Published online Aug 18, 2023. doi: 10.5312/wjo.v14.i8.598
Peer-review started: April 22, 2023
First decision: May 25, 2023
Revised: June 7, 2023
Accepted: July 11, 2023
Article in press: July 11, 2023
Published online: August 18, 2023
Processing time: 117 Days and 1.3 Hours
The musculoskeletal system involves multiple tissues which are constantly exposed to being exposed to various biological and mechanical stimuli. As such, isolating and studying a particular system from a complex human clinical environment is not always a realistic expectation. On top of that, recruitment limitations, in addition to the nature of orthopaedic interventions and their associated cost, sometimes preclude consideration of human trials to answer a clinical question. Therefore, in this mini review, we sought to rationalize the rapid evolution of biomedical research at a basic scientific level and explain why the perception of orthopaedic conditions has fundamentally changed over the last decades. In more detail, we highlight that the number of orthopaedic in vitro publications has soared since 1990. Last but not least, we elaborated on the minimum requirements for conducting a scientifically sound infection-related laboratory experiment to offer valuable information to clinical practitioners. We also explained the rationale behind implementing molecular biology techniques, ex vivo experiments, and artificial intelligence in this type of laboratory research.
Core Tip: This paper highlights some major orthopaedic research advances at a basic science level. On top of that, it is highlighted that the perception of orthopaedic conditions has fundamentally changed recently, reflecting on clinical practice. We also described the basic aspects of a successful in vitro infection laboratory experiment and expanded on recent evidence relating to molecular biology, ex vivo investigations, and artificial intelligence in orthopaedics.
- Citation: Tsikopoulos K, Drago L, Meroni G, Kitridis D, Chalidis B, Papageorgiou F, Papaioannidou P. In vitro laboratory infection research in orthopaedics: Why, when, and how. World J Orthop 2023; 14(8): 598-603
- URL: https://www.wjgnet.com/2218-5836/full/v14/i8/598.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i8.598
Barriers to orthopaedic research: It is widely recognized that high-quality randomized evidence is lacking in orthopaedics. The main reasons behind that include the increased cost of conducting a trial, the lack of funding and resources, the limited availability of time from the clinicians[1], and the overall recruitment limitations. Although significant musculoskeletal concerns are fraught with ambiguity in the scientific community, it has been demonstrated that many surgeons are insufficiently motivated to discuss trial participation with their patients[2]. On top of that, orthopaedic trainees may show limited interest in conducting research, feel unsupported, and lack mentorship and/or opportunities to get involved in experimental projects[3]. According to the above, when assessing the feasibility of orthopaedic projects, researchers should have realistic expectations and carefully select the appropriate study design.
Is in vitro research the appropriate remedy? It should be noted that in vitro models are laboratory-based experiments that imitate biological processes outside a real organism. In vitro models are frequently utilized in orthopaedic research to investigate many aspects of musculoskeletal diseases, tissue engineering, and medication development[4]. Conducting in vitro laboratory research enables researchers to overcome the complexity of multiple organ interactions in human beings[4]. Furthermore, funding for clinical and translational animal research is usually insufficient to address the unanswered orthopaedic research questions. Therefore, opting for basic science projects appears beneficial, particularly when investigating topics that have not been dealt with before. Nevertheless, we should underline that although basic science orthopaedic research intends to bridge the gap between the absence of knowledge on a given topic and clinical practice, we advocate that the results of in vitro trials must be interpreted cautiously and should not be exclusively extrapolated in human setting.
Infection outcomes and administration/training requirements: First, it must be pointed out that the standard strain of a given microorganism is investigated for reproducibility and transparency reasons. Initial testing should include planktonic growth evaluation not only in the presence of antimicrobial agents as well as untreated control, in order for the researcher to reliably evaluate the antimicrobial capacity of a potential antimicrobial agent. Subsequently, biofilm studies should include intervention(s) group(s) and an untreated biofilm control.
When assessing the results of the exposure of microorganisms to potential anti-bacterial agents, there are plenty of options to investigate their effectiveness in the lab. The most common method features a colorimetric evaluation of cell viability with the 2-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide reduction assay, which is a highly reliable method and particularly useful for investigating multiple technical replications in the lab[5,6].
Regarding the infection prevention experiments, attention should be paid to the difference in cell viability between the intervention and control groups. The minimum inhibition threshold of 80% must be surpassed for this difference to be clinically meaningful[7].
From an administrative perspective, attaining Institutional Review Board Approval before performing a formal microbiological experiment is advisable to ensure Good Laboratory Practice. According to our experience and literature, substantial up-front investment in infrastructure and lab consumables is suggested to ensure an uninterrupted flow of experiments. From a scientific point of view, collaboration with well-established infection labs as well as ongoing lab training of the personnel are essential steps for the successful execution of experiments.
Guidelines, statistical considerations, pictorial presentations and extrapolation of results: Similar to other types of research, reporting guidelines should be provided along with the experimental studies[8]. More specifically, the primary and secondary outcomes of a given in vitro experiment should be clearly reported in the methods sections.
From a statistical point of view, researchers should bear in mind that achieving sufficient study power applies to clinical papers and basic science research. In other words, implementing a sufficient sample size based on the primary outcomes of an in vitro study enables the authors to reach consistent and clinically meaningful conclusions. Moreover, authors are encouraged to comment on any potential biases which may compromise the reliability of their findings[8].
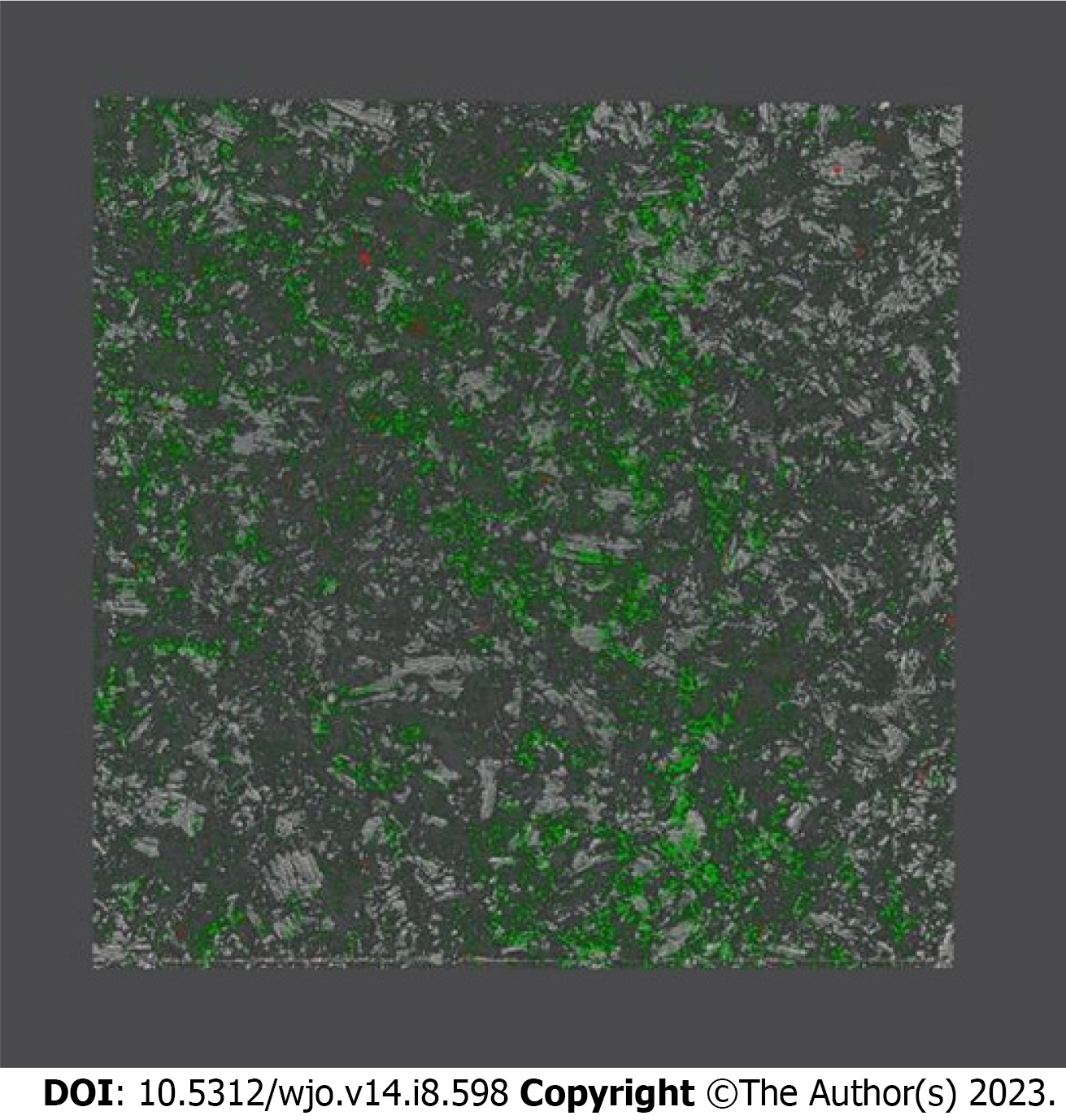
Pictorial and graphical presentation of the results is essential: Regarding infection-related in vitro pre-clinical research, it is proposed that the authors produce a graphical representation of microbial development that will serve as the basis for their future tests[9] (Figure 1).
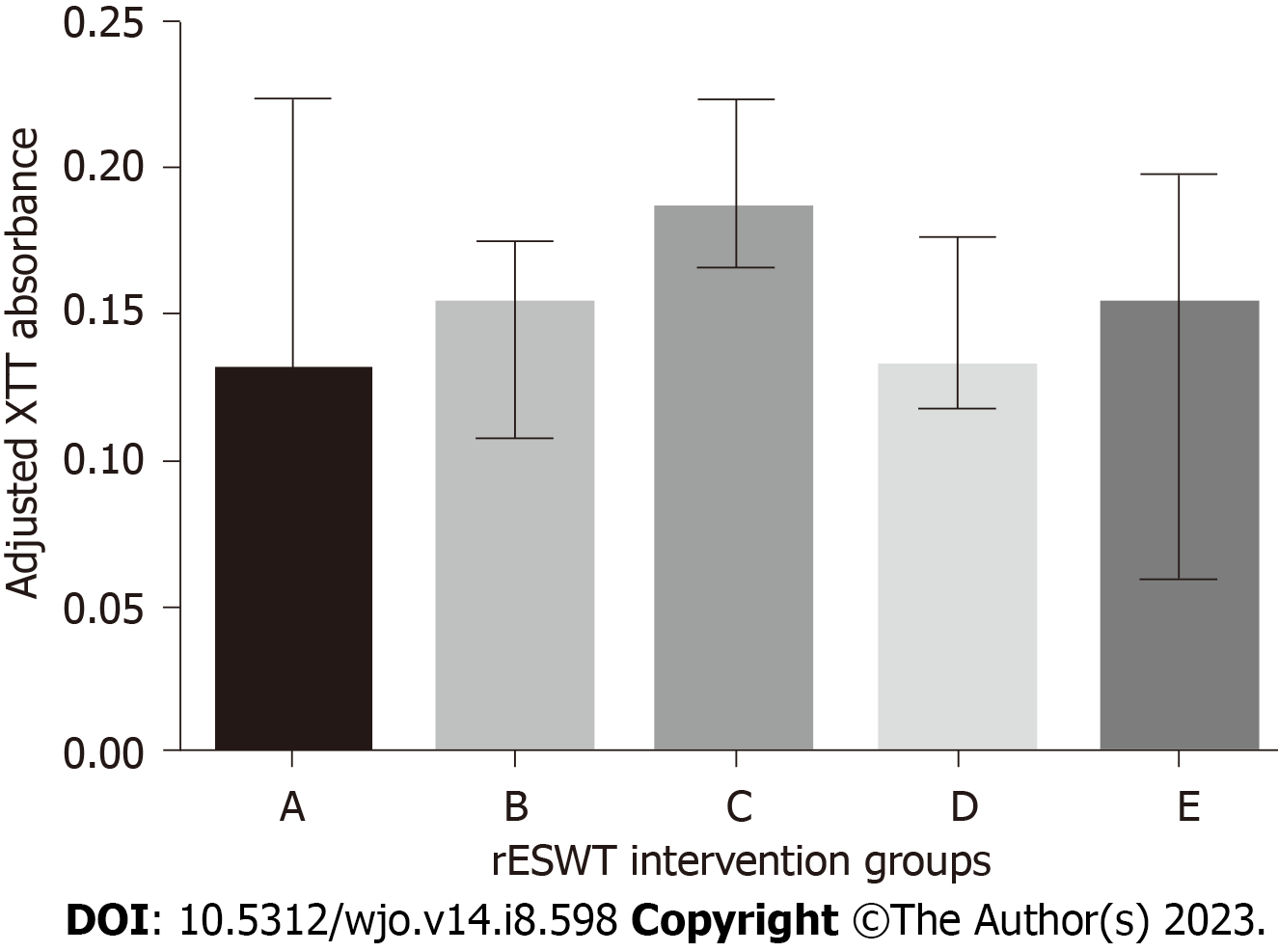
In doing so, the credibility of the work and the establishment of future, more advanced and sophisticated investigations can be improved. Furthermore, in case of comparisons with control groups, implementation of a dedicated piece of software such as Prism (GraphPad Software, Inc, La Jolla, CA, United States) is advocated to generate scientifically sound figures[9] (Figure 2).
Furthermore, considering that in vitro research is the first step of the evidence-based procedure, researchers and clinicians alike should adopt a stepwise approach when validating the results of an in vitro laboratory experiment. More specifically, a proper animal model study should be generated following a successful in vitro experiment[10], so that the authors can assess their primary findings against in vivo conditions. As a last step, human testing should be performed for confirmation purposes[4].
Molecular biology techniques: Molecular-biology-based techniques have rapidly evolved in the last years in orthopedic infections and other fields, such as in diagnosing severe acute respiratory syndrome coronavirus 2[11] and water surveillance industry. In particular, more sophisticated molecular methods, including but not limited to clustered regularly interspaced short palindromic repeats, next-generation sequencing and droplet-digital polymerase chain reaction (PCR) are currently being investigated to contribute to the detection of the above mentioned virus. Moreover, rapid detection of harmful bacteria in wastewater is achievable when utilising DNA microarray and sequencing-based methods[12].
Similar progression has been recorded over the past years in orthopedic infections, not only in experimental laboratory settings but also in clinical practice. To illustrate, utilisation of PCR-based techniques, including but not limited to DNA microarrays and multiplex PCR, has been one of the most important advances in periprosthetic joint infections (PJI) diagnosis during the last decades[13]. The readily available multiplex polymerase chain reaction techniques (i.e., commercial and bespoke ones) feature satisfactory diagnostic accuracy, with a specificity approaching 100% and sensitivity varying between 70% to 80%[13]. In addition, newer diagnostic modalities such as metagenomic next-generation sequencing enable DNA sequencing directly from synovial fluid[14]. It has been claimed that metagenomic next-generation sequencing may revolutionise PJI diagnosis as it demonstrates increased sensitivity relative to PCR, while maintaining specificity at the same ultimate levels[13].
Is artificial intelligence applicable to in vitro models? Artificial intelligence (AI) may be implemented into orthopaedic in vitro models. For example, AI may aid in evaluating massive volumes of information from in vitro studies, such as gene expression data, protein profiles, and biomechanical data, to detect trends and make predictions. Another example would be application of AI to tumor models to monitor tumorigenesis progression in addition to real-time modelling[15]. Furthermore, AI could also aid in controlling the quality of organoids in the field of organogenesis and bioprinting technology[16]. In general, in vitro models frequently generate enormous volumes of data, including pictures and signals from several imaging modalities such as microscopy, radiography, and biomechanical sensors. AI enables assessing those data, extracting relevant information, and spotting small changes that human eyes may miss. This can facilitate measuring cellular activity, tissue shape, and mechanical characteristics, thus resulting in a more complete knowledge of the underlying biology[17,18]. Therefore, researchers may better understand the underlying process of orthopaedic illness, predict disease development, and consider prospective therapy efficacy[19]. In addition, AI can optimize experimental research designs by finetuning experimental settings, addressing sample size issues, and recommending the most important experiment variables that should be prioritised during analysis. Therefore, researchers may become more effective and productive, eliminate trial-and-error and save time and money at the same time[17]. Another example of AI application is referred to personalized/individualised medicine. AI may utilise patient-specific data, such as genetic information and medical history and construct tailored in vitro models that simulate the patient’s clinical condition. Researchers can monitor illness development and explore individualized treatment strategies, leading to more effective and targeted therapeutic approaches.
Additionally, AI can enhance the potential of medication research by evaluating enormous databases of chemical substances and predicting their possible effects on orthopaedic diseases. This can further identify interesting drug candidates and optimize their chemical structures, improving effectiveness and safety. Moreover, AI can generate virtual simulations of in vitro models, thus allowing researchers to test various scenarios and treatments in a controlled and cost-effective setting[20]. As a result, it could potentially refine experimental methods, minimise the requirement for physical experiments, and reduce the need to implement animal models.
Overall, AI has the potential to significantly improve orthopaedic in vitro models by enhancing predictive modeling, optimizing experimental design, assisting in image and signal analysis. Therefore, it improves customized therapy, expedites drug development, and facilitates virtual simulations. According to the above, orthopaedic researchers may obtain deeper insights into disease causes, devise more effective therapies, and ultimately enhance patient outcomes by utilizing the potential of AI.
Are in vitro “ex-vivo” infection models viable options?Ex vivo orthopaedic models allow using bone or muscle tissue as a substrate to form biofilm infections such as osteomyelitis[21]. These models appear particularly advantageous relative to their in vitro counterparts as they maintain important biological factors from the hosts[22]. Ex vivo experiments are cheaper than animal models and can spare animal lives[23]. However, careful consideration prior to selecting this type of laboratory research is advisable since no immune system interactions occur, and therefore the results may be suboptimal compared to in vivo settings[24].
Over the last years, the perception of orthopaedic conditions has shifted towards a more basic science-oriented approach. Therefore, the value of conducting high-quality basic science research tends to be increasingly appreciated by the orthopaedic community. In addition, when appropriate, favouring basic science over clinical investigations could mitigate the clinical research obstacles to some extent. However, it is important to highlight that unwarranted extrapolations of basic science research to human biology should be avoided.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: He YF, China; LI L, China; Rotondo JC, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Realpe AX, Blackstone J, Griffin DR, Bing AJF, Karski M, Milner SA, Siddique M, Goldberg A. Barriers to recruitment to an orthopaedic randomized controlled trial comparing two surgical procedures for ankle arthritis: a qualitative study. Bone Jt Open. 2021;2:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Carter S, Liew S, Brown G, Moaveni AK. Barriers to Completion of Research Projects Among Orthopaedic Trainees. J Surg Educ. 2018;75:1630-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Schindeler A, Mills RJ, Bobyn JD, Little DG. Preclinical models for orthopedic research and bone tissue engineering. J Orthop Res. 2017;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 743] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods. 2008;72:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 791] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 7. | Opperman TJ, Kwasny SM, Williams JD, Khan AR, Peet NP, Moir DT, Bowlin TL. Aryl rhodanines specifically inhibit staphylococcal and enterococcal biofilm formation. Antimicrob Agents Chemother. 2009;53:4357-4367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Faggion CM Jr. Guidelines for reporting pre-clinical in vitro studies on dental materials. J Evid Based Dent Pract. 2012;12:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 9. | Tsikopoulos K, Drago L, Koutras G, Givissis P, Vagdatli E, Soukiouroglou P, Papaioannidou P. Radial Extracorporeal Shock Wave Therapy Against Cutibacterium acnes Implant-Associated Infections: An in Vitro Trial. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Lovati AB, Bottagisio M, de Vecchi E, Gallazzi E, Drago L. Animal Models of Implant-Related Low-Grade Infections. A Twenty-Year Review. Adv Exp Med Biol. 2017;971:29-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Rotondo JC, Martini F, Maritati M, Caselli E, Gallenga CE, Guarino M, De Giorgio R, Mazziotta C, Tramarin ML, Badiale G, Tognon M, Contini C. Advanced Molecular and Immunological Diagnostic Methods to Detect SARS-CoV-2 Infection. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 12. | Zhang S, Li X, Wu J, Coin L, O’Brien J, Hai F, Jiang G. Molecular Methods for Pathogenic Bacteria Detection and Recent Advances in Wastewater Analysis. Water. 2021;13:3551. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Esteban J, Gadea I, Pérez-Jorge C, Sandoval E, García-Cañete J, Fernandez-Roblas R, Blanco A, Prieto-Borja L, Cordero-Ampuero J. Diagnosis of spacer-associated infection using quantitative cultures from sonicated antibiotics-loaded spacers: implications for the clinical outcome. Eur J Clin Microbiol Infect Dis. 2016;35:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Indelli PF, Ghirardelli S, Violante B, Amanatullah DF. Next generation sequencing for pathogen detection in periprosthetic joint infections. EFORT Open Rev. 2021;6:236-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Lee RY, Wu Y, Goh D, Tan V, Ng CW, Lim JCT, Lau MC, Yeong JPS. Application of Artificial Intelligence to In Vitro Tumor Modeling and Characterization of the Tumor Microenvironment. Adv Healthc Mater. 2023;12:e2202457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 16. | Lee H. Engineering In vitro Models: Bioprinting of Organoids with Artificial Intelligence. Cyborg Bionic Syst. 2023;4:0018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 17. | Mackay BS, Marshall K, Grant-Jacob JA, Kanczler J, Eason RW, Oreffo ROC, Mills B. The future of bone regeneration: integrating AI into tissue engineering. Biomed Phys Eng Express. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Longo UG, De Salvatore S, Candela V, Zollo G, Calabrese G, Fioravanti S, Giannone L, Marchetti A, De Marinis MG, Denaro V. Augmented Reality, Virtual Reality and Artificial Intelligence in Orthopedic Surgery: A Systematic Review. Appl Sci. 2021;11:3253. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Zaniletti I, Larson DR, Lewallen DG, Berry DJ, Maradit Kremers H. How to Develop and Validate Prediction Models for Orthopedic Outcomes. J Arthroplasty. 2023;38:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 20. | Basile AO, Yahi A, Tatonetti NP. Artificial Intelligence for Drug Toxicity and Safety. Trends Pharmacol Sci. 2019;40:624-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 21. | Junka A, Szymczyk P, Ziółkowski G, Karuga-Kuzniewska E, Smutnicka D, Bil-Lula I, Bartoszewicz M, Mahabady S, Sedghizadeh PP. Bad to the Bone: On In Vitro and Ex Vivo Microbial Biofilm Ability to Directly Destroy Colonized Bone Surfaces without Participation of Host Immunity or Osteoclastogenesis. PLoS One. 2017;12:e0169565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Yang Q, Phillips PL, Sampson EM, Progulske-Fox A, Jin S, Antonelli P, Schultz GS. Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen. 2013;21:704-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Mao Y, Singh-Varma A, Hoffman T, Dhall S, Danilkovitch A, Kohn J. The Effect of Cryopreserved Human Placental Tissues on Biofilm Formation of Wound-Associated Pathogens. J Funct Biomater. 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Chuang-Smith ON, Wells CL, Henry-Stanley MJ, Dunny GM. Acceleration of Enterococcus faecalis biofilm formation by aggregation substance expression in an ex vivo model of cardiac valve colonization. PLoS One. 2010;5:e15798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |