Published online Sep 18, 2023. doi: 10.5312/wjo.v14.i9.698
Peer-review started: July 3, 2023
First decision: August 4, 2023
Revised: August 17, 2023
Accepted: August 23, 2023
Article in press: August 23, 2023
Published online: September 18, 2023
Processing time: 72 Days and 20.7 Hours
Aneurysmal bone cysts (ABC) are benign cystic bone tumors of an osteolytic and locally aggressive nature. As an alternative to the primary treatment of choice, which consists of curettage with bone grafting, alternative treatment methods with promising results have been described. At our department, we have, in recent years, used percutaneous sclerotherapy with polidocanol. The objective of this study was to identify the healing rate and safety of sclerotherapy with polidocanol.
To identify the efficacy and safety of sclerotherapy with polidocanol in primary and recurrent ABC.
Twenty-two consecutive patients (median age 12.5 years; range 1-27) with 23 ABCs treated with sclerotherapy with polidocanol from 2016-2021 were included retrospectively. Eleven patients (48%) had undergone different forms of previous treatment with recurrence. Under general anesthesia and fluoroscopic guidance, repeated percutaneous injections of 4mg polidocanol/kg body weight were performed. Through review of the electronic medical records, the following were identified: healing and recurrence rate, number of treatments, gender, age, comorbidity, location of the tumor and side effects / complications, as well as any previous surgery for ABC. The median length of radiographic follow-up was 19.5 mo.
All ABCs except one (96%) showed healing or stable disease after a median of 4 (range 1-8) injections. Complete clinical and radiographic healing was observed in 16 cysts (70%), while partial radiographic healing with resolution of pain was seen in 6 cases (26%) and considered as stable disease. The cyst that failed to heal had previously undergone curettage twice with recurrence. One patient with a large pelvic ABC experienced, right after two injections, a sudden drop in blood pressure, which could quickly be reversed. One patient with a juxtaphyseal ABC in the femoral neck showed a minor limb length discrepancy because of deformity. Beyond that, no complications were observed.
Percutaneous sclerotherapy with polidocanol appears to be a safe alternative for treatment of aneurysmal bone cysts. In our series of both primary and recurrent cysts, it showed the ability to achieve healing or stable disease in 22 of 23 cases (96%). Further studies are needed to decide if this provides a long-lasting effect.
Core Tip: This retrospective study presents the first outcomes after implementation of sclerotherapy for aneurysmal bone cysts (ABC) at our department. Compared to other series, 48% of our cohort consisted of patients with recurrent ABC after previous treatment or failed surgery. Sclerotherapy showed a high potency to achieve healing or stable disease and a low rate of adverse events in both treatment groups. We can recommend it as standard treatment both for primary and recurrent ABC, keeping in mind that randomized multicenter studies would be needed to provide evidence for the superiority of one treatment method.
- Citation: Weber KS, Jensen CL, Petersen MM. Sclerotherapy as a primary or salvage procedure for aneurysmal bone cysts: A single-center experience. World J Orthop 2023; 14(9): 698-706
- URL: https://www.wjgnet.com/2218-5836/full/v14/i9/698.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i9.698
Aneurysmal bone cysts (ABC) are rare benign bone tumors, usually affecting children and adolescents1. Patients mostly present with pain, with or without swelling, and sometimes with a pathological fracture.
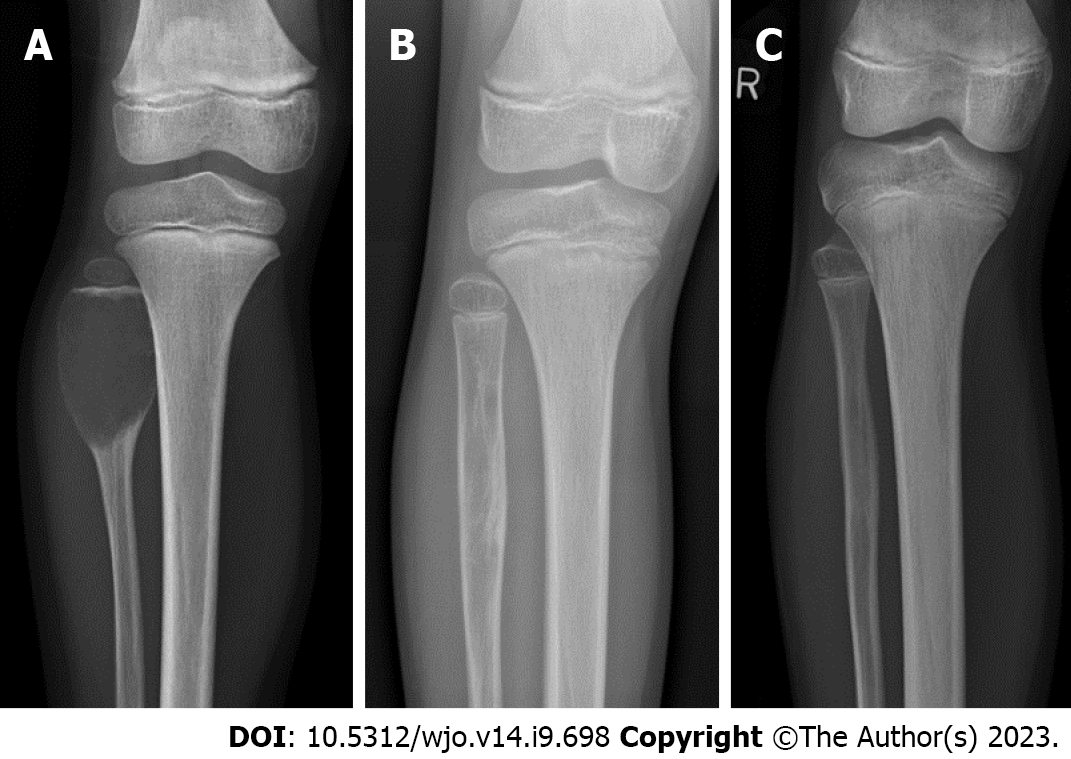
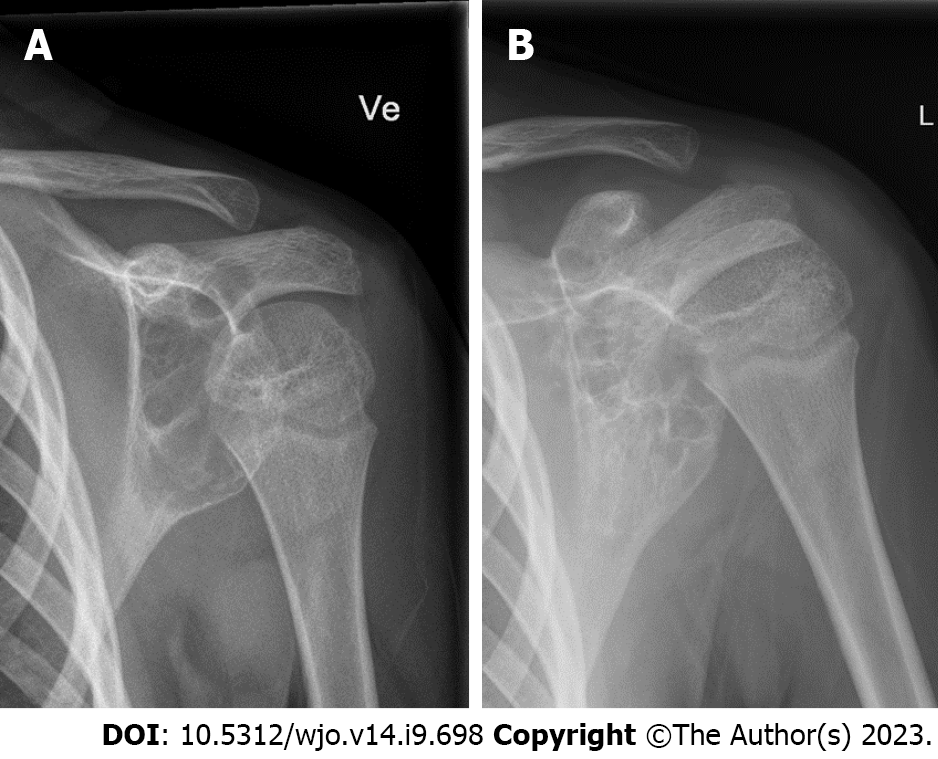
Radiologically, these lesions are expansile and osteolytic and can be locally aggressive (Figure 1). On magnetic resonance imaging (MRI), fluid-fluid levels are diagnostic, and soft tissue expansion can be seen. It is the gold standard to verify the diagnosis with biopsy.
The traditional treatment, curettage and bone grafting, has shown high rates of morbidity and recurrence[1-4]. As a result of that, several other treatment options have been explored: embolization, radiotherapy, wide excision and sclerotherapy with different agents and more[5-9]. In recent years, percutaneous sclerotherapy, especially with polidocanol, alcohol or doxycycline, has shown promising results with similar recurrence rates and low morbidity compared to open surgery[10-17]. There are several series published with polidocanol as primary treatment, but to our knowledge, there are no larger reported series on polidocanol as a salvage procedure. In our series, 48% of the cysts were treated with polidocanol for failed surgery or recurrence after previous treatment.
In this study, we present our experience with percutaneous polidocanol treatment for primary and recurrent ABC with focus on healing rate, recurrence and complications.
In this single-center retrospective study design, all patients treated for ABC with percutaneous sclerotherapy from 2016-2021 were included. In 2016, sclerotherapy was introduced as standard therapy for primary and recurrent ABCs at our institution. Through review of the electronic medical records, the following data was identified: healing and recurrence rate, number of treatments, gender, age, comorbidity, location of the tumor, side effects and complications, as well as any previous surgery for ABC. Twenty-two patients with a combined number of 23 ABCs were identified. The various characteristics are shown in Table 1. Imaging at first presentation included conventional radiographs and MRI in all patients. Furthermore, a histological sample was available in all patients, either from previous surgery or collected with open biopsy. In five patients, open biopsy was performed simultaneously with the first sclerosant injection (Figure 2).
| Patient-ID | Age at presentation | Gender | Location | No. of injections | Ossification | Previous treatment | Follow-up (mo) | Histologic result |
| 1 | 13 | M | Pubic bone | 5 | Complete | Curettage + bone graft | 13 | Classic ABC |
| 1 | 13 | M | Sacrum | 3 | Partial | None | 13 | Consistent with ABC |
| 2 | 13 | F | Distal fibula | 5 | Absent | Two curettages + bone graft | 37 | Classic ABC |
| 3 | 10 | M | Ischium | 6 | Partial | None | 29 | Classic ABC |
| 4 | 6 | M | Scapula | 7 | Partial | None | 24 | Classic ABC |
| 5 | 25 | F | Proximal femur | 3 | Partial | Two curettages + bone graft | 8 | Classic ABC |
| 6 | 14 | M | Ileum | 6 | Partial | None | 16 | Consistent with ABC |
| 7 | 4 | M | Distal fibula | 3 | Complete | None | 4 | Consistent with ABC |
| 8 | 12 | F | Distal tibia | 1 | Complete | Curettage + bone substitute | 31 | Classic ABC |
| 9 | 16 | M | Proximal tibia | 4 | Complete | Curettage + bone graft | 33 | Classic ABC |
| 10 | 6 | M | Distal tibia | 8 | Complete | Triamcinolone injection | 47 | Consistent with ABC |
| 11 | 5 | F | Proximal fibula | 3 | Complete | Curettage + bone substitute | 38 | Classic ABC |
| 12 | 15 | M | Ischium | 2 | Partial | None | 4 | Consistent with ABC |
| 13 | 14 | M | Distal fibula | 4 | Complete | Curettage + bone graft | 23 | Classic ABC |
| 14 | 25 | M | Acetabulum | 1 | Complete | Two curettages + bone graft | 35 | Classic ABC |
| 15 | 10 | F | 3. metatarsal | 4 | Complete | Curettage + bone substitute | 19 | Classic ABC |
| 16 | 10 | M | Proximal femur | 3 | Complete | None | 26 | Classic ABC |
| 17 | 27 | M | Proximal radius | 1 | Complete | None | 11 | Classic ABC |
| 18 | 16 | M | Proximal fibula | 5 | Complete | None | 6 | Classic ABC |
| 19 | 18 | M | Proximal femur | 2 | Complete | Three curettages + bone graft | 20 | Consistent with ABC |
| 20 | 9 | M | Ischium | 4 | Complete | None | 16 | Classic ABC |
| 21 | 7 | F | Distal tibia | 6 | Complete | None | 5 | Classic ABC |
| 22 | 1 | F | Proximal femur | 6 | Complete | None | 9 | Classic ABC |
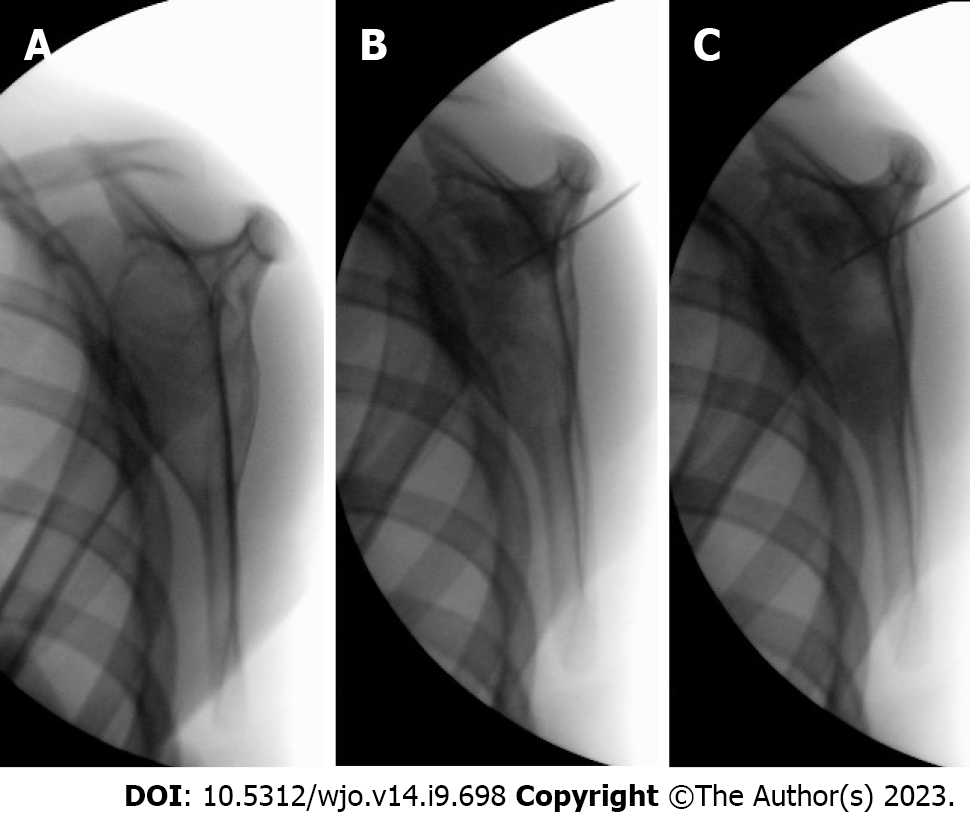
Sclerotherapy was performed under general anesthesia. The cyst was punctured percutaneously with a 1.0 or 1.2 mm trocar needle under fluoroscopic guidance. In classic ABC, bloody fluid from the cyst can be aspirated at the beginning of treatment. The cyst was injected with 4 mg Polidocanol (Aethoxysklerol®) per kilogram bodyweight, as described by Brosjö et al[10], mixed with contrast agent Iohexol (Omnipaque®). Before injection, the needle with trocar was directed in all cystic areas to break down possible septae. During injection, contrast agent was used to ensure that all parts of the cyst were treated (Figure 3). After injection, the needle was flushed with saline or previously aspirated blood, and after needle removal, pressure was applied at the site for at least two minutes to prevent extravasation.
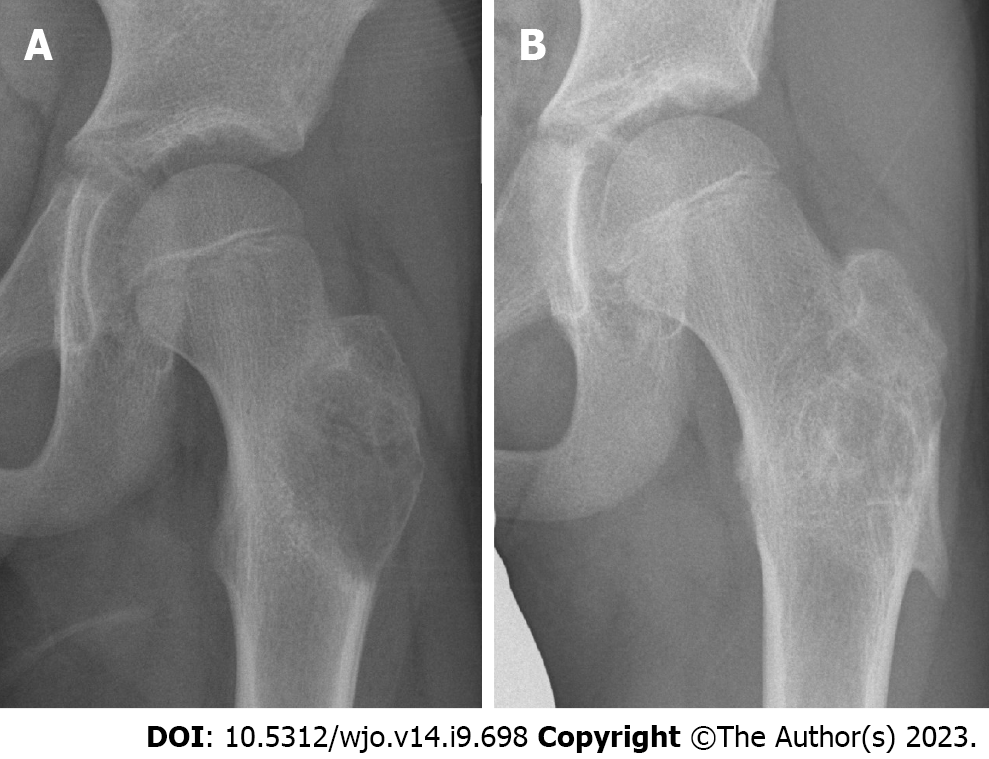
The procedure was repeated every 4-6 wk, following the example of Brosjö et al[10], until satisfactory radiographical and clinical healing of the cyst was seen. Healing was defined as resolution of pain on clinical examination in addition to complete ossification on radiographs (Figures 1 and 4). Partial ossification or stable disease was determined as resolution of pain on clinical examination in addition to thickening of cortex and partial ossification without increase in cyst size on radiographs (Figure 5). The median radiographic follow-up was 19.5 mo (range: 4-47 mo).
The summary of the patient characteristics and treatment results is shown in Table 1. The median age at beginning of treatment was 12.5 years (range: 1-27), and males accounted for 68% of patients. Of the 23 cysts, eleven had undergone previous surgical intervention (48%) with subsequent recurrence, wherein ten cysts had been treated with curettage and bone grafting/bone substitute and one with glucocorticoid injection (for details see Table 1). Histopathologically, the diagnosis was confirmed in 17 cases, and in the remaining six cases, the sample was consistent with aneurysmal bone cyst. There were no tumors with an ABC-like area (previously called secondary ABC) or malignancies.
All cysts except one (96%) showed healing or stable disease after a median of four (range: 1-8) injections. Complete ossification was observed in 16 cysts (70%), while partial ossification with complete resolution of pain were seen in six cases (26%), which were considered as stable disease. There were no recurrences in the follow-up period.
The cyst in the distal fibula that failed to heal had previously undergone curettage twice with recurrence. During the course of five polidocanol injections, it showed recurring radiolucency in different parts of the cyst, and the patient displayed persistent pain. Finally, extended curettage with high-speed burring and bone grafting with polidocanol as adjuvant was performed, and the cyst healed without recurrence.
Due to a possible allergic reaction, one patient with a large cyst in the ischium showed a sudden drop in blood pressure to 60 mmHg systolic right after injection of polidocanol/iohexol in two settings, which could quickly be reversed after a bolus of ephedrine and fluid.
One patient with a juxtaphyseal ABC in the femoral neck, who was only 18 mo old at the beginning of treatment, developed a minor limb shortening because of deformity in the trochanteric area at 9 mo follow-up, possibly requiring further orthopedic treatment in the future. Beyond that, no complications, such as skin necrosis or infections, were observed.
In this study, we present our experience with percutaneous sclerotherapy of ABC with polidocanol. In our series, 96% of all cysts showed satisfactory treatment response with complete resolution of pain, with 70% showing complete ossification and 26% showing partial ossification without increase in cyst size. There were no recurrences in the follow-up period, but one cyst failed to heal, and treatment was converted to extended curettage and bone grafting with polidocanol as adjuvant. The healing rate of 96% in this study is comparable to the results published in recent literature, with healing rates ranging from 83%-100%[10-13,17-20], except for one study discussed further below. The studies comparing sclerotherapy with polidocanol with curettage found healing rates slightly in favor of sclerotherapy (93.3% vs 84.8% and 100% vs 82%, respectively) and showed lower morbidity in the sclerotherapy group[11,17].
There is no agreement about the clear definition of successful treatment, or healing, in the literature. As applied in this study, most authors accept the following clinical and radiological signs as successful treatment: Resolution of pain, restoring of function, increasing sclerosis and cortical thickness without increase in cyst size[12,13,17,18].
One study showed less favorable results in both groups: Failure of sclerotherapy with polidocanol and need for further treatment in 34.5% of cases as well as a 38.5% recurrence rate with need for further treatment after intralesional curettage[21]. The authors find a possible explanation for these less satisfactory results in the more accurate cyst size measurement based on MRI scans and higher initial cyst volume in their study compared to similar studies[21]. Furthermore, MRI scans might be more sensitive to detect residual cyst activity (i.e., fluid-fluid levels) than conventional biplanar radiographs, though the clinical importance of this finding remains unclear.
Apart from polidocanol, other sclerosing agents have been used for ABC with promising results. Publications on sclerotherapy with ethanol (in one study combined with Surgiflo®) and doxycycline showed success rates ranging from 68%-100% and 86%-100%, respectively, making them comparable to treatment with polidocanol[14,15,22-25]. At this stage, no sclerotherapy modality has been proven superior, and the treatment choice is usually dependent on regional preference and experience.
The general disadvantage of sclerotherapy is the need for repeated treatments in most cases, which, in this patient age group, usually requires general anesthesia.
Our cohort consisted of 12 primary ABCs and 11 cysts that had undergone previous surgical intervention with subsequent recurrence. To our knowledge, there are no larger reported series on polidocanol as a salvage procedure. In this series, 10 of the 11 recurrent ABCs (92%) showed satisfactory treatment response after sclerotherapy (Table 1).
One recurrent cyst, which had previously undergone curettage twice, failed to heal. Five injections of polidocanol were performed, but the patient showed persistent pain, and radiologically recurrent osteolytic areas were observed in different parts of the cyst. Finally, extended curettage with high-speed burring and bone grafting with polidocanol as adjuvant was performed, and the cyst healed without recurrence. We argue that it can be difficult to address all areas of a recurrent cyst, which in some cases consists of many subcavities. Especially if there are bony septae, it can be challenging to reach a certain subpart of the cyst percutaneously.
There are other described methods for ABC as salvage treatment or in patients where sclerotherapy or open surgery are contraindicated or not feasible. Selective arterial embolization has shown good results and is primarily used in the spine and other anatomically difficult locations, as well as a preoperative adjuvant to surgery. However, it is a technically demanding, operator-dependent procedure with potentially serious side effects due to ischemia, and it requires that a feeding vessel to the cyst can be identified[26,27].
Furthermore, there are pharmacological treatments available as rescue therapy for ABC. In several studies, denosumab, a monoclonal antibody inhibiting osteoclast formation and activity, has been shown to achieve re-ossification of ABC[6,28-30]. However, serious side effects have been described. Some patients demonstrated severe hypo- or hypercalcemia under or after denosumab treatment[30,31]. Moreover, knowledge regarding the long-term consequences of denosumab on the growing skeleton is lacking so far. A related medical therapy is the use of bisphosphonates, such as zoledronic acid, which also inhibits osteoclast activity. They are widely used to successfully treat and manage various unresectable or metastatic bone tumors. Two smaller studies, with five and eight patients, respectively, have shown positive clinical and radiological effects of bisphosphonates in ABC without adverse events[32,33]. The authors even suggest bisphosphonates as treatment of choice in spinal ABC without neurological deficit or instability[32].
Based on the results of the present study, sclerotherapy with polidocanol might be another alternative salvage procedure to stabilize refractory or recurrent ABC. It can be considered before advancing to salvage treatments with a spectrum of more serious adverse events, as described above. However, further studies with more patients and longer follow-ups are needed to decide whether this is of lasting effect.
There was one adverse event observed in our cohort: A ten-year-old boy with a large cyst in the ischium showed a sudden drop in blood pressure to 60 mmHg systolic right after injection of polidocanol/iohexol in two settings. The reaction could quickly be reversed after a bolus of ephedrine and fluid. This phenomenon has also been described by other authors[18,34]. Like in the present study, it is usually harmless and quickly reversible, but in two cases, severe reactions have been described: One patient developed hypovolemic shock and cardiac arrest needing resuscitation, and the other one presented ventricular tachycardia with a sharp drop in blood pressure[18,34]. Both patients are reported to have recovered completely, but these observations necessitate this procedure only to be performed in a controlled setting with monitoring of vital signs.
Other authors reported minor complications to sclerotherapy, e.g., skin induration, hypopigmentation, minor inflammatory reactions, ulceration or temporary pain[10,12,13,17,19,20]. On the other hand, the studies comparing sclerotherapy to curettage and/or resection found a higher rate of clinically pertinent complications and poorer functional outcomes in the surgery group[11,17,21].
One important limitation of this study is the short radiological follow-up in some patients. The retrospective study design meant that the follow-up was planned individually by the treating surgeon, not following a standard plan. Some patients showing signs of healing and resolution of pain in the first year after treatment were discharged and informed to contact our department again should pain or other symptoms recur. For these patients, our department is the responsible center for treating ABCs. The electronic medical journal showed no contacts to our or other hospitals in the region concerning their previous ABC in the following months and years. Furthermore, it was ensured that the place of residence was still in our region. The authors therefore argue that recurrence with clinical signs most likely would have been detected in these patients.
The other limitations of this study consist primarily of the number of patients, the retrospective study design and the lack of a control group. Randomized multicenter studies would be needed to provide evidence for the superiority of one treatment method for aneurysmal bone cysts.
This study supports evidence in the current literature that percutaneous sclerotherapy with polidocanol is an efficient and safe alternative to conventional surgery for the treatment of aneurysmal bone cysts. It can especially be recommended for cysts in difficult anatomic locations where open surgery would cause significant morbidity. Based on the present study, it may also be considered as a salvage procedure for failed surgery or multiple recurrences. Further studies are needed to decide if this provides a long-lasting effect and which of the discussed treatment methods is superior for primary or recurring aneurysmal bone cysts.
As an alternative to the traditional treatment of aneurysmal bone cysts (ABC), which consists of curettage with bone grafting, alternative treatment methods with promising results have been described. At our department, we have been using percutaneous sclerotherapy with polidocanol for primary and recurrent ABC. To our knowledge, this is the first larger series reporting on polidocanol as a salvage procedure.
The main challenge with aneurysmal bone cysts is their locally aggressive nature and a high risk of recurrence. Therefore, there is a need to find evidence for the best available treatment, which optimally shows high efficacy and at the same time low morbidity and low rates of recurrence.
The main objective was to identify the efficacy and safety of sclerotherapy with polidocanol in primary and recurrent ABC. The outcomes of this study, especially regarding recurrent ABC, propose sclerotherapy as a relevant treatment method to be considered.
This is a single-center retrospective study, where all patients treated for ABC with percutaneous sclerotherapy from 2016-2021 were included. The data was collected through review of the electronic medical records.
In our series, sclerotherapy with polidocanol showed the ability to achieve healing or stable disease in 96% of cases (100% in primary ABC and 92% in recurrent ABC). These results support the positive experience with sclerotherapy for primary ABC in the recent literature and open the debate about sclerotherapy as a possible salvage treatment. The median length of radiographic follow-up was 19.5 mo. Further studies with longer follow-up are needed to decide if this provides a long-lasting effect.
Based on the present study, sclerotherapy with polidocanol may also be considered as a salvage procedure for failed surgery or multiple recurrences. This can be a valuable alternative to other salvage treatment methods, which may have higher morbidity.
Given the variety of possible treatment methods, future research should focus on randomized clinical trials to identify the gold standard treatment for primary and recurrent ABC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeyaraman M, India; Li JM, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Grahneis F, Klein A, Baur-Melnyk A, Knösel T, Birkenmaier C, Jansson V, Dürr HR. Aneurysmal bone cyst: A review of 65 patients. J Bone Oncol. 2019;18:100255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Park HY, Yang SK, Sheppard WL, Hegde V, Zoller SD, Nelson SD, Federman N, Bernthal NM. Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med. 2016;9:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 3. | Mankin HJ, Hornicek FJ, Ortiz-Cruz E, Villafuerte J, Gebhardt MC. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol. 2005;23:6756-6762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Cottalorda J, Bourelle S. Current treatments of primary aneurysmal bone cysts. J Pediatr Orthop B. 2006;15:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Tsagozis P, Brosjö O. Current Strategies for the Treatment of Aneurysmal Bone Cysts. Orthop Rev (Pavia). 2015;7:6182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Deventer N, Deventer N, Gosheger G, de Vaal M, Vogt B, Budny T. Current strategies for the treatment of solitary and aneurysmal bone cysts: A review of the literature. J Bone Oncol. 2021;30:100384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Cruz GS, Cuevas-Suárez CE, Saavedra JPA, Giorgis R, Teixeira MRK, Muniz FWMG. Percutaneous treatments of primary aneurysmal bone cysts: systematic review and meta-analysis. Eur J Orthop Surg Traumatol. 2021;31:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | van Geloven TPG, van de Sande MAJ, van der Heijden L. The treatment of aneurysmal bone cysts. Curr Opin Pediatr. 2023;35:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Cottalorda J, Louahem Sabah D, Joly Monrigal P, Jeandel C, Delpont M. Minimally invasive treatment of aneurysmal bone cysts: Systematic literature review. Orthop Traumatol Surg Res. 2022;108:103272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Brosjö O, Pechon P, Hesla A, Tsagozis P, Bauer H. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts. Acta Orthop. 2013;84:502-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Varshney MK, Rastogi S, Khan SA, Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin Orthop Relat Res. 2010;468:1649-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Rastogi S, Varshney MK, Trikha V, Khan SA, Choudhury B, Safaya R. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polidocanol. A review of 72 cases with long-term follow-up. J Bone Joint Surg Br. 2006;88:1212-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Puri A, Hegde P, Gulia A, Parikh M. Primary aneurysmal bone cysts. Bone Joint J. 2020;102-B:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Ulici A, Florea DC, Carp M, Ladaru A, Tevanov I. Treatment of the aneurysmal bone cyst by percutaneous intracystic sclerotherapy using ethanol ninety five percent in children. Int Orthop. 2018;42:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Shiels WE 2nd, Beebe AC, Mayerson JL. Percutaneous Doxycycline Treatment of Juxtaphyseal Aneurysmal Bone Cysts. J Pediatr Orthop. 2016;36:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Liu X, Han SB, Si G, Yang SM, Wang CM, Jiang L, Wei F, Wu FL, Liu XG, Liu ZJ. Percutaneous albumin/doxycycline injection vs open surgery for aneurysmal bone cysts in the mobile spine. Eur Spine J. 2019;28:1529-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Puthoor D, Francis L, Ismail R. Is sclerotherapy with polidocanol a better treatment option for aneurysmal bone cyst compared to conventional curettage and bone grafting? J Orthop. 2021;25:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Jasper J, van der Heijden L, van Rijswijk CSP, van de Sande MAJ. Efficacy of Sclerotherapy With Polidocanol (Ethoxysclerol) in Primary Aneurysmal Bone Cysts in Children and Adolescents. J Pediatr Orthop. 2021;41:e555-e562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Kumar D, Kumar S, Kumar D, Patel BM, Kumar A, Waliullah S. Sclerotherapy for Aneurysmal Bone Cyst: A Single-Center Experience. Cureus. 2021;13:e18469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Rai AK, Rathod TN, Bansal D, Hadole BS, Rahman SH, Kumar K G G, Prabhu RM. Clinicoradiological outcome of percutaneous intralesional polidocanol in Aneurysmal Bone Cysts: A prospective study of 43 patients in a single tertiary care centre. J Orthop. 2022;32:72-77. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Deventer N, Schulze M, Gosheger G, de Vaal M, Deventer N. Primary Aneurysmal Bone Cyst and Its Recent Treatment Options: A Comparative Review of 74 Cases. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Wong MN, Braswell LE, Murakami JW. Doxycycline sclerotherapy of cervical spine aneurysmal bone cysts: single-institution 13-year experience. Pediatr Radiol. 2022;52:1528-1538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Arleo TL, Hawkins CM, Fabregas JA, Gill AE. Percutaneous image-guided treatment of aneurysmal bone cysts: is there a superior treatment option? Pediatr Radiol. 2022;52:1539-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Marie-Hardy L, El Sayed L, Alves A, Brunelle F, Ouchrif Y, Naggara O, Breton S, Mascard E, Glorion C, Pannier S. Percutaneous alcohol-based sclerotherapy in aneurysmal bone cyst in children and adolescents. Orthop Traumatol Surg Res. 2020;106:1313-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Ghanem I, Nicolas N, Rizkallah M, Slaba S. Sclerotherapy using Surgiflo and alcohol: a new alternative for the treatment of aneurysmal bone cysts. J Child Orthop. 2017;11:448-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Terzi S, Gasbarrini A, Fuiano M, Barbanti Brodano G, Ghermandi R, Bandiera S, Boriani S. Efficacy and Safety of Selective Arterial Embolization in the Treatment of Aneurysmal Bone Cyst of the Mobile Spine: A Retrospective Observational Study. Spine (Phila Pa 1976). 2017;42:1130-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Rossi G, Mavrogenis AF, Facchini G, Bartalena T, Rimondi E, Renzulli M, Andreone A, Durante S, Angelini A, Errani C. How effective is embolization with N-2-butyl-cyanoacrylate for aneurysmal bone cysts? Int Orthop. 2017;41:1685-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Kurucu N, Akyuz C, Ergen FB, Yalcin B, Kosemehmetoglu K, Ayvaz M, Varan A, Aydin B, Kutluk T. Denosumab treatment in aneurysmal bone cyst: Evaluation of nine cases. Pediatr Blood Cancer. 2018;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Palmerini E, Ruggieri P, Angelini A, Boriani S, Campanacci D, Milano GM, Cesari M, Paioli A, Longhi A, Abate ME, Scoccianti G, Terzi S, Trovarelli G, Franchi A, Picci P, Ferrari S, Leopardi MP, Pierini M. Denosumab in patients with aneurysmal bone cysts: A case series with preliminary results. Tumori. 2018;104:344-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Alhumaid I, Abu-Zaid A. Denosumab Therapy in the Management of Aneurysmal Bone Cysts: A Comprehensive Literature Review. Cureus. 2019;11:e3989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Deodati A, Fintini D, Levtchenko E, Rossi M, Ubertini G, Segers H, Battafarano G, Cappa M, Del Fattore A. Mechanisms of acute hypercalcemia in pediatric patients following the interruption of Denosumab. J Endocrinol Invest. 2022;45:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Kieser DC, Mazas S, Cawley DT, Fujishiro T, Tavolaro C, Boissiere L, Obeid I, Pointillart V, Vital JM, Gille O. Bisphosphonate therapy for spinal aneurysmal bone cysts. Eur Spine J. 2018;27:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Cornelis F, Truchetet ME, Amoretti N, Verdier D, Fournier C, Pillet O, Gille O, Hauger O. Bisphosphonate therapy for unresectable symptomatic benign bone tumors: a long-term prospective study of tolerance and efficacy. Bone. 2014;58:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Gupta G, Pandit RS, Jerath N, Narasimhan R. Severe life-threatening hypersensitivity reaction to polidocanol in a case of recurrent aneurysmal bone cyst. J Clin Orthop Trauma. 2019;10:414-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |