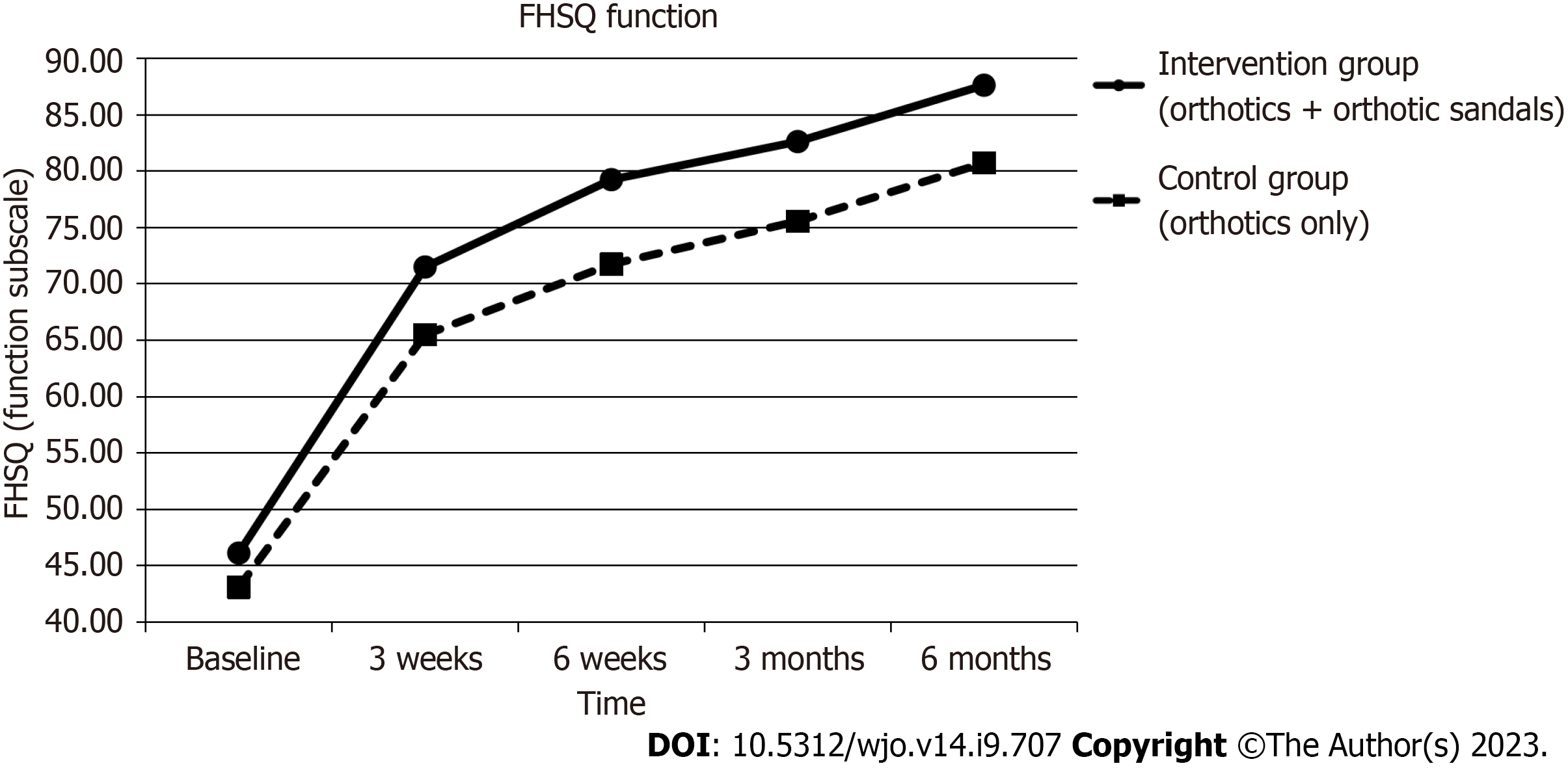
Foot functionality, measured using the function sub-scale of the FHSQ, improved during the six-month period in the interventional group by 41.50 (95% CI: 34.44 to 48.56, P < 0.001) and in the control group by 37.64 (95% CI: 30.19 to 45.09, P = 0.001). Between-group analysis showed that there was again greater improvement of function in the intervention group with the combined use of orthotics and orthotic sandals as compared to the control group with the sole use of orthotics, the improvement was not significant (P = 0.46).
The findings for the GROC scale demonstrated statistically significant improvement in symptoms over time for both groups (Median improvement in intervention group 2 (P < 0.05) vs Median improvement in control group 2 (P < 0.05). Between-group analysis showed that there was again greater improvement of symptoms in the intervention group with the combined use of orthotics and orthotic sandals as compared to the control group with the sole use of orthotics, but the improvement was not significant (P = 0.093).
Figures 4-7 show how the study outcomes varied for each group over time. Figures 4, 5 and 6 show that, for both the intervention group with the combined use of the orthotics and orthotic sandals and the control group with the sole use of orthotics, the greatest improvements in foot pain reported via NRS and FHSQ pain sub-scale and foot functionality reported via the FHSQ function sub-scale, were between baseline and 3 wk. Following the 3-wk period, there was a slower but sustained improvement in both groups. Additionally, for these three outcomes, the rate of initial improvement between baseline and week 3 was greater for the intervention group with the combined use of the orthotics and orthotic sandals than the control group with the sole use of orthotics. Figure 7 shows the median change in GROC scale scores for each group over time. At each time point that data were collected, a greater improvement in symptoms was reported by the intervention group with the combined use of the orthotics and orthotic sandals than by the control group with the sole use of orthotics.
This article presents the results from a RCT. The study investigated whether the combined use of prefabricated orthotics and orthotic sandals provided enhanced symptom relief from PF in comparison to the sole use of orthotics.
The main findings show that both the intervention group with the combined use of orthotics and orthotic sandals and the control group with the sole use of orthotics experienced significant decreases in foot pain at 6 mo as assessed via both the NRS and FHSQ pain sub-scale. Decreases in foot pain on both measures were significantly greater in the intervention group with the combined use of orthotics and orthotic sandals, as compared to the control group with the sole use of orthotics. However, between-group differences in pain scores reached statistical significance only on the NRS pain score (P < 0.05). Foot function, as assessed by the FHSQ function sub-scale, also showed statistically significant improvement in both the intervention and control groups at 6 mo. Similarly, there was greater improvement in foot function in the intervention group with the combined use of orthotics and orthotic sandals compared to the control group with the sole use of orthotics but it did not reach statistical significance. At 6 mo, the GROC showed a statistically significant improvement in each of the two groups but between-group analysis did not reach statistical significance.
Baldassin et al[30] evaluated the effectiveness of both prefabricated and customised insoles on PF in 142 symptomatic adults. The study employed the visual analogue score (VAS) and the foot function index (FFI) to compare pain and function at baseline, 4 wk, and 8 wk. Results showed that both the use of prefabricated and customised orthotics significantly reduced pain and improved function at 8 wk. However, no significant difference was found between the two groups, suggesting that the costlier customised orthotics were not superior to prefabricated orthotics.
Conversely, other studies have had mixed results. Landorf et al[7] conducted an RCT containing 136 participants who were randomised into one of three groups to receive either prefabricated foot orthotics, custom foot orthotics or sham orthotics (placebo). Like the current study, this employed the FHSQ to evaluate pain and function. Findings revealed that, at 3 mo, both prefabricated and custom orthotics significantly improved function. However, no continued significant effects were observable at 12 mo for any outcome in either group. This lack of continued significant improvement at 12 mo may have been caused by the self-limiting nature of PF, in which spontaneous resolution of symptoms may occur with the passage of time. Nevertheless, the findings did show that prefabricated orthotics are effective in the period when the symptoms of PF are potentially most severe. Furthermore, results indicate that more cost-effective prefabricated orthotics obtain similar results to the costlier custom orthotics.
Wrobel et al[18] conducted a RCT to compare the use of custom foot orthoses (CFO), prefabricated foot orthoses (PFO) and sham insoles for the treatment of PF. Outcomes measured were first-step pain, end-of-day pain, Revised FFI short form (FFI-R), and a short form health survey. Following 3 mo of orthoses/sham insole use, the results showed that both the CFO and PFO groups demonstrated significant improvements in morning and evening pain. All groups, including the sham orthotics group, also reported significant improvements in FFI-R pain and short form health survey at 3 mo.
Costa et al[31] evaluated the efficacy of flip-flop sandals, adapted with insoles, on pain and function in PF patients. Sixty-six participants were randomised to receive either a pair of adapted flip-flop sandals with custom insoles or a pair of un-adapted plain sandals. Participants were instructed to use the flip-flops for at least 4 h a day for 12 wk. Data regarding first-step pain, as assessed by VAS, and function, as assessed by the FFI, were collected at baseline and 12 wk. Results showed that the group with the adapted flip-flop sandals with custom insoles had significant improvements with first-step pain and function compared to the un-adapted plain sandals group.
In addition, Chuter et al[25] investigated the effects of sandals with a moulded footbed on pain and function in PF. Results showed that, after 12 wk of intervention, there were significant improvements in the primary outcome of foot pain, also measured using FHSQ. In further agreement with our study findings, secondary outcomes of function and pain measured using VAS also improved significantly.
Vicenzino et al[24] compared the effects of a contoured sandal, a flat flip-flop and contoured in-shoe orthotics for plantar heel pain. The study contained 50 participants who had experienced plantar heel pain for at least 4 wk. Like our study, this used a GROC scale to determine change in symptoms. The lower extremity function scale (LEFS) was also used to assess function. Findings showed that participants who had been provided with the contoured sandals were 68% more likely to experience symptom improvement using the GROC compared to those who used the flat flip-flop. The contoured sandal group was also 61% more likely to report improvements on the LEFS. No significant differences were observed between the effects of the contoured sandals and the contoured in-shoe orthotics. The findings of these investigations, along with the findings of our study, suggest that the use of orthotic sandals is effective in the treatment of PF.
In our study, the intervention group with the combined use of orthotics and orthotic sandals reported greater improvements on all measures than the control group with the sole use of orthotics. However statistical significance in between-group differences was only reached on the NRS pain scale. We hypothesise that these enhanced benefits were due to the extended periods of support to the feet, and consequent symptomatic relief, provided by the orthotic sandals. Prior to this study, the combined use of prefabricated orthotics and prefabricated orthotic sandals for PF had not been investigated. Nevertheless, studies have assessed the effects of sandals whose weight-bearing surface is contoured like an orthotic on PF as an independent intervention.
The current study investigated the long-term benefits of the intervention, with final data collection at 6 mo. In comparison, other studies only investigated short-term symptom relief, such as 8 wk[25], and 12 wk[26]. The natural history of PF is that most cases resolve spontaneously within 6 to 18 mo[8], and hence symptom relief is required during the active or symptomatic phase of the study. This study demonstrates that both the combined use of orthotics and orthotic sandals or the sole use of orthotics alone can be beneficial for relieving symptoms of PF when it is most symptomatic. In addition, both orthotics and orthotic sandals are drug-free and non-invasive modality to treat PF. Hence it has a lower cost burden and decreased long-term risk profile than other treatment strategies. The combined use of orthotics and orthotic sandals were better on all outcome measures as compared to the sole use of orthotics but between group differences only reached statistical significance on the NRS pains scales.
The findings of this study contribute to existing literature surrounding the use of orthotics and orthotic sandals in the treatment of PF. By combining the use of prefabricated orthotics and prefabricated orthotic sandals, this study has taken a novel approach. To our knowledge, no study has previously been conducted into the combined effects of prefabricated orthotics and orthotic sandals. We wish to propose that the combined use of orthotics and orthotics sandals resulted in greater improvement due to the increased time that the foot is supported.
A strength of this study is the inclusion of participants with a wide age range of 18-75 years old. This increases the generalisability of our findings to the wider population of patients with PF. The cohort recruited for this trial is similar to other studies in terms of mean age and female predominance[30,31], and reflects global prevalence[3,32].
However, as participants were recruited voluntarily through social media adverts, the sample may not be representative of the general population. This might have limited our participants to individuals who use the computer, internet and social media whilst excluding a large segment of the population that do not use digital media.
In addition, the study design was drafted in the periods of national lockdowns due to the coronavirus pandemic. It was not known for how long the national lockdowns would last and therefore the protocol and the study design were created to accommodate social distancing by introducing telephone/virtual consultation with the lead author on inclusion into the study. The telephone/virtual consultation and the lack of a physical examination could have led to both over and under-diagnosis of PF in the study population.
In this study, both groups showed significant improvements in foot pain, foot function and symptoms. Significant improvements in control groups may be associated with factors such as regression to the mean and Hawthorne effects[31]. Due to the nature of this study, blinding participants to group allocation was not possible. Therefore, participants in the intervention group may have expectations that the combined use of orthotics and orthotic sandals would improve pain and functionality. In future studies, the use of a sham orthotic placebo may be beneficial in reducing bias. However, a sham intervention should provide as little an effect as possible whilst being perceived as equally credible compared to the real intervention[33]. Hence, a sham orthotic should not provide the same mechanical benefits as the real orthotics, and yet participants should expect both to provide similar effects. This may be difficult when attempting to blind participants, as they may detect the sham as a fake, causing their results to be influenced by the nocebo effect. Mitigating this would depend greatly on the design of the sham orthotic to convince the participants that the sham they receive is credible. However, inconsistencies have been found in the construction, blinding and biomechanical validation of sham orthotics in research. Therefore, the quality and reliability of the findings from studies which utilised sham orthotics have been questioned[34]. NRS was selected as the outcome measure to score pain, and though this has been shown to be valid, reliable and appropriate when used for the assessment of pain[35], it is still subjective and therefore has the potential for bias.
In future studies, it would be valuable to collect data on how long each participant has been experiencing plantar heel pain before the study commenced. PF is a self-limiting condition that spontaneously resolves within 12 mo in 75% of cases[36]. Therefore, a natural improvement would be expected regardless of intervention. Hence, data on the duration of participants’ pre-study PF would have allowed for this to be factored into the analysis. In addition, monitoring of how often each participant wore orthotic devices would be advised in future studies to determine study compliance and reveal the effect on outcomes. This study was not designed to collect information on risk factors for PF, such as structural foot abnormalities, activity levels, high body mass index etc. Future studies may find a correlation between the prevalence of these risk factors and their influence on PF symptom control when using orthotics and orthotic sandals. The current study also did not collect data on concurrent or past treatments like physiotherapy and exercises. This could also be considered in future studies.
However, the main purpose of the study was to empower patients to choose a non-invasive and over-the-counter treatment to address PF or plantar heel pain without the need to seek professional help and thereby reduce the burden on the health care system.
The within-group results of this study indicate that both the combined use of prefabricated orthotics and orthotic sandals, as well as the sole use of prefabricated orthotics, significantly improved pain and function in PF in all the four outcome measures utilised in this study. Between-group analysis showed that the combined use of orthotics and orthotic sandals provided better benefits than the sole use of orthotics in all four outcome measures, but the improvement was statistically significant only for foot pain on the NRS scale.
Prefabricated orthotics with arch support provides symptom relief in plantar fasciitis (PF) but are only effective when shoes are worn. Hence, the foot may be left unsupported when it is impractical to wear shoes, such as in the morning or evening at home. Utilising orthotic sandals in conjunction with prefabricated orthotics may enhance symptom relief for PF patients, as they can be worn inside the home, thereby extending the period in which the foot is supported. Prefabricated orthotics and orthotic sandals have been investigated as treatment methods for PF independently, but not in combination.
PF affects around 10% of the population. The resulting pain can cause activity avoidance, disability, and reduced quality of life. However, the natural history of PF is that it resolves naturally with time. Unfortunately, it remains symptomatic during the active phase and requires intervention for pain relief and symptom improvement. As most cases of PF spontaneously resolve with the passage of time, it is needless to burden the already overburdened healthcare system to address this disorder. This trial sought to identify the superiority between two drug-free and non-invasive treatment modalities to address plantar heel pain which can be used as a self-help measure by patients.
To compare the combined use of orthotics and orthotic sandals vs the sole use of orthotics in the treatment of PF.
104 participants were randomly assigned to the intervention group, who received both prefabricated orthotics and orthotics sandals, or the control group, who received prefabricated orthotics only. Participants were instructed to use the devices as much as possible. Data were collected at baseline, three weeks, six weeks, three months, and six months. Foot pain was assessed using an 11-point numerical rating scale (NRS). Foot pain and functionality were assessed using the foot pain and foot functionality sub-scales of the foot health status questionnaire (FHSQ). The global rating of change score (GROC) was provided at three weeks, six weeks, three months and six months to assess PF symptom change. A series of Wilcoxon signed-rank tests, Mann-Whitney U tests, Paired T-tests and independent sample t-tests were performed for analysis.
Foot pain scores significantly improved in both groups, as assessed and measured by the NRS and FHSQ pain sub-scale. Significant improvements in function by the FHSQ function subscale and changes in the level of symptoms by the GROC scale were also observed in both groups. The combined use of orthotics and orthotic sandals showed superior outcomes on all four measures but only reached statistical significance on the NRS pain scales.
This study provides evidence that both the combined use of orthotics and orthotic sandals and the sole use of orthotics alone, improve pain and function significantly in PF patients. Between-group differences show that the combined use does provide a greater decrease in foot pain compared to using orthotics alone.
Though this study provides evidence that the combined use of prefabricated orthotics and orthotic sandals improves foot pain in PF patients more than the use of prefabricated orthotics alone, it was not without limitation. Hence, future research should aim to address these limitations, including collecting data on participants’ duration of PF symptoms on enrolment, and risk factors for the condition.
The researchers would like to thank all participants who took part in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: British Orthopaedic Association, 15546.
Specialty type: Orthopedics
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang L, United States; Nambi G, Saudi Arabia S-Editor: Qu XL L-Editor: A P-Editor: Xu ZH
| 1. | Radwan A, Wyland M, Applequist L, Bolowsky E, Klingensmith H, Virag I. ULTRASONOGRAPHY, AN EFFECTIVE TOOL IN DIAGNOSING PLANTAR FASCIITIS: A SYSTEMATIC REVIEW OF DIAGNOSTIC TRIALS. Int J Sports Phys Ther. 2016;11:663-671. [PubMed] |
| 2. | Wheeler P, Boyd K, Shipton M. Surgery for Patients With Recalcitrant Plantar Fasciitis: Good Results at Short-, Medium-, and Long-term Follow-up. Orthop J Sports Med. 2014;2:2325967114527901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Rasenberg N, Bierma-Zeinstra SM, Bindels PJ, van der Lei J, van Middelkoop M. Incidence, prevalence, and management of plantar heel pain: a retrospective cohort study in Dutch primary care. Br J Gen Pract. 2019;69:e801-e808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician. 2011;84:676-682. [PubMed] |
| 5. | Zhang J, Nie D, Rocha JL, Hogan MV, Wang JH. Characterization of the structure, cells, and cellular mechanobiological response of human plantar fascia. J Tissue Eng. 2018;9:2041731418801103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Bolívar YA, Munuera PV, Padillo JP. Relationship between tightness of the posterior muscles of the lower limb and plantar fasciitis. Foot Ankle Int. 2013;34:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Landorf KB, Keenan AM, Herbert RD. Effectiveness of foot orthoses to treat plantar fasciitis: a randomized trial. Arch Intern Med. 2006;166:1305-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Dyck DD Jr, Boyajian-O'Neill LA. Plantar fasciitis. Clin J Sport Med. 2004;14:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Hansen L, Krogh TP, Ellingsen T, Bolvig L, Fredberg U. Long-Term Prognosis of Plantar Fasciitis: A 5- to 15-Year Follow-up Study of 174 Patients With Ultrasound Examination. Orthop J Sports Med. 2018;6:2325967118757983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Riddle DL, Pulisic M, Pidcoe P, Johnson RE. Risk factors for Plantar fasciitis: a matched case-control study. J Bone Joint Surg Am. 2003;85:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 411] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 11. | Schwartz EN, Su J. Plantar fasciitis: a concise review. Perm J. 2014;18:e105-e107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Arshad Z, Aslam A, Razzaq MA, Bhatia M. Gastrocnemius Release in the Management of Chronic Plantar Fasciitis: A Systematic Review. Foot Ankle Int. 2022;43:568-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Siriphorn A, Eksakulkla S. Calf stretching and plantar fascia-specific stretching for plantar fasciitis: A systematic review and meta-analysis. J Bodyw Mov Ther. 2020;24:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol Drug Saf. 2014;23:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 15. | Biswas C, Pal A, Acharya A. A comparative study of efficacy of oral non-steroidal anti-inflammatory agents and locally injectable steroid for the treatment of plantar fasciitis. Anesth Essays Res. 2011;5:158-161. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Podolsky R, Kalichman L. Taping for plantar fasciitis. J Back Musculoskelet Rehabil. 2015;28:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Probe RA, Baca M, Adams R, Preece C. Night splint treatment for plantar fasciitis. A prospective randomized study. Clin Orthop Relat Res. 1999;190-195. [PubMed] |
| 18. | Wrobel JS, Fleischer AE, Crews RT, Jarrett B, Najafi B. A randomised controlled trial of custom foot orthoses for the treatment of plantar heel pain. J Am Podiatr Med Assoc. 2015;105:281-294. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Seo K, Park S, Park K. Impact of wearing a functional foot orthotic on the ankle joint angle of frontal surface of young adults with flatfoot. J Phys Ther Sci. 2017;29:819-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Aboutorabi A, Bahramizadeh M, Arazpour M, Fadayevatan R, Farahmand F, Curran S, Hutchins SW. A systematic review of the effect of foot orthoses and shoe characteristics on balance in healthy older subjects. Prosthet Orthot Int. 2016;40:170-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Kogler GF, Solomonidis SE, Paul JP. Biomechanics of longitudinal arch support mechanisms in foot orthoses and their effect on plantar aponeurosis strain. Clin Biomech (Bristol, Avon). 1996;11:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Rasenberg N, Riel H, Rathleff MS, Bierma-Zeinstra SMA, van Middelkoop M. Efficacy of foot orthoses for the treatment of plantar heel pain: a systematic review and meta-analysis. Br J Sports Med. 2018;52:1040-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Whittaker GA, Munteanu SE, Menz HB, Tan JM, Rabusin CL, Landorf KB. Foot orthoses for plantar heel pain: a systematic review and meta-analysis. Br J Sports Med. 2018;52:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Vicenzino B, McPoil TG, Stephenson A, Paul SK. Orthosis-Shaped Sandals Are as Efficacious as In-Shoe Orthoses and Better than Flat Sandals for Plantar Heel Pain: A Randomized Control Trial. PLoS One. 2015;10:e0142789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Chuter VH, Searle A, Spink MJ. Flip-flop footwear with a moulded foot-bed for the treatment of foot pain: a randomised controlled trial. BMC Musculoskelet Disord. 2016;17:468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2218] [Cited by in RCA: 2795] [Article Influence: 186.3] [Reference Citation Analysis (0)] |
| 27. | Stoermer MJ, Pinhey JT. Ethyl (Z)-3-(2-Methoxyphenyl)-2-butenoate. Molecules. 1998;3:M72. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Chesterton LS, Thomas MJ, Hendry G, Chen Y, Goddin D, Halliday N, Lawton SA, Lewis M, Mallen CD, Menz HB, Foster NE, Roddy E. Self-management advice, exercise and foot orthoses for plantar heel pain: the TREADON pilot and feasibility randomised trial. Pilot Feasibility Stud. 2021;7:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Landorf KB, Radford JA, Hudson S. Minimal Important Difference (MID) of two commonly used outcome measures for foot problems. J Foot Ankle Res. 2010;3:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Baldassin V, Gomes CR, Beraldo PS. Effectiveness of prefabricated and customized foot orthoses made from low-cost foam for noncomplicated plantar fasciitis: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Costa ARA, de Almeida Silva HJ, Mendes AAMT, Scattone Silva R, de Almeida Lins CA, de Souza MC. Effects of insoles adapted in flip-flop sandals in people with plantar fasciopathy: a randomized, double-blind clinical, controlled study. Clin Rehabil. 2020;34:334-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Scher DL, Belmont PJ Jr, Bear R, Mountcastle SB, Orr JD, Owens BD. The incidence of plantar fasciitis in the United States military. J Bone Joint Surg Am. 2009;91:2867-2872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Herbert R, Jamtvedt G, Hagen K B, Mead J. Practical Evidence-Based Physiotherapy. Edinburgh (UK): Elsevier Churchill Livingstone (UK). May 17, 2005. [cited 17 May 2005]. Available from: https://www.amazon.co.uk/Practical-Evidence-Based-Physiotherapy-Robert-Herbert/dp/0750688203. |
| 34. | Morrow EM, Theologis T, Kothari A. Construction and validation of sham insoles used in clinical trials: A systematic review. Prosthet Orthot Int. 2022;46:121-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Karcioglu O, Topacoglu H, Dikme O. A systematic review of the pain scales in adults: Which to use? Am J Emerg Med. 2018;36:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 604] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 36. | Buchanan BK, Kushner D. Plantar Fasciitis. In: StatPearls. Treasure Island (FL) : StatPearls Publishing, 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431073/. |