Published online Aug 18, 2023. doi: 10.5312/wjo.v14.i8.651
Peer-review started: April 24, 2023
First decision: June 1, 2023
Revised: June 26, 2023
Accepted: August 1, 2023
Article in press: August 1, 2023
Published online: August 18, 2023
Processing time: 114 Days and 22 Hours
Spinal metallosis is a rare complication following spinal instrumentation whereby an inflammatory response to the metal implants results in the development of granulomatous tissue.
We describe the case of a 78-year-old woman who had recurrence of back pain 5 years after lumbar spine posterior decompression and instrumented fusion. Lumbar spine radiographs showed hardware loosening and magnetic resonance imaging showed adjacent segment disease. Revision surgery revealed evidence of metallosis intraoperatively.
Spinal metallosis can present several years after instrumentation. Radiography and computed tomography may demonstrate hardware loosening secondary to metallosis. Blood metal concentrations associated with spinal metallosis have yet to be established. Hence, metallosis is still an intraoperative and histopathological diagnosis. The presence of metallosis after spinal instrumentation likely indicates a more complex underlying problem: Pseudarthrosis, failure to address sagittal balance, infection, and cross-threading of set screws. Hence, identifying metallosis is important, but initiating treatment promptly for symptomatic implant loosening is of greater paramount.
Core Tip: This paper describes a rare case of metallosis after spinal instrumentation and discusses the methods of diagnosing and risk factors contributing to spinal metallosis. A review of the current literature as presented in this paper demonstrates the scarcity of studies on spinal metallosis after spinal instrumentation, despite the fact that a diagnosis of spinal metallosis should be promptly identified and treated by revision surgery. It is also important to understand that the presence of metallosis after spinal instrumentation likely indicates a more complex underlying problem, such as instability of the spinal implants.
- Citation: Kwan YH, Teo HLT, Dinesh SK, Loo WL. Metallosis with spinal implant loosening after spinal instrumentation: A case report. World J Orthop 2023; 14(8): 651-661
- URL: https://www.wjgnet.com/2218-5836/full/v14/i8/651.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i8.651
Metallosis is postulated to occur due to the corrosion of metal implants leading to metal debris build-up in periprosthetic soft tissue and bone. This precipitates a granulation-type reaction involving phagocytosis of metal particles resulting in osteolysis and local reactions, namely, aseptic fibrosis, local tissue necrosis, and implant loosening, as well as systemic toxicity such as cardiomyopathy and abnormal thyroid function[1]. Posterior spinal fusion involves the placement of metallic rods and screws and these fixtures are not routinely removed except for reasons such as localised pain over implant site, prominent hardware, implant failure, or infection[2]. Metallosis is not uncommon following joint arthroplasties but only a few cases of spinal metallosis have been described in the literature. Hence, we report a case of spinal metallosis with implant loosening after posterior spinal instrumentation.
A 78-year-old woman was admitted in 2019 to the Department of Orthopaedic Surgery in Changi General Hospital, a tertiary hospital in Singapore, for a 4-mo duration of worsening lower back pain with claudication and right lower limb radiculopathy, with onset 5 years after a spinal surgery.
The patient’s past medical history was significant for well-controlled hypertension and type 2 diabetes mellitus, gout, gastroesophageal reflux disease, and obesity [body mass index (BMI) of 31.2].
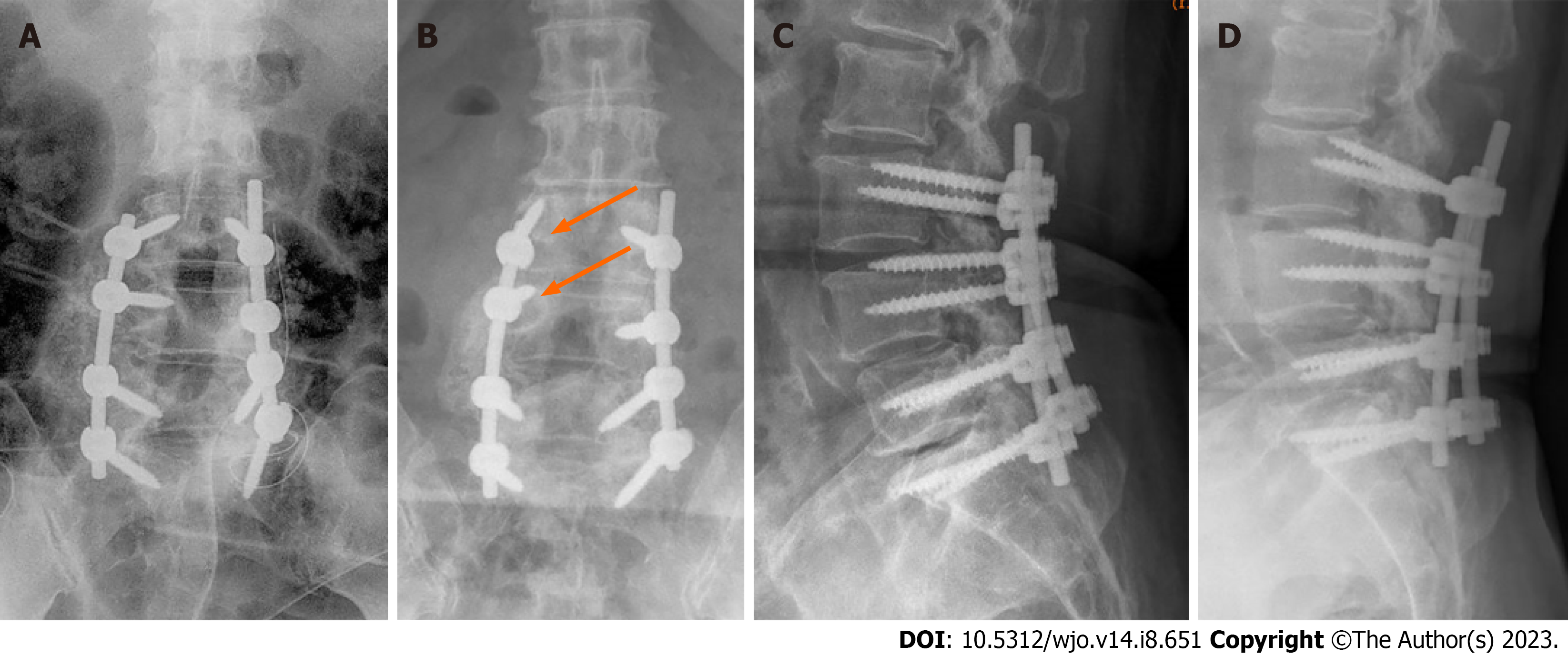
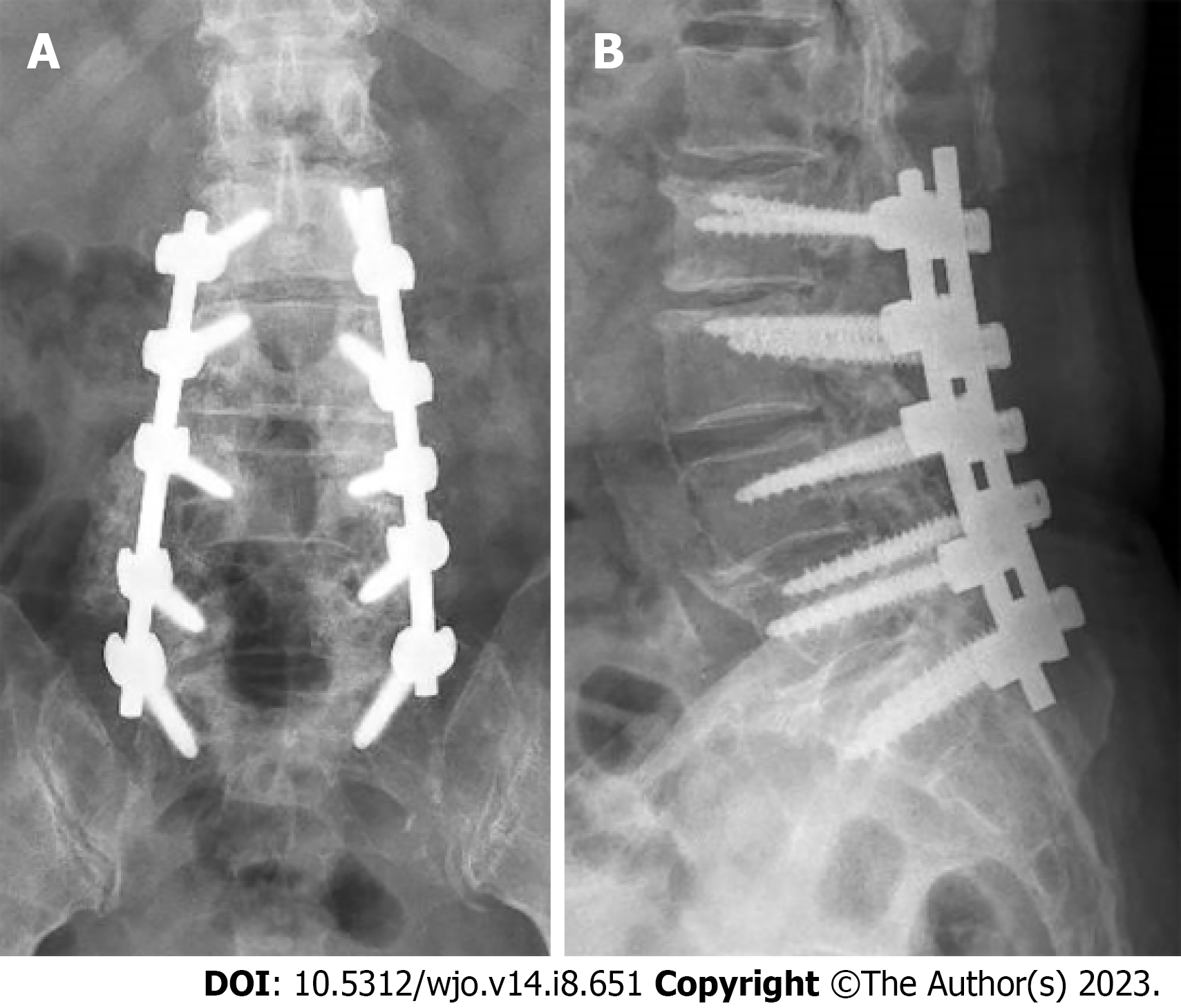
She also had a significant past surgical history of L3 to S1 posterior decompressive laminectomy, stabilisation with pedicle screws, and posterolateral fusion with local bone grafting performed at the same hospital 5 years ago. The surgery was performed for a diagnosis of lumbar spondylosis with central canal stenosis, for which she presented with chronic lower back pain radiating down her bilateral lower limbs. Titanium polyaxial screws (DePuy Synthes) were used in that surgery. The patient’s symptoms were relieved after the surgery and she recovered. Postoperative lumbar spine radiographs showed the proper positioning of the spinal implants (Figure 1).
The patient was followed up in the orthopaedic surgery specialist outpatient clinic regularly after her spine surgery. Five years after the surgery, she started to develop progressively worsening lower back pain which radiated to her right lateral thigh and calves. Four months later, her symptoms had slowly deteriorated to the point where she required a motorized wheelchair as she was unable to ambulate long distances due to pain.
The patient had no relevant personal and family history.
The most significant finding on examination was reduced power in the right L2 and L3 myotomes [grade 4 out of 5 on the Medical Research Council (MRC) scale for muscle strength]. The rest of the myotomes from L2 to S1 were normal, with a grade 5 out of 5 power on the MRC scale. She also had a large body habitus. Her gait was slow but steady over a short distance. The rest of the physical examination was unremarkable: There was no spinal tenderness or significant muscle wasting of her back or lower limbs. The range of movement of her cervical and thoracolumbar spine was normal. Sensation was intact over all dermatomes. Bilateral knee and ankle reflexes were normal. The straight leg raise test was negative as well.
Laboratory blood tests showed normal values, including a white blood cell count of 8.2 × 109/L and C-reactive protein level of 4.1 mg/L. Other biochemical parameters were within the normal range. Serum metal concentrations were not performed for the patient due to cost issues.
Lumbar spine radiographs and magnetic resonance imaging (MRI) were performed prior to the revision surgery. Lumbar spine anteroposterior and lateral radiographs revealed stable periprosthetic radiolucency surrounding the right upper two screws (L3 and L4 pedicle screws) that suggested loosening of the instrumentation (Figure 1). There was no evidence of fracture of the pedicle screws and rod instrumentation. Narrowing of the L4-L5 and L5-S1 intervertebral disc spaces was noted but vertebral body heights were largely maintained. Spondylotic changes and facet arthropathy were seen. There was no instability noted in the flexion and extension views.
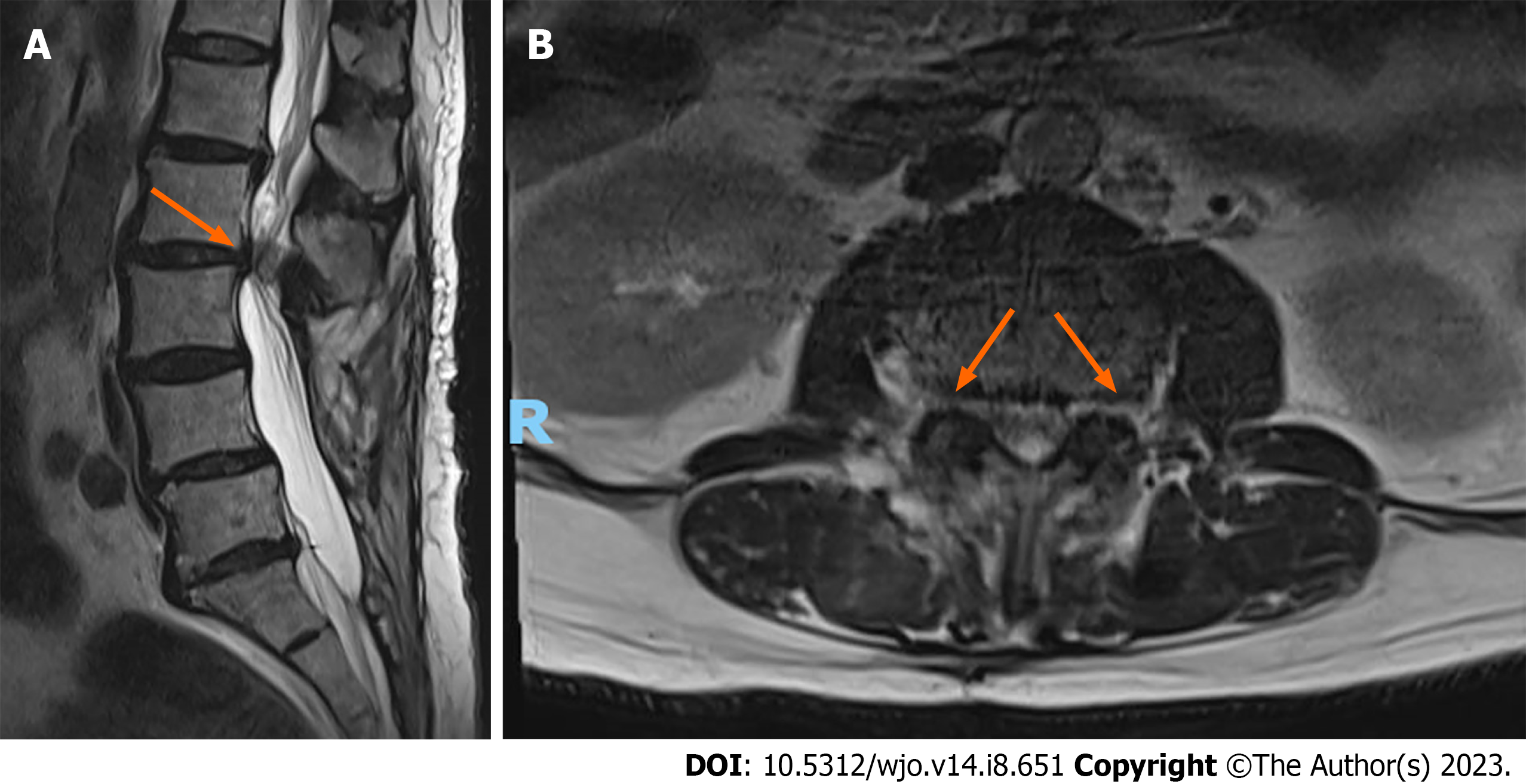
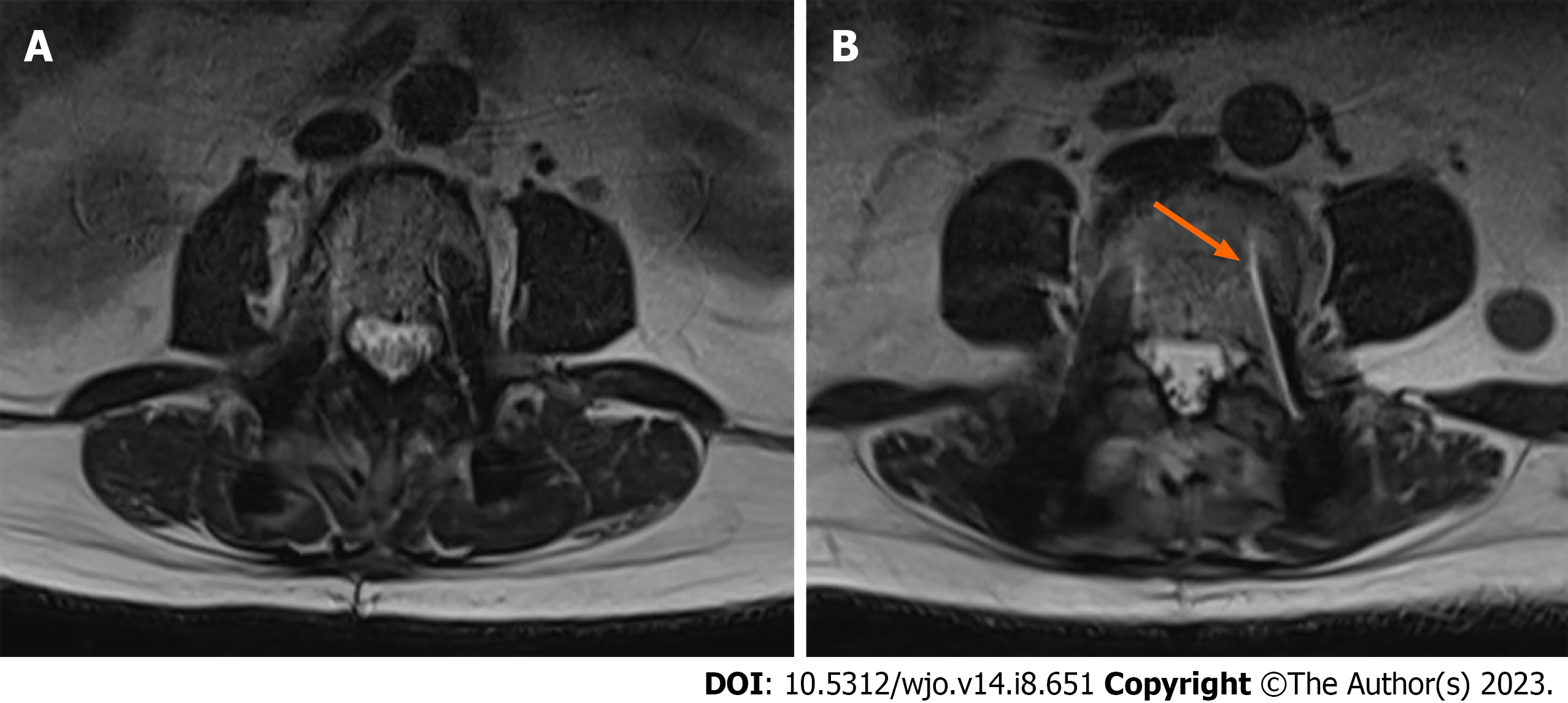
Lumbar spine MRI revealed severe spinal canal stenosis at the L2-L3 level with compression of the cauda equina nerve roots, severe bilateral lateral recess, and neural foraminal stenosis (Figure 2). There was moderate spinal canal stenosis with crowding of the cauda equina nerve roots at L1-L2. Mild peri-screw bony edema was observed around the left L4 screw (Figure 3), otherwise there was no significant evidence of peri-screw edema and screw loosening around the rest of the L3 to S1 screws.
Histopathology of the stained tissues that were excised revealed fibroadipose tissue and occasional striated muscle bundles exhibiting degenerative changes. There were aggregates of non-refractile, non-polarisable black granular foreign material mostly in a perivascular location, that were consistent with metallosis. There was no evidence of malignancy. Intra-operative tissue cultures were negative for bacterial growth.
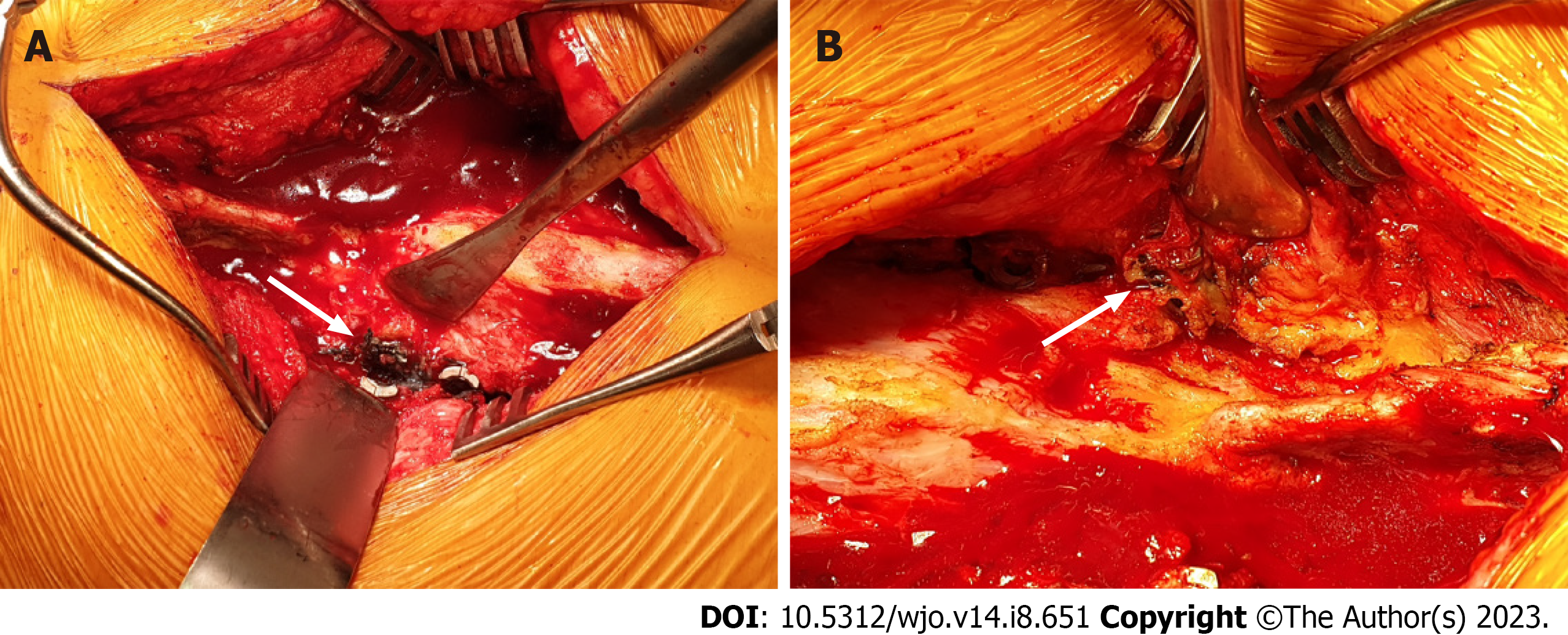
L2 to S1 posterior decompression and instrumented fusion with O-Arm computer-guided navigation (Medtronic, StealthStation® S7®) were performed. Posterior elements were exposed from L2 to S1, revealing loosened L3 to L5 screws bilaterally, at both the set screw-rod interface and the bone-implant interface. The tissues surrounding the bilateral L3 to L5 polyaxial screw heads and tulips were observed to be stained dark grey (Figure 4). All previous DePuy Synthes screws were removed uneventfully. The loosened screws showed evidence of fretting at the contact surfaces. Newtitanium pedicle screws (Medtronic, CD Horizon® Solera®) were inserted with new trajectories under O-Arm computer-guided navigation. L2 and L3 Laminectomy was carried out. The thecal sac was well decompressed at L2-L3 where there was severe stenosis. A drain was inserted, and the surgical site was closed in layers.
The patient’s postoperative recovery was uneventful. At the 2-mo follow up, her back pain and lower limb weakness had almost completely resolved. Her postoperative lumbar spine radiographs showed that the new implants were intact (Figure 5).
Metallosis was first identified as a complication of metal-on-metal total hip arthroplasty[1]. The incidence of metallosis following total hip arthroplasty has been described to be 5.3% and 0.3% after lumbar arthroplasty[3], but the incidence following spinal instrumentation such as posterior spinal fusion is not well estimated currently due to the scarce literature. A literature search detailing other cases of metallosis after spinal instrumentation revealed that it is a rare occurrence. A detailed look of the reported cases of metallosis after spinal instrumentation is summarised in Table 1.
| Ref. | Patient biodata | Type of surgery undergone | Instrumentation | Symptoms leading to revision surgery | Radiological findings | Revision surgery | Intraoperative findings | Histopathological findings | Patient outcome |
| Takahashi et al[4], 2001 (case series) | 1 Female, aged 58 | Posterior correction and stabilisation T10-L3 (no decompression) for degenerative thoracolumbar scoliosis | Stainless steel Cotrel-Dubousset | Left L4-L5 radicular pain several months post-op | Plain radiographs: No implant dislodgement or spinal instability; myelography: Shadow defect adjacent to the tip of the L3 infralaminar hook and dura mater was compressed from the posterior | 11 mo post-op; removal of L3 pedicular screws and left L3 infralaminar hook, L3 laminectomy, excision of metallotic mass, instrumentation elongated to L4 with connecting pieces, posterolateral fusion | L3 hook loose from rod, macroscopic metallosis (8 mm mass of dark grey granulation tissue) at hook-rod junction extending to surrounding fibrous tissues, L2-L3 pseudarthrosis | Not described | Immediate resolution of radicular pain, but continued to have slight low back pain during active trunk motion |
| 2 Female, aged 54 | Posterior correction and arthrodesis T12-L4 for symptomatic degenerative lumbar scoliosis | Stainless steel Cotrel-Dubousset | Right L5 sciatic pain 4 yr post-op | Plain radiographs: No implant dislodgement or spinal instability; myelography: Stenotic lesion at lowest level of instrumented lumbar spine but undisplaced implants; myelotomography: No migration of the hooks in the spinal canal, stenotic lesion adjacent to tip of L4 supralaminar hook | 5 yr post-op; L4 and L5 laminectomy, excision of metallotic mass, instrumentation elongated down to sacrum | 1 cm × 1 cm × 2 cm dark grey granulation tissue under L4 lamina continuous with fibrous membrane of the same colour surrounding right L4 supralaminar hook and compressing right L5 root, loosening implant connection and wear of rod at hook-rod junction, no pseudarthrosis | Granulation tissue consisting of metallic debris – iron staining showed widespread intracellular iron, spectrometry analysis of metal concentrations showed presence of iron, nickel and chromium | Radicular symptoms resolved | |
| Tezer et al[5], 2005 (Case report) | Male, aged 57 | Posterior spinal instrumentation for T8-9 compression fracture | Stainless steel pedicle screw-hook combination system | Progressive paraparesis 3 yr post-op | Myelography and myelo-CT: Focal image of a mass at T6-7 antero- laterally displacing the dural sac and spinal cord; CT and MRI could not be done due to diffuse metal artefacts | Posterior surgical procedure, complete removal of implants, excision of mass, all metallic debris cleaned | Corroded, black-coloured pedicle hook, no loosening or colour change of other implanted parts, construct stable and strong, fusion complete, granuloma formation in centre of metallic construct, metallic debris had pushed dural sac and spinal cord to anterior and contralateral side resulting in defect of 1.5 cm in diameter in lamina and pedicle | Hematoxylineosin stained sections of paraffin-embedded material showed dense fibrotic tissue heavily stained with black metal debris, foreign body giant cells seen around metallic debris, iron staining by Perls method showed widespread iron within macrophages | Symptom-free 3 mo post-op |
| Goldenberg et al[6], 2016 (Systematic review) | Male, aged 75 | Single-level lumbar laminectomy, posterior instrumentation and fusion | Bilateral L4 and L5 titanium alloy pedicle screws, dual interconnecting vertical rods, single interconnecting horizontal rod using the DENALI K2M system, interbody device containing bone graft admixed with bone morphogenetic protein, high speed burr used but no contact between metalwork and drill | Persistent and progressive severe lower back pain since the surgery, associated with severe left-sided sciatica | CT myelography: Posterior epidural mass causing canal stenosis, no features suggestive of corrosion or loosening of metalwork; SPECT: Increased uptake in keeping with discovertebral disease; MRI not done as incompatible cardiac pacemaker | Explorative lumbar canal decompression and nerve root neurolysis, dissected down to area of previous surgery, removal of scar tissue and rostral part of L5 lamina and spinous process, debulking of mass | Scar tissue in area of previous surgery, intermixed dark brown and pale pink roughened firm tissue compressing thecal sac, no implant loosening or corrosion | Dense fibrohistiocytic reaction and cystic change associated with granulomas and calcification, multinucleated giant cells both encasing and adjacent to foreign black pigmented particles, presence of degenerate bone, cartilaginous material and skeletal muscle, no micro-organisms identified | Satisfactory clinical improvement in back pain and sciatica |
| Li et al[7], 2016 (Case report) | Male, aged 58 | Posterior decompression and instrumented fusion | Titanium implant (surgery was done at another institution) | Recurrent lower back pain radiating to left lower limb, dysesthesia, neurogenic claudication | MRI: Severe adjacent stenosis at L3-4, intraspinal extradural tumor-like mass with compression of the neurological elements | Spinal decompression, excision of mass, and extension of instrumented fusion | Metallic soft tissue and a well-capsulated tumor-like mass | Hematoxylin and eosin staining of mass showed many spindle-shaped; fibroblasts. Many macrophages containing dark metallic wear particulates with phagocytosis | Follow-up not reported |
| Ayers et al[8], 2017 (Case series) | Male, aged 74 | Multiple previous spinal surgeries including limited lumbar fusion complicated by pseudarthrosis, revision with extension of fusions and infection at subsequent operations | Mix of alloy rods (CoCrMoC, ASTM F-1537 specification) and titanium alloy (Ti6Al4V ASTM F-136 specification) screws | Continued mechanical back and radicular pain | CT: Hardware failure with bilateral fractured L5 screws and sagittal plane deformity | Staged revision surgery; (1) Initial surgery - removal and cleaning of T10-S1 hardware, evacuation of fluid collection, wound debridement, intra-op cultures, and exploration of the fusion, subfascial drains inserted; (2) then 2 further irrigation and debridement procedures until cultures negative and tissues appeared viable; and (3) after 6 wk, final stage – evacuation of smaller fluid collection, revision posterior instrumentation with L3 pedicle subtraction osteotomy | (1) Initial surgery: Large fluid pocket containing approximately 500 mL of grey-black fluid, black discolouration of posterior soft tissues, all rods showed significant evidence of fretting, galling, pitting and crevice corrosion; and (2) final stage: Smaller fluid collection of 300 mL in posterior soft tissues, gram stain negative | Excised tissue consisted of necrotic fibrous tissue with areas of viable fibrous tissue and particle laden histiocytes. Soft tissue, pseudomembrane from L3-S1 consisted of fibrous tissue with refractile material and calcification. Cell culture of infected tissue/fluid showed presence of propioni-bacterium acnes and staphylococcus aureus | Significant reduction in pain and symptoms 1 yr post-op |
| Male, aged 47 | Multiple previous lumbar spine procedures complicated by pseudarthrosis and infection | Titanium alloy (Ti6Al4V) components | Recurrent pulmonary infections and continued back and radicular leg symptoms | CT: Likely pseudarthrosis at multiple lumbar spine levels | 2 yr post-op; Staged surgery; (1) Initial surgery – wound exploration, removal of hardware, formal irrigation-and-debridement, deep drains placed; (2) another irrigation-and-debridement with post-op antibiotics × 6 wk; (3) after 6 wk, instrumented fusion from T10-Ilium with revision TLIF at L2-3 and Smith-Petersen Osteotomy; (4) irrigation-and-debridement; and (5) removal of right S1 screw as it was causing right nerve root irritation | (1) Initial surgery: Significant fluid collection, soft tissues stained black, all rods showed significant evidence of fretting, galling, pitting and crevice corrosion | (1) Initial surgery: Excised tissue comprised of necrotic adipose and fibrotic connective tissue; and (2) instrumented fusion stage: Cultures grew Mycobacterium phlei | No back or leg pain at follow up (recent to when paper was written) | |
| Female, aged 61 | Single level lumbar stabilisation procedure including instrumentation with pedicle screws and PEEK rod | Titanium alloy (Ti6Al4V) components | Significant sagittal plane deformity and significant back/radicular leg symptoms | CT: Significant sagittal plane deformity | Instrumentation from T4-pelvis with hardware removal and pedicle subtraction osteotomy, including removal of L2-3 disc to allow greater correction | Significant black staining of the posterior soft tissues, all rods showed significant evidence of fretting, galling, pitting and crevice corrosion | Tissues not submitted to pathology | Complete symptomatic relief at 6 mo post-op | |
| Richman et al[9], 2017 (Case report) | Male, aged 19 | Posterior spinal fusion | Stainless steel implants | Low back pain, urinary hesitancy, and parasthesias on bilateral anterior thighs, that quickly progressed to flaccid paraparesis, hypoesthesis, and urinary retention | CT: Cavitation around right L1 pedicle screw CT myelogram: Irregular and inadequate opacification of the thecal sac at L1 | (1) Initial surgery: Removal of screw; and (2) posterior laminectomy and decompression from T12 to L2 with removal of all instrumentation | (1) Initial surgery: Black and yellowish corrosive film and tissue around right L1 screw; and (2) subsequent surgery: Gritty yellow-black material tracking through the L1 foramen around left L1 pedicle screw, causing thecal saccompression at T12-L2 | Pathologic diagnosis was consistent with metallosis | Pain and urinary retention resolved, complete motor and sensory recovery, but presence of bilateral clonus 3 yr post-discharge |
| Mazur-Hart et al[10], 2022 (Case report) | Male, aged 79 | 2 previous lumbar decompression, posterior instrumentation and fusion 2 yr apart. Right hip arthroplasty 1 yr later | First surgery: Cobalt chrome rods and titanium screws. Second surgery: PEEK spacer and titanium screws and plates | Worsening falls, ataxia and pseudoclaudication | CT and MRI: T1 and T2 hypointense non–enhancing mass around right-sided paraspinal rod extending into spinal canal and surrounding bones and muscle on the same side | L4-S1 biopsy and subtotal resection of paraspinal mass with removal of hardware at L2-S1 | Dense fibrotic tissue, black granular material on screws and rods, black staining of adjacent soft tissues and lumbar bone | Extensive necrosis with surrounding inflammation and fibrosis with focal deposition of black pigment of exogenous origin (metallic vs carbonaceous), lymphohistiocytic reaction with giant cell formation in rare areas. Gram stain and culture negative | Weaned off walker, reduced dysesthesia but leg weakness still present 3 mo post-op. Leg strength and ambulation continued to improve 7 mo post-op |
The earliest cases of metallosis following spinal instrumentation were reported by Takahashi et al[4]. The authors described two cases of delayed neurological deficits secondary to intraspinal metalloma adjacent to loosened infralaminar hooks. One of the patients had undergone posterior correction and stabilisation and the other had undergone posterior correction and arthrodesis for degenerative scoliosis. Radicular symptoms resolved entirely after revision surgery. The authors speculated that metallosis was caused by abnormal implant movements and chemical reactions from the metal particles. Tezer et al[5] then described a case whereby paraparesis secondary to intraspinal metallosis adjacent to the pedicular hook occurred 3 years after posterior spinal instrumentation and fusion for a vertebral compression fracture. The patient’s neurological symptoms resolved completely following the excision of the metalloma and removal of the affected instrumentation. The authors concurred with the pathophysiology of metallosis described by Takahashi et al[4], and recommended using transpedicular screws sufficiently while carrying out further research to improve the corrosive resistance of spinal instrumentation. Goldenberg et al[6] also reported a case of spinal metalloma 18 mo after lumbar laminectomy, posterior spinal instrumentation, and fusion using titanium instead of stainless-steel alloy components. The authors concluded that the metallosis in their case occurred due to the interaction between titanium and the surrounding tissue structures rather than as a result of implant failure, corrosion, or infection as described in previous cases. Li et al[7] described another case of metalloma attributed to the wear and loosening of implant. A 2-cm large metalloma could be visualised on MRI. Prior to this study, MRI had not demonstrated much utility in the investigation of metallosis. Subsequently, Ayers et al[8] described three more cases of spinal metallosis, two of which had undergone multiple previous spine surgeries complicated by pseudarthrosis and infection and the last had undergone single-level lumbar stabilisation. Neurological symptoms improved in all three cases following revision surgery. The authors hypothesised that biologic mechanisms such as bacterial growth could influence fretting and corrosion of spinal instrumentation leading to metallosis. Richman et al[9] then described a young patient with acute onset pain and neurological deficits that progressed quickly. Previous instrument made of stainless steel was removed. They also noted high serum chromium levels. Most recently, Mazur-Hart et al[10] reported a case of unilateral metalloma from mixed-metal (titanium and cobalt chrome) instrumentation that resulted in progressive neurological deficit, but not hardware failure. Another unusual finding was the absence of metallosis on the side where the patient had also undergone a hip arthroplasty comprising of the same materials.
Metallosis is often diagnosed incidentally through intraoperative findings of grey-stained local tissue[8], and definitively through histopathological evidence of macrophages containing metal debris[11]. This is because of the non-specificity of clinical presentations, such as pain, symptoms of infection, and neurological deficits[9]. Based on case studies in the literature (summarised in Table 1), patients with spinal metallosis most commonly presented with lower back or radicular pain. Other symptoms included neurogenic claudication and progressive paraparesis of the lower limbs. It is also challenging to visualise metallosis through standard radiographic evaluation[8]. MRI and computed tomography (CT) are not able to definitively diagnose spinal metallosis due to the presence of artifacts around the metal implants[4,5]. Ayer et al’s study showed that all three cases did not have evidence of metallosis on CT prior to surgery[8]. This differs from the usefulness of CT in the diagnosis of metallosis in total hip arthroplasties, in which metallic debris or a high-density material outlining the joint capsule or bursa can be visualised[12]. On the other hand, CT myelography has been the diagnostic imaging modality of choice in a number of studies on spinal metallosis, showing stenotic lesions adjacent to previous instrumentation[4,5,6,9]. In our case, lumbar spine radiographs showed evidence of loosening of pedicle screws. Screw loosening can be secondary to a variety of factors including metal wear debris, microfracture, infection, tumour, and metabolic diseases, with a greater incidence in patients with osteoporosis[13]. Our patient had type 2 diabetes mellitus but was not known to have osteoporosis. She did not have constitutional symptoms and her preoperative routine blood tests were unremarkable. Therefore, osteolysis secondary to fretting of instrumentation was a more probable mechanism for the loosening of screws in this case. MRI was not useful in identifying hardware loosening or spinal metallosis in our case. In a recent clinical trial by Spirig et al[14], CT was more sensitive and specific in detecting screw loosening despite applying metal artifact reduction techniques with MRI. However, MRI may be of utility in cases where the metalloma is large enough with compression or extension into surrounding structures, such as in the cases reported by Li et al[7] and Mazur-Hart et al[10]. Comparing plain radiographs and CT findings of previous studies (Table 1), we suggest adopting a high index of suspicion of metallosis when screw loosening is evident on radiographical imaging in a patient with persistent postoperative back pain or radiculopathy but otherwise medically well. If plain radiographs do not show hardware abnormalities, it may be prudent to proceed with a CT scan or myelogram instead.
In regards to the relationship between serum metal concentrations and the development of metallosis, Richman et al[9] noted that in asymptomatic patients, serum chromium levels of more than 0.6 ng/mL and more than 3.75 μg/L were indications of implant malfunction and corrosion, respectively. Fernández Bances et al[15] found a significant rise (P = 0.00049) in serum titanium concentrations from the levels prior to posterior spinal fusion using titanium instrumentation, similarly to previous studies; however, the correlation of serum titanium concentrations and metallosis was not explored in their study. Cundy et al[16] found that serum titanium and niobium levels in children 2 years after instrumented spinal fusion were significantly increased but their clinical significance was not explored. Ayers et al[8] reported that muscle concentrations of various metals, namely, aluminium, cobalt, vanadium, and molybdenum, were higher than normal levels in their cases with spinal metallosis due to observed wear and corrosion of the metal instrumentation. However, none of the patients had elevated concentrations of metal in blood. So far, serum metal levels indicating metallosis have yet to be well-defined. We did not check serum metal concentrations in our patient as she did not have symptoms of metal poisoning, there was no baseline data prior to her previous spinal surgery, and investigating metal concentrations this time would be costly and purely academic. However, in patients exhibiting symptoms of metal poisoning, serum metal concentrations will be helpful in confirming a diagnosis of metallosis, potentially leading to timely intervention.
The mechanism for the development of metallosis following spinal instrumentation is yet to be well-ascertained. It has been postulated that the rigidity that results from instrumentation leads to accelerated degeneration at adjacent spinal levels[7]. The metallic debris from the degeneration of the spinal implants in turn results in a chronic inflammatory process involving a foreign-body granulation-type reaction[1,7]. Spinal metallosis has been described in studies that involved titanium or stainless steel implants[3]. Spinal instrumentation of different metals is commonly used in combination as each metal has a particular mechanical and physical property; for example, cobalt chromium rods have been described to provide stronger correctional forces for scoliotic curves as compared to rods made of other materials[17]. Titanium may be more resistant to crevice corrosion than stainless steel but it has less mechanical resistance and may even stimulate osteolysis[4]. Singh et al[18] demonstrated that posterior spinal fusion constructs made of stainless steel were more prone to fretting corrosion as compared to those made with a combination of cobalt chrome with titanium alloy or pure titanium with titanium alloy in a simulated in vitro experiment using normal saline. However, the study was unable to account for the actual inflammatory environments present in the human body. Panagiotopoulou et al’s study on retrieved spinal implants demonstrated that the risk of corrosion was not increased when two dissimilar metals, namely, cobalt chromium alloy rods and titanium screws, were used in combination[17]. The authors suggested that metallosis may be more dependent on patient factors rather than the corrosiveness of the metals. However, the main limitation of that study was its small sample size, whereby a combination of metals was employed only in two out of seven patients[17].
Vieweg et al[19] in an early study on the corrosion of the internal spinal fixator system described that corrosion occurred due to not only the metallurgical composition but the specific construction of the instrument as well. Cundy et al[16] described that crevice corrosion was more likely to occur at rod junctions with increased metal-on-metal sites, contributed by micromovements prior to spinal fusion. Takahashi et al[4] noted that the lower end of an instrumented fusion was subjected to greater stress hence predisposing the release of metal debris from the hook-rod junction during flexion-extension movement of the lumbar spine. Comparatively, Tezer et al[5] felt that metallosis occurred at the middle levels of the spinal construct in their case because of the unequal distribution of chemical properties and degeneration of micromovements in the long term. Interestingly, metallosis occurred at the upper levels of the spinal construct in our patient, where the burden of flexion-extension is not particularly high in day-to-day activities.
Patient factors such as a high BMI as evident in our patient could have accelerated the wear of the titanium screws. As described in the literature review, previous studies have postulated that metallosis results from abnormal micromove
Metallosis is also likely to be a by-product of unstable spinal instrumentation. The increased cyclical loading as a result of implant loosening causes increased fretting at the contact surfaces. This not only produces the characteristic metal debris in metallosis, but ultimately can lead to implant failure. Pseudarthrosis after lumbar spine fusion is a common cause of spinal implant loosening requiring revision surgery[21]. Chronic low-grade spinal surgical site infection is another potential cause for instrumentation loosening, hence stressing the importance of sterile instrumentation[22,23]. Several procedure-related risk factors for implant loosening have also been described. First, inadequate correction of sagittal imbalance has been associated with a negative prognosis for implant anchorage in bone[24], increasing the risks of screw loosening in posterior spine fusion[25]. Inadequate set screw tightening or cross-threading of screw tulips due to improper insertion of set screws into the screw tulip can also predispose to coupling failure, which can occur at any level of a spinal construct[26]. Hence, spinal surgery should be performed only by well-trained spinal surgeons with vast experience and undertaking these operations regularly to minimise these mechanical risk factors. Other possible risk factors for instrumentation loosening are osteoporosis and cobalt chromium rods[23]. Our patient was not known to have osteoporosis, but it may be prudent to commence osteoporosis treatment prior to surgery in patients who have been diagnosed with osteoporosis so as to improve bone density and potentially increase the strength of screw fixation. Utilising a material that is less rigid than cobalt chromium rods may reduce the risk of implant loosening; however, current options are limited, and cases of metallosis including our patient have mainly involved titanium or stainless steel instrumentation. Our patient’s symptoms of progressive lower back and radicular pain were due to severe spinal stenosis and compression of the cauda equina nerve roots. Although implant loosening may present similarly even in the absence of metallosis, there was both intraoperative (corrosion of screws and grey-stained surrounding tissues) as well as histopathological (metallic debris in macrophages) evidence of metallosis at the same spinal levels. Ultimately, it is important to recognise symptoms of spinal implant instability and initiate treatment for patients timely.
Identifying metallosis prior to surgical exploration is challenging. Clinical presentation tends to be non-specific, most commonly being lower back or radicular pain. CT appears to be the modality of choice to observe for aseptic hardware loosening and pseudarthrosis, while myelography and MRI are able to suggest the presence of a metalloma. A definitive diagnosis of metallosis can only be made from histopathological results, where metallic debris is seen in macrophages.
To date, the relationship between serum metal levels and the presence of metallosis has yet to be established. Currently, implants of various metallic compositions are used, but the individual metallic properties confer theoretical benefits and disadvantages and no particular material has been identified to be least likely to cause metallosis thus far. Furthermore, patient factors may contribute to metallosis but further studies are required to establish an association.
Finally, we would like to highlight that the presence of metallosis after spinal instrumentation likely indicates a more complex underlying problem. Metallosis can occur due to instability of the spinal implants, which may be secondary to pseudarthrosis, failure to address sagittal balance, infection, and cross-threading of set screws. Spinal implant instability manifests commonly as pain and weakness, which were present in most cases involving instrumentation loosening described within this report to varying degrees. However, regardless of the cause for metallosis, the only definitive treatment to date for symptomatic implant loosening is the removal and replacement of the implants. The rate at which metallosis progress and the onset of symptoms is not known. However, it undoubtedly can lead to significant pain and mobility issues. Hence, it is prudent to identify the underlying cause of implant loosening early and commence treatment promptly.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fernandez-Fairen M, Spain; Zhang M, China; Zhou Y, China S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Xu ZH
| 1. | Marinescu R, Socoliuc C, Botezatu L, Laptoiu D, Voinescu D. Metallosis – literature review and particular cases presentation. Key Eng Mater. 2017;745:77-90. [DOI] [Full Text] |
| 2. | Alpert HW, Farley FA, Caird MS, Hensinger RN, Li Y, Vanderhave KL. Outcomes following removal of instrumentation after posterior spinal fusion. J Pediatr Orthop. 2014;34:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Yang CC, Tang CL, Tzeng CY, Tsou HK. Metallosis after traumatic loosening of Bryan cervical disc arthroplasty: a case report and literature review. Eur Spine J. 2018;27:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Takahashi S, Delécrin J, Passuti N. Intraspinal metallosis causing delayed neurologic symptoms after spinal instrumentation surgery. Spine (Phila Pa 1976). 2001;26:1495-8; discussion 1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Tezer M, Kuzgun U, Hamzaoglu A, Ozturk C, Kabukcuoglu F, Sirvanci M. Intraspinal metalloma resulting in late paraparesis. Arch Orthop Trauma Surg. 2005;125:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Goldenberg Y, Tee JW, Salinas-La Rosa CM, Murphy M. Spinal metallosis: a systematic review. Eur Spine J. 2016;25:1467-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Li YC, Yang SC, Hsu CT, Tu YK. Capsulated Metallic Debris Tumor Mass Mimicking Adjacent Segment Disease: A Case Report. Clin Spine Surg. 2016;29:E532-E535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Ayers R, Miller M, Schowinsky J, Burger E, Patel V, Kleck C. Three cases of metallosis associated with spine instrumentation. J Mater Sci Mater Med. 2017;29:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Richman SH, Razzano AJ, Morscher MA, Riley PM Sr. Metallosis Presenting as a Progressive Neurologic Deficit Four Years After a Posterior Spinal Fusion for Adolescent Idiopathic Scoliosis: A Case Report. Spine (Phila Pa 1976). 2017;42:E56-E59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Mazur-Hart DJ, Larson EW, Yaghi NK, Halfpenny AM, Pettersson DR, Yam DA. Neurological emergency from rare spinal metalloma: Case report and literature review. Radiol Case Rep. 2022;17:1540-1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Natu S, Sidaginamale RP, Gandhi J, Langton DJ, Nargol AV. Adverse reactions to metal debris: histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. J Clin Pathol. 2012;65:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Kirkham JR, Petscavage JM, Richardson ML. Metallosis: CT findings in a total hip arthroplasty. Radiol Case Rep. 2010;5:410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Wu X, Shi J, Wu J, Cheng Y, Peng K, Chen J, Jiang H. Pedicle screw loosening: the value of radiological imagings and the identification of risk factors assessed by extraction torque during screw removal surgery. J Orthop Surg Res. 2019;14:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Spirig JM, Sutter R, Götschi T, Farshad-Amacker NA, Farshad M. Value of standard radiographs, computed tomography, and magnetic resonance imaging of the lumbar spine in detection of intraoperatively confirmed pedicle screw loosening-a prospective clinical trial. Spine J. 2019;19:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Fernández Bances I, Paz Aparicio J, Alvarez Vega MA. Evaluation of Titanium Serum Levels in Patients After Spine Instrumentation: Comparison Between Posterolateral and 360º Spinal Fusion Surgery. Cureus. 2019;11:e5451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Cundy TP, Cundy WJ, Antoniou G, Sutherland LM, Freeman BJ, Cundy PJ. Serum titanium, niobium and aluminium levels two years following instrumented spinal fusion in children: does implant surface area predict serum metal ion levels? Eur Spine J. 2014;23:2393-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Panagiotopoulou VC, Hothi HS, Anwar HA, Molloy S, Noordeen H, Rezajooi K, Sutcliffe J, Skinner JA, Hart AJ. Assessment of corrosion in retrieved spine implants. J Biomed Mater Res B Appl Biomater. 2018;106:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Singh V, Shorez JP, Mali SA, Hallab NJ, Gilbert JL. Material dependent fretting corrosion in spinal fusion devices: Evaluation of onset and long-term response. J Biomed Mater Res B Appl Biomater. 2018;106:2858-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Vieweg U, van Roost D, Wolf HK, Schyma CA, Schramm J. Corrosion on an internal spinal fixator system. Spine (Phila Pa 1976). 1999;24:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Sheng B, Feng C, Zhang D, Spitler H, Shi L. Associations between Obesity and Spinal Diseases: A Medical Expenditure Panel Study Analysis. Int J Environ Res Public Health. 2017;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Raizman NM, O'Brien JR, Poehling-Monaghan KL, Yu WD. Pseudarthrosis of the spine. J Am Acad Orthop Surg. 2009;17:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Prinz V, Bayerl S, Renz N, Trampuz A, Czabanka M, Woitzik J, Vajkoczy P, Finger T. High frequency of low-virulent microorganisms detected by sonication of pedicle screws: a potential cause for implant failure. J Neurosurg Spine. 2019;31:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Chang HK, Huang WC, Wu JC. Commentary: Low-Grade Infection and Implant Failure Following Spinal Instrumentation: A Prospective Comparative Study. Neurosurgery. 2020;87:E541-E542. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Marie-Hardy L, Pascal-Moussellard H, Barnaba A, Bonaccorsi R, Scemama C. Screw Loosening in Posterior Spine Fusion: Prevalence and Risk Factors. Global Spine J. 2020;10:598-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 25. | Berjano P, Bassani R, Casero G, Sinigaglia A, Cecchinato R, Lamartina C. Failures and revisions in surgery for sagittal imbalance: analysis of factors influencing failure. Eur Spine J. 2013;22 Suppl 6:S853-S858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Pihlajämaki H, Myllynen P, Böstman O. Complications of transpedicular lumbosacral fixation for non-traumatic disorders. J Bone Joint Surg Br. 1997;79:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 3.4] [Reference Citation Analysis (0)] |