Published online Jun 18, 2023. doi: 10.5312/wjo.v14.i6.443
Peer-review started: March 6, 2023
First decision: March 24, 2023
Revised: April 19, 2023
Accepted: May 12, 2023
Article in press: May 12, 2023
Published online: June 18, 2023
Processing time: 104 Days and 10 Hours
Oral treatment of glucosamine (GA) combined with chondroitin sulfate (CS) was reportedly effective for pain relief and function improvement in osteoarthritis patients with moderate to severe knee pain in clinical trials. While the effectiveness of GA and CS on both clinical and radiological findings has been demon
To investigate the impact of GA + CS on clinical outcomes of patients with knee and hip osteoarthritis in routine clinical practice.
A multicenter prospective observational cohort study included 1102 patients of both genders with knee or hip osteoarthritis (Kellgren & Lawrence grades I-III) in 51 clinical centers in the Russian Federation from November 20, 2017, to March 20, 2020, who had started to receive oral capsules of glucosamine hydrochloride 500 mg and CS 400 mg according to the approved patient information leaflet starting from 3 capsules daily for 3 wk, followed by a reduced dosage of 2 capsules daily before study inclusion (minimal recommended treatment duration is 3-6 mo). Changes in subscale scores [Pain, Symptoms, Function, and Quality of Life (QOL)] of the Knee Injury and Osteoarthritis Outcome Score (KOOS)/Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaires during the observational period (up to 54-64 wk with a total of 4 visits). Patients’ treatment satisfaction, data on the combined oral use of glucosamine hydrochloride and CS, concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs), and adverse events (AEs) were also evaluated.
A total of 1102 patients with knee and hip osteoarthritis were included in the study. The mean patient age was 60.4 years, most patients were women (87.8%), and their average body mass index was 29.49 kg/m2. All subscale scores (Pain, Symptoms, Function, and QOL) of the KOOS and HOOS demonstrated clinically and statistically significant improvements. In patients with knee osteoarthritis, the mean score increases from baseline to the end of Week 64 were 22.87, 20.78, 16.60, and 24.87 on Pain, Symptoms, Physical Function (KOOS-PS), and QOL subscales (P < 0.001 for all), respectively. In patients with hip osteoarthritis, the mean score increases were 22.81, 19.93, 18.77, and 22.71 on Pain, Symptoms, Physical Function (HOOS-PS), and QOL subscales (P < 0.001 for all), respectively. The number of patients using any NSAIDs decreased from 43.1% to 13.5% (P < 0.001) at the end of the observation period. Treatment-related AEs occurred in 2.8% of the patients and mainly included gastrointestinal disorders [25 AEs in 24 (2.2%) patients]. Most patients (78.1%) were satisfied with the treatment.
Long-term oral GA + CS was associated with decreased pain, reduced concomitant NSAID therapy, improved joint function and QOL in patients with knee and hip osteoarthritis in routine clinical practice.
Core Tip: Long-term 64-wk treatment by oral capsules of glucosamine hydrochloride 500 mg and chondroitin sulfate 400 mg three times a day for the first 3 wk, then twice daily, was associated with clinically significant improvements in all subscale scores (Pain, Symptoms, Function, and Quality of Life) of the Knee Injury and Osteoarthritis Outcome Score/Hip Disability and Osteoarthritis Outcome Score and a decreasing number of patients with knee and hip osteoarthritis receiving any non-steroidal anti-inflammatory drugs in real-world clinical practice. Incidence of drug-related adverse events was low, and their nature was consistent with known safety profile of glucosamine hydrochloride and chondroitin sulfate combination.
- Citation: Lila AM, Alekseeva LI, Baranov AA, Taskina EA, Kashevarova NG, Lapkina NA, Trofimov EA. Chondroitin sulfate and glucosamine combination in patients with knee and hip osteoarthritis: A long-term observational study in Russia. World J Orthop 2023; 14(6): 443-457
- URL: https://www.wjgnet.com/2218-5836/full/v14/i6/443.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i6.443
Osteoarthritis (OA), the most common musculoskeletal disease worldwide, is associated with significant morbidity and mortality[1]. According to the 2017 Global Burden of Disease study, OA is the 12th leading cause of years lived with disability (YLDs) of all ages. YLDs due to OA increased substantially by 31.5% from 2006 to 2016 in the world[2]. This burdensome syndrome has become more common due to the combined effects of the aging population, the increasing proportion of obese individuals worldwide, and the increasing number of joint injuries. By estimate, 250 million people are currently suffering from OA worldwide[3]. In an epidemiological study, approximately 13% of the Russian population over the age of 18 suffered from knee or hip OA[4].
Knee or hip OA is one of the most frequent and burdensome joint diseases, with knee OA being more common than hip OA. The clinical manifestations and symptoms of OA include pain, stiffness and impaired joint function, gradual pain onset, crunching, muscle atrophy, joint deformation, and joint enlargement[5]. By estimate, OA treatment costs in some high-income countries were 1%-2.5% of the gross domestic product, with hip and knee replacements accounting for the major share of such healthcare costs[3].
Management guidelines for OA patients by the Osteoarthritis Research Society International (OARSI) and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases recommend a combination of non-pharmacological and pharmacological interventions[6,7]. Non-pharmacological options include exercise therapy, walking aids, weight loss, physical therapy, patient education, and self-help programs. Non-steroidal anti-inflammatory drugs (NSAIDs), paracetamol, opioids, duloxetine, intra-articular injections of corticosteroids and hyaluronic acid are the most common medicinal treatment products. Long-term usage of symptomatic slow-acting drugs for OA (SYSADOAs) is also considered a treatment option for symptomatic OA by several medical associations[7-10]. SYSADOAs reportedly had a structure-modifying effect in OA, based on their ability to activate anabolic processes in the cartilage matrix, suppress the activity of lysosomal enzymes, and stimulate chondrocyte function[11]. Regarding structure-modifying effects, standalone therapies of glucosamine (GA) as well as chondroitin sulfate (CS) achieved a statistically significant reduction in joint space narrowing[12].
The SYSADOA class comprises many products (GA, CS, diacerein, and unsaponifiables of soy and avocado oils), with various degrees of clinical efficacy. The most commonly investigated and recommended SYSADOAs are GA, CS, and their combinations. The increase in effectiveness of the combination of GA and CS as compared to each drug alone could be explained by the differences in their mechanisms of action[11]. However, their efficacy in clinical studies remains inconsistent for various reasons[7,13-17].
While many randomized controlled trials (RCTs) showed a significant treatment effect and remarkable safety for GA and CS, controversy regarding their relative effectiveness as compared to placebo or other treatments, their cost-effectiveness, and the need for insurance coverage of the cost of the therapy remains[18-20]. A recent meta-analysis[21] demonstrated clinical and radiological effectiveness of GA and CS. However, only a few high-quality trials exist, and the validity of these results was limited by a high risk of bias introduced in the studies[21]. Efficacious GA and CS treatment dosages, regimen of administration and treatment duration to provide symptom- and structure-modifying effects are not well investigated and properly justified[21], which emphasizes the importance of obtaining additional data on the effectiveness and safety of SYSADOA treatment in long-term studies, particularly in real-world clinical practice. The lower satisfaction with effectiveness of medication was strongly associated with treatment adherence[22].
Currently, GA and CS combination have been registered as an over-the-counter medicinal product in some countries (Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan, Kyrgyzstan, Mongolia, Russian Federation, Tajikistan, Turkmenistan, Ukraine, and Uzbekistan). GA and CS combination showed anti-inflammatory, analgesic, and chondroprotective activities in several clinical studies, including randomized studies[23,24]. Data on efficacy and safety of GA and CS combination therapy in routine clinical practice, particularly among patients with knee and hip OA receiving a long-term treatment, are of important practical interest.
This observational study aimed to assess pain dynamics, daily functional activity of joints, quality of life, and treatment satisfaction in patients with knee and hip OA, who received long-term GA and CS combination in routine clinical practice, and to collect data on the characteristics of OA patients receiving GA and CS combination treatment.
This multicenter prospective observational cohort study was conducted in 51 clinical centers in the Russian Federation from November 20, 2017, to March 20, 2020. Patients with OA who had been prescribed GA and CS combination by a doctor during routine care or bought the medicine in a pharmacy on their own care were invited to participate in the study. The study planned to enroll no less than 1100 participants with OA (Kellgren & Lawrence stages I-III) in 80 centers, who were treated with GA and CS combination (capsules, 500 mg + 400 mg) for no longer than 2 wk at the time of study enrollment.
Patients used GA and CS combination according to the approved patient information leaflet, starting from 3 capsules daily for 3 wk, followed by a reduced dosage of 2 capsules daily. Theraflex® [capsules, glucosamine hydrochloride 500 mg and CS 400 mg, (GA + CS)] was authorized in Russia in 2008 for treatment of OA (grades I-III).
Inclusion criteria were as follows: Patients aged 45-75 years with hip or knee OA of stages I-III according to the Kellgren & Lawrence classification; patient who started GA + CS treatment (capsules, 500 mg + 400 mg) no longer than 2 wk before study enrollment; and patients who personally signed and dated informed consent. For OA of several joints or bilateral knee or hip OA, only one “target” joint, in which the patient experienced the most pronounced pain at the time of study enrollment, was selected for evaluation.
Patients were considered ineligible for the study if: They had participated in any other studies involving interventions other than standard clinical practice; they had hip or knee OA stage 0 or stage IV; they had received GA + CS or other slow-acting drug for symptomatic treatment within the past 5 mo; they had received intra-articular corticosteroid injections within the past 3 mo, or hyaluronic acid injections, and/or intra-articular lower extremity autologous platelet-rich plasma injections within the past 6 mo; and women who were pregnant or breastfeeding.
Observational visits were scheduled based on routine clinical practice and GA + CS prescription by a consulting physician. The study protocol included an initial visit, two follow-up visits (Weeks 16-24 and Weeks 36-44 after GA + CS treatment initiation), and a final visit (Weeks 56-64 after treatment initiation). Once consent was obtained, patient demographics, lifestyle, medical history, and OA grades according to the Kellgren & Lawrence classification were recorded. During all visits, data on GA + CS usage were collected, patients’ basic vital signs and body mass index (BMI) were recorded, a physical examination was performed, and concomitant therapy and adverse events (AEs) were noted. Patients completed the Knee Injury and Osteoarthritis Outcome Score (KOOS) or the Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaires (depending on selected “target” joint). HOOS is used to evaluate patients' opinions about their hip joint and related problems. This scale is intended for adults with hip joint disability with or without osteoarthritis[25]. KOOS is used to evaluate the opinion of patients about their knee and related problems. It can be used for short-term and long-term monitoring[26]. HOOS and KOOS are widely used questionnaires for patient reported joint-specific assessment of symptoms and functions in subjects with knee/hip injury and osteoarthritis. Both questionnaires are available on-line.
KOOS/HOOS consist of 5 subscales: Pain, other Symptoms, Function in daily living (ADL), Function in sport and recreation (Sport/Rec) and knee/hip related Quality of life (QOL). Standardized answer options are given (5 Likert boxes) and each question gets a score from 0 to 4. Questionnaire scores range from 0 to 100 with a score of 0 indicating the worst possible knee/hip symptoms and 100 indicating no knee/hip symptoms. To simplify the process of completing the questionnaires and reduce the amount of information provided, the subscales “Function in daily living” and “Function in sport and recreation” of the KOOS/HOOS questionnaires were replaced by the KOOS Physical Function (KOOSPS) and the HOOS Physical Function (HOOSPS) short forms, respectively.
Additionally, during the post-baseline visit, patients were asked to rate their satisfaction with the treatment on a 5point scale (very satisfied - 5, satisfied - 4, neither satisfied nor dissatisfied - 3, dissatisfied - 2, very dissatisfied - 1).
An interim analysis after completion of follow-up visits on Week 16-24 by the first 550 enrolled patients was performed during the study. Its main purpose was to investigate changes in pain, functions of daily living, and quality of life in patients with knee and hip OA after the first treatment course with GA + CS. A report on the results of the interim analysis was presented at the European League Against Rheumatism online congress in 2020[23].
This study was approved by the Intercollegiate Ethics Committee of the Russian Federation (protocol number 08/17, dated on September 14, 2017) and by the Bayer Protocol Review Committee (held on June 14, 2017). Patients provided informed consent for data collection before starting any study-related procedures. Study was registered on ClinicalTrials.gov resource before patient enrollment with the study Identifier NCT03330288 on November 6, 2017[27].
Changes in parameters of the KOOS/HOOS subscales were assessed as the primary outcome of the study. Other outcomes, such as patient satisfaction with the study treatment, data on the use of GA + CS, frequency of concomitant use of analgesics and other pain medications, were also evaluated as additional study outcomes.
Data for the study were collected through clinical interviews with patients and from source documents available at the center. Source documents were original documents, data, and records, which could include laboratory data/information or assessment checklists, pharmacy records, etc. The KOOS/HOOS and treatment satisfaction questionnaires were completed by the patients themselves with touch-screen tablets provided to them.
Statistical analysis was performed using SAS 9.4 software package for Windows (SAS Institute Inc., Cary, NC, United States). Since the study was observational, descriptive analysis was used to process the data. All variables were analyzed in a full analysis set (FAS) that included all screened patients who received at least one dose of GA + CS and who had data to evaluate at least one efficacy and/or safety parameter. Categorical data were expressed as absolute numbers and percentages, and continuous data were expressed as mean values with standard deviations or 95% Wald confidence intervals (CI). The Student’s paired t-test and the Bonferroni-Holm correction for P value were used in the analysis of the dynamics of KOOS and HOOS scales. The null hypothesis was tested: No changes on the visits. According to the results of testing, the hypothesis is rejected on all visits. The analysis of treatment satisfaction was carried out using median values and 25 and 75 quantiles.
A total of 1111 participants were screened over the course of the study, of which 1102 participants were enrolled. The study group included male and female patients aged 45-75 years with knee (824 participants) or hip (278 participants) OA.
A total of 38 patients (3.4%) discontinued the study prematurely. The main reason was loss of contact with patient (33 patients), the remaining 5 patients discontinued participation by withdrawal of consent due to treatment switch to another SYSADOA, planned hip arthroplasty, or patient inability to visit the study center. A total of 1102 patients received at least one dose of GA + CS and were included in the FAS population for efficacy and safety analysis. Among these patients, 97.1% completed the visit for Weeks 16-24, and 96.6% completed visits for Weeks 36-44 and Weeks 56-64 (Figure 1).
Patient demographics and baseline characteristics were summarized in Table 1. Most patients (74.8%) had knee OA. Approximately, 87.8% of patients were women, 99.6% were Caucasians, and their mean age was 60.4 years. Notably, the average BMI of patients was 29.49 kg/m2, and most patients were obese or overweight.
| Knee osteoarthritis (n = 824) | Hip osteoarthritis (n = 278) | All (n = 1102) | |
| Age, (yr), mean (SD) | 60.4 (6.9) | 60.3 (7.2) | 60.4 (7.0) |
| Gender | |||
| Female | 728 (88.3) | 240 (86.3) | 968 (87.8) |
| Male | 96 (11.7) | 38 (13.7) | 134 (12.2) |
| Ethnicity | |||
| Caucasian | 820 (99.5) | 278 (100.0) | 1098 (99.6) |
| Black | 1 (0.1) | 0 | 1 (0.1) |
| Asian | 3 (0.4) | 0 | 3 (0.3) |
| BMI, mean (SD) | 29.77 (5.5) | 28.66 (5.3) | 29.49 (5.5) |
| Scale of stages of osteoarthritis according to Kellgren & Lawrence | |||
| Stage 1 | 81 (9.8) | 35 (12.6) | 116 (10.5) |
| Stage 2 | 617 (74.9) | 211 (75.9) | 828 (75.1) |
| Stage 3 | 125 (15.2) | 32 (11.5) | 157 (14.2) |
| No data available | 1 (0.1) | 1 (0.1) |
At study enrollment, data on concomitant diseases were collected. The most common diseases were cardiovascular diseases (52.6%), metabolic and nutritional disorders (34.3%), and gastrointestinal disorders (22.1%). Hypertension (44.6%), obesity (23.2%), chronic gastritis (15.6%), varicose veins (9.3%), and type 2 diabetes mellitus (8.4%) were most often reported. Treatment of concomitant diseases was the main reason for the prescription of concomitant therapy during the observation period. At least one concomitant treatment was reported by 813 (73.8%) patients, the most commonly used concomitant medications were selective betaadrenergic blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers (in 19%, 13.9% and 12.9% of patients, respectively).
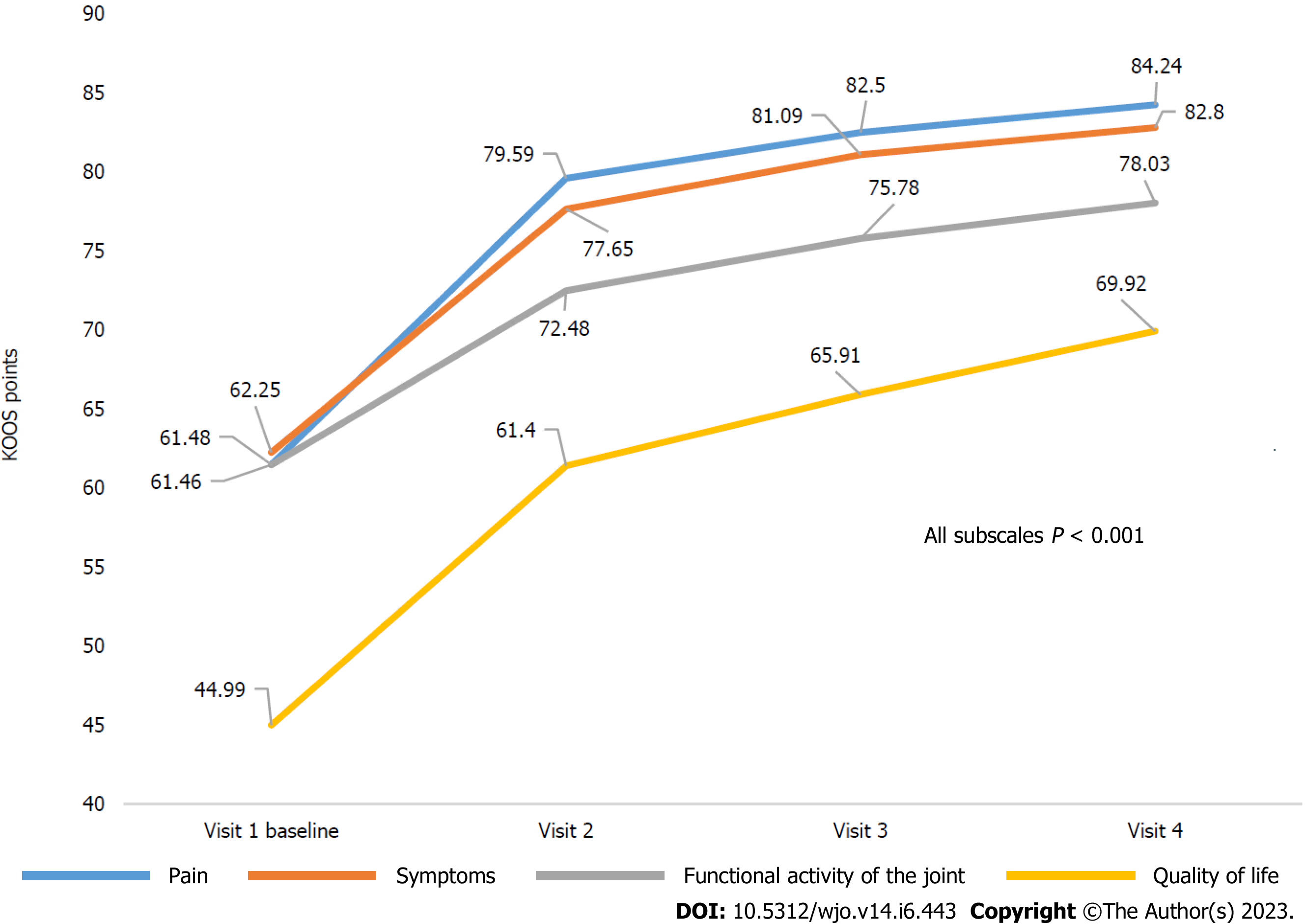
According to the KOOS questionnaire, patients with knee OA noted a significant improvement during the observation period. The mean score increases from baseline to the end of Week 64 were 22.87 (95%CI: 21.56-24.18), 20.78 (95%CI: 19.43-22.13), 16.60 (95%CI: 15.59-17.61), and 24.87 (95%CI: 23.41-26.34) on Pain, Symptoms, Physical Function (KOOS-PS), and Quality of Life subscales, respectively, (P < 0.001 for all). Notably, for all KOOS subscales, the largest score increases were achieved by Week 16-24 (observation for 4-6 mo), and the achieved effects further remained at the mean values for all subscales with a tendency to increase (P < 0.001 for all) (Table 2, Figure 2).
| Subscale | Knee osteoarthritis (n = 824) – KOOS scale | P valuea | ||||
| Visit 1, baseline, n = 824 | Visit 2, week 16-24, n = 798 | Visit 3 week 36-44, n = 794 | Visit 4, week 56-64, n = 794 | Changes from baseline on visit 4 | ||
| Pain | 61.48 (60.36-62.61) | 79.59 (78.48-80.70) | 82.50 (81.45-83.56) | 84.24 (83.15-85.34) | 22.87 (21.56-24.18) | < 0.001 |
| Symptoms | 62.25 (61.02-63.48) | 77.65 (76.46-78.84) | 81.09 (79.97-82.21) | 82.80 (81.66-83.93) | 20.78 (19.43-22.13) | < 0.001 |
| Functional activity of the joint | 61.46 (60.73-62.18) | 72.48 (71.63-73.34) | 75.78 (74.86-76.69) | 78.03 (77.06-78.99) | 16.60 (15.59-17.61) | < 0.001 |
| Quality of life | 44.99 (43.83-46.16) | 61.40 (60.10-62.69) | 65.91 (64.54-67.27) | 69.92 (68.45-71.39) | 24.87 (23.41-26.34) | < 0.001 |
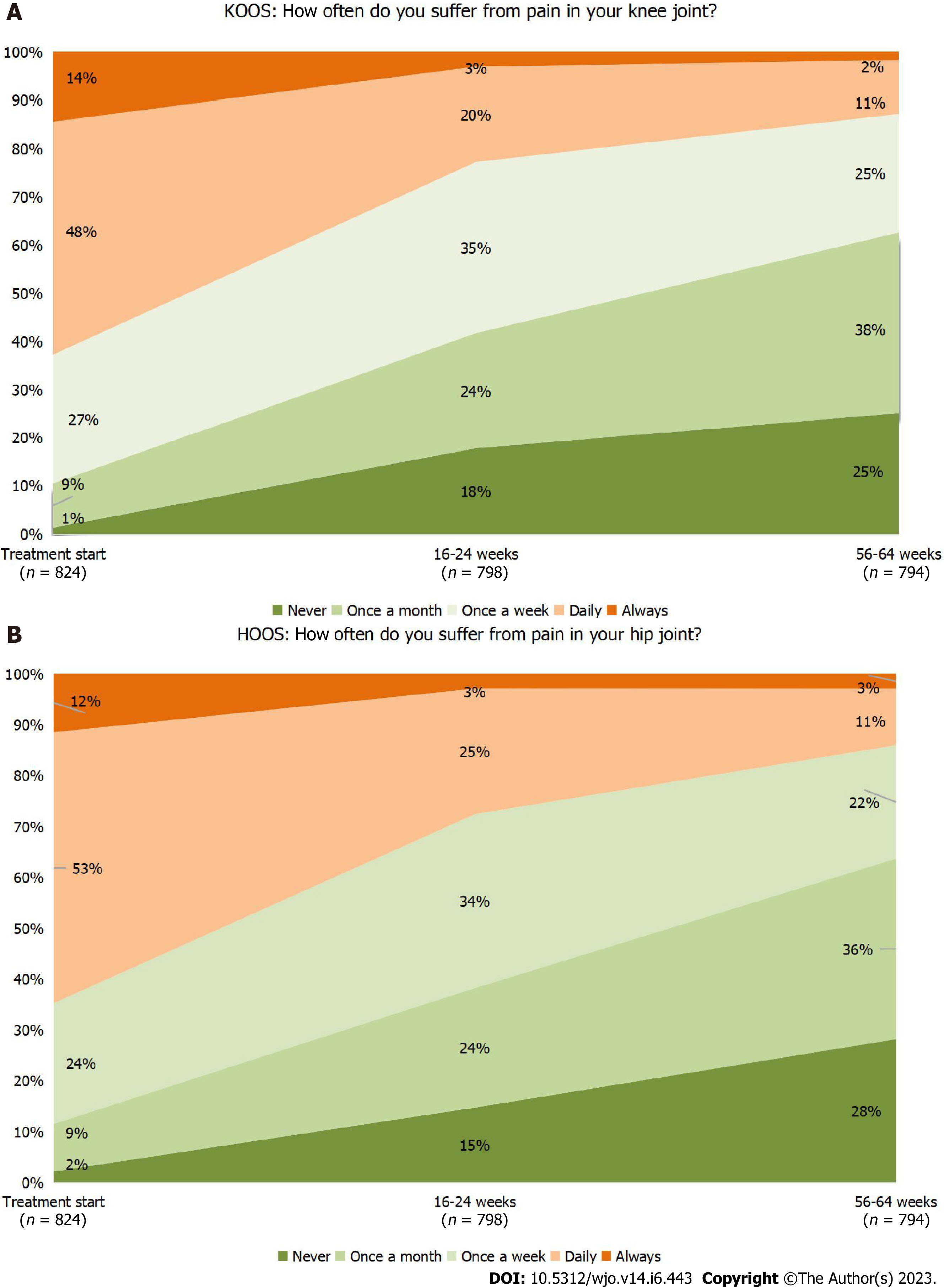
The most important questions (pain frequency and knee stiffness) of the KOOS questionnaire were analyzed separately. By the end of the 64-week observation period, the percentage of patients who reported pain frequency as “daily” or “always” decreased from 62.7% to 12.9% (Figure 3A). The percentage of patients who reported knee stiffness in the morning as “moderate”, “severe” or “extremely severe” decreased from 55.3% to 18.1%, and the percentage of patients with knee joint stiffness in the evening decreased from 60.9% to 18.6%. A significant improvement of quality of life was observed. According to answers to the question of how much the patient’s life was complicated by knee joint problems, the percentages of patients reported “not at all” and “slightly” increased from 1.9% to 28.7%, and from 19.5% to 38.5%, respectively, while the percentages of patients with “moderate”, “severe, and “extremely severe” decreased from 47.0% to 27.0%, 27.7% to 4.8%, and 3.9% to 1.0%, respectively.
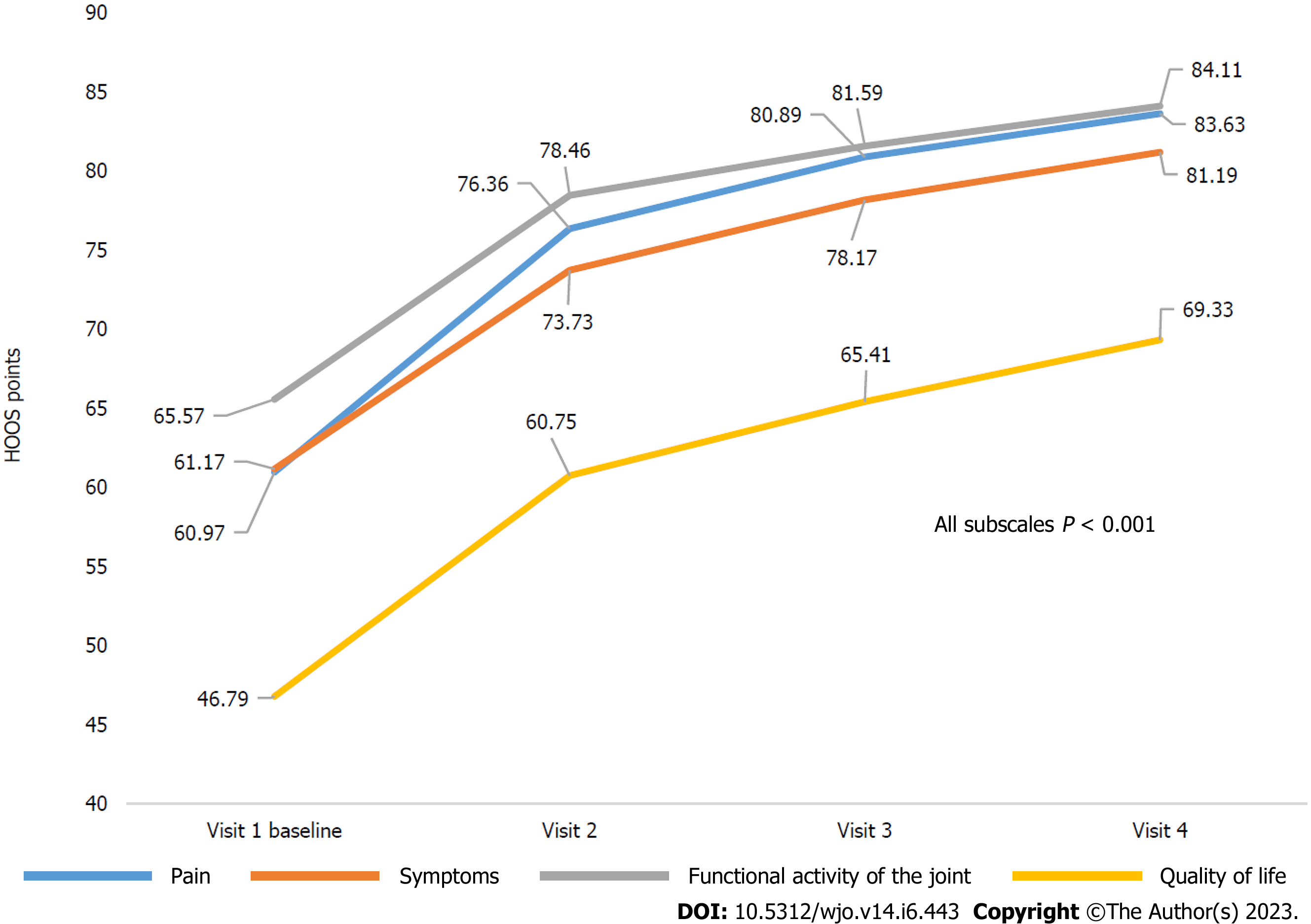
Patients with hip OA showed positive dynamics in all HOOS subscales during the observation period. The mean increases from baseline to the end of Week 64 were 22.81 (95%CI: 20.47-25.16), 19.93 (95%CI: 17.49-22.36), 18.77 (95%CI: 16.61-20.93), and 22.71 (95%CI: 20.14-25.28) on Pain, Symptoms, Physical Function (HOOS-PS), and Quality of Life subscales, respectively. Similar to changes in the KOOS scale, the highest score increases in the HOOS subscales were achieved by Week 16-24 with a tendency to increase during further follow-up (P < 0.001 for all) (Table 3, Figure 4).
| Subscale | Hip osteoarthritis (n = 278) – HOOS scale | P valuea | ||||
| Visit 1, baseline, n = 278 | Visit 2, week 16-24, n = 272 | Visit 3, week 36-44, n = 271 | Visit 4, week 56-64, n = 270 | Changes from baseline on visit 4 | ||
| Pain | 60.97 (58.93-63.02) | 76.36 (74.34-78.38) | 80.89 (78.95-82.84) | 83.63 (81.64-85.62) | 22.81 (20.47-25.16) | < 0.001 |
| Symptoms | 61.17 (59.08-63.26) | 73.73 (71.62-75.84) | 78.17 (76.21-80.14) | 81.19 (79.21-83.16) | 19.93 (17.49-22.36) | < 0.001 |
| Functional activity of the joint | 65.57 (63.62-67.52) | 78.46 (76.72-80.21) | 81.59 (79.93-83.26) | 84.11 (82.45-85.76) | 18.77 (16.61-20.93) | < 0.001 |
| Quality of life | 46.79 (44.72-48.85) | 60.75 (58.50-63.01) | 65.41 (63.09-67.72) | 69.33 (66.83-71.82) | 22.71 (20.14-25.28) | < 0.001 |
Similar to patients with knee OA, the percentage of subjects with less frequent and less severe symptoms, problems, or difficulties in the specified joint during the observation period increased among patients with hip OA. By the end of the 64-week follow-up, the percentage of patients with “daily” or “always” pain frequency decreased from 64.7% to 13.7% (Figure 3B), and those of patients with “moderate”, “severe”, or “extremely severe” morning hip stiffness and evening knee stiffness decreased from 55.4% to 15.9%, and from 61.9% to 17.0%, respectively. The percentages of patients who answered “not at all” and “slightly” in response to the HOOS question on complications due to hip joint problems increased from 1.8% to 23.7%, and from 22.7% to 41.5%, respectively, while the percentages of patients with “moderate”, “severe”, and “extremely severe” complications decreased from 46.4% to 28.1%, 25.5% to 4.8%, and 3.6% to 1.9%, respectively.
During the observation period, most patients (749/1102, 68%) were treated with GA + CS capsules for more than 6 mo. Treatment duration was 3-6 mo for 261 patients (23.7%), 1-3 mo for 78 patients (7.1%), and less than 1 mo for 14 patients (1.3%). A total of 1006 (91.3%) patients complied with the recommendations for the use of GA + CS.
The patient satisfaction response rates of “satisfied” or “very satisfied” on Visits 2, 3, and 4 were 78%, 79.9%, and 78.1%, respectively. Notably, over the visits, the proportion of patients who gave a rating of “very satisfied” tended to increase. In this regard, at Visit 2, 218/1070 (20.4%) patients rated treatment satisfaction as “very satisfied”. This rating was given by 261/1065 (24.5%) patients and 312/1064 (29.3%) patients at Visits 3 and 4, respectively. The results of patients with knee OA and hip OA were generally comparable.
At the end of each patient’s observation period, the investigators were asked for their opinion on the need for concomitant analgesic therapy for patients during the study. According to the data at the initiation of GA + CS treatment, 200/1102 patients (18.1%) with knee or hip OA received regular NSAID/analgesic therapy, and 276 patients (25.0%) received analgesic therapy episodically. For the majority of these cases, oral treatment was used (375 patients). However, some patients required only topical anti-inflammatory products (45 patients) or a combination of topical and oral analgesics (56 patients).
At the end of the study, the number of patients using any types of analgesics or NSAIDs decreased from 43.6% to 12.68% (P < 0.001) in the knee OA group, and from 44.36% to 10.58% (P < 0.001) in the hip OA group (Figure 5). The majority of patients [953/1102 (86.5%) patients] did not require analgesic therapy by NSAIDs at the end of follow-up, showing significant reduction in the need of concomitant NSAIDs or analgesics therapy. Notably, a decrease in the need for the use of topical and oral analgesic therapy was observed in both knee OA and hip OA groups (P < 0.001 for both) (Figure 5).
During the study, 307 AEs were reported in 190 (17.2%) participants. The most common AEs for systemorgan classes of MedDRA were: “Musculoskeletal and connective tissue disorders” (5.3%), “Gastrointestinal disorders” (4.7%), and “Infections and infestations”4.4%). The most common AEs by the Preferred Term included OA (worsening of the main diagnosis) (1.2%), upper abdominal pain (1.3%), upper respiratory tract infections (2.3%), and headache (1.3%). In most patients, AEs were mild [108 patients (9.8%)] or moderate [78 patients (7.1%)].
AEs considered by the investigators as “related to the study product” were reported for 31 (2.8%) patients (a total of 33 AEs) and mainly included gastrointestinal disorders [25 AEs in 24 (2.2%) patients]. The most common AE was upper abdominal pain [12 (1.1%) patients].
The dosage of GA + CS was reduced in 7 (0.6%) patients due to AEs (upper abdominal pain, diarrhea, dyspepsia, flatulence, stomach upset, hypothyroidism secondary to diffuse nodular goiter, and hyperuricemia). In 19 (1.7%) patients, AEs led to the cancellation of therapy with GA + CS. The most common cause was gastrointestinal disorders [12 (1.1%) patients]. Additionally, AEs, such as myalgia, cerebrovascular disorder, hypertension, essential hypertension, angina pectoris, peripheral edema, and hip arthroplasty were recorded.
Serious AEs (SAEs) were reported in 14 (1.3%) patients, including atrial fibrillation (2 patients), cholecystectomy, coronary bypass, hip arthroplasty, knee arthroplasty, hemorrhoids, ischemic thrombotic stroke, radicular pain syndrome, cholelithiasis, foot fracture, and myositis (one patient for each SAE). Additionally, one patient had several cardiac-related SAEs (atrial fibrillation, angina pectoris, chronic heart failure, ischemic cardiomyopathy, myocardial ischemia, and tachycardia) and another patient had gastrointestinal disorders (gastric ulcer and gastric ulcer bleeding, which was fatal). None of the SAEs were considered “related to the study product” by the investigator.
OA of knee and hip joints is one of the most common causes of disability and chronic pain worldwide. OA also implies significant medical and social costs, both directly due to treatment and indirectly because of reduced productivity and early retirement[3].
The main objectives of OA medication treatment are to alleviate pain, support quality of life, and maintain functional independence. Additionally, patients and doctors are concerned about possible adverse events caused by long-term use of NSAIDs[28,29]. The meta-analysis of the preferences of OA patients demonstrated, that patients evaluate side effects in the first place, when choosing medications, and the effectiveness of treatment significantly less affects the choice of therapy[29].
The meta-analysis of the preferences of OA patients demonstrated, that patients evaluate side effects in the first place, when choosing medications, and the effectiveness of treatment significantly less affects the choice of therapy[29].
SYSADOAs, including the combination of GA and CS, have been observed as effective and safe. SYSADOAs exert a delayed effect that persists after they are discontinued. These drugs have a symptomatic effect and could potentially slow down OA progression by influencing several pathways in the pathogenesis of the disease[30]. The effectiveness of GA or CS as monotherapies has been confirmed by several studies, which provided the prerequisites for the combination therapy[31,32]. In an experimental model, the combination of GA and CS increased the production of glycosaminoglycans in chondrocytes by 96.6% as compared to a 32% increase with the administration of each agent alone[33].
One of the most recent RCTs showed that combination of GA and CS was non-inferior to celecoxib in terms of reduction of pain, stiffness, and functional limitation after 6 mo in patients with painful knee OA, with a good safety profile[14]. In contrast, there are studies demonstrating inconsistent effects of GA and CS for OA treatment. For example, one of the largest studies [Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT)] showed no difference among the response rates, following the criteria recommended by OARSI, of CS alone, GA alone, and their combination in all OA patients[34]. However, a potentially high clinical efficacy was demonstrated in patients with moderate to severe knee pain. The proportion of patients whose pain syndrome had decreased by ≥ 20% by Week 24 was higher in the combination therapy group than in the placebo group (79.2% vs 54.3%, P = 0.002)[34]. Notably, a significant effect was demonstrated for the combination treatment, but not for the monotherapy of GA or CS[34]. The safety of GA and CS in OA has also been confirmed in a recent meta-analysis[35].
The aim of this prospective multicenter observational cohort study was to assess changes in pain, functions of daily living, and quality of life in patients with knee and hip OA who received long-term treatment with GA + CS (capsules, 500 mg + 400 mg) in a real-world clinical setting, using the KOOS and HOOS questionnaires. We consider that the initial characteristics of patients and the treatment results are consistent with the data accumulated to date on the effectiveness of the drug.
During this real-world clinical study, patients with knee OA and hip OA showed positive dynamics in all subscales of the KOOS and HOOS questionnaires (increases in the mean scores relative to the baseline values) at each visit during the observation period, with the most pronounced changes observed during the visit after the first treatment course of GA + CS (week 16-24). Hereafter, the effect achieved by the therapy was maintained with a tendency to increase during the entire observation period (Visit 3/week 36-44, Visit 4/week 56-64). Importantly, mean score increases in all KOOS subscales exceeded 8-10 points, which were consistent with the Minimal Clinically Significant Change (MCSC) according to Roos et al[36]. For the HOOS questionnaire, the mean score increases across all subscales were also higher than 8-10 points. While the MCSC score for the HOOS questionnaire currently remains under assessment, the results obtained in this study on the HOOS questionnaire could be considered clinically significant according to literature data[37,38]. It’s also worth noting that positive dynamics was observed for each question of the KOOS and HOOS questionnaires. In this regard, the proportion of patients with less frequent and less intense symptoms and difficulties associated with OA increased.
Data on patients’ satisfaction with the long-term treatment also demonstrated beneficial efficacy and safety profile. At the end of the treatment, almost 80% patients with knee or hip OA were “very satisfied” or “satisfied”. Satisfaction with the result of treatment is an important guideline in the choice of therapy tactics. The Guidance for Osteoarthritis by The National Institute for Health and Care Excellence (NICE) says that OA patients may be able to self-manage their condition effectively after getting information and guidance on management strategies. So, healthcare professionals should focus on the person's needs, so there are some situations in which planned follow-up may be necessary[39].
In general, during the observation period, patients showed a decrease in the need for concomitant pain therapy. Initially, 18.1% of the patients received regular analgesic therapy, and 25.0% of the patients received this therapy on demand (episodically). In most cases, systemic oral drug products were taken. By the end of the observation period, most patients (86.5%) did not require analgesic therapy with NSAIDs. This decrease in the need of analgesics over the observation period might be attributed to the overall improvements in functionality and well-being of the joints.
Notably, patients complied with the recommendations for the treatment duration of GA + CS. According to the evaluation results of treatment duration, therapy lasted for more than 6 mo in most patients (68.0%), 3-6 mo in 23.7%, 1-3 mo in 7.1%, and up to 1 mo in 1.3%. Therefore, the majority of patients (91.7%) generally complied with the treatment recommendations given in approved local patient information leaflet (minimal recommended treatment duration is 3-6 mo).
The baseline characteristics of our patients were consistent with the population of patients with knee and hip OA. Most patients (75.1%) had secondstage OA according to the Kellgren & Lawrence classification. The most frequent comorbidities were primary arterial hypertension (44.6%) and obesity (23.2%).
AEs associated with GA + CS usage were registered only in 31 patients (2.8%), and mainly included gastrointestinal events, which was consistent with available information on the side effects of GA + CS. SAE was reported in 14 (1.3%) patients, which could be explained by the prevalence of older people with many comorbidities in the study population (average patient age: 60.4 years). However, no SAEs were related to GA + CS therapy.
Notably, usage of patient-reported outcomes (KOOS and HOOS questionnaires) is one of the strengths of this study. Patient used touch-screen tablets to report their daily functionality and quality of life during OA treatment. Currently, data regarding patient-reported outcomes in long-term observation of OA patients are lacking.
This study had several limitations. First, it was not possible to directly assess the effectiveness of GA + CS, since the study was observational in its nature and did not include a control group. However, long-term follow-up of patients up to 64 wk greatly offsets this limitation and increases value of received real-world data. Additionally, the standard clinical setting implied certain limitations. For example, the inability to control the use of concomitant treatment and the inability to obtain data at all time points, which could adversely affect data interpretation. To simplify the process of completing the questionnaires and reduce the amount of information provided, the subscales “Everyday Life” and “Sports and Health Activities” of KOOS/HOOS questionnaires were replaced by the KOOS-PS and HOOS-PS short forms, respectively. Nonetheless, these scales demonstrated changes in OA symptoms, improvements in functionality of the joints and quality of life, and safety.
In the framework of routine clinical practice, after long-term treatment with a fixed combination of GA and CS, a decrease in pain syndrome and significant improvements in the functional state and quality of life of patients were observed. There was a significant reduction in the need for concomitant usage of analgesics. Most patients (approximately 80%) were satisfied with the treatment. The results of this real-world clinical study confirmed the potential benefit of the combination of GA and CS for the treatment of knee and hip OA.
Long-term supplementation with GA and CS combination could be considered a standard pharmacological option on top of non-pharmacological treatment measures during any disease stage. The results of this study were considered during the preparation of national clinical guidelines on the treatment of knee and hip OA in Russia[8,9].
High prevalence of osteoarthritis (OA) forces healthcare professionals to look for efficient and safe long-term treatment options. Combined oral treatment with glucosamine (GA) and chondroitin sulfate (CS) was shown to be efficient for pain relief and function improvement in OA patients with moderate to severe knee pain in clinical trials. There is still need in additional data regarding their effectiveness in routine clinical practice.
Considering high prevalence of OA and its frequent co-existence with concurrent diseases, as well as the absence of proven long-term disease-modifying treatment options, the authors aimed to evaluate the effectiveness and safety of long-term therapy of GA and CS in the framework of real clinical practice. One of the key questions was to assess the effectiveness of this therapy in the treatment of the two most common OA affected joints – knee and hip. It was important to assess the dynamics of OA symptoms, including patients who were using non-steroidal anti-inflammatory drugs (NSAIDs), and assessment of the following need of concomitant analgesic therapy.
The main objective of the study was to evaluate dynamics of pain syndrome, functions of living, quality of life and satisfaction of patient as well as of actual study product utilization by patients with OA for an observation period up to 64 wk. The study was also aimed to evaluate the HCP approach to the treatment and management of patients with OA to understand the place of the combination of GA and CS in their recommendations and formed the basis for the development of clinical practice guidelines.
An open-label multicenter non-interventional prospective cohort study enrolled patients with Hip or Knee OA stage I to III who started a treatment with combination of GA and CS to evaluate health status by physical examination and validated patient questionnaires for an observation period up to 64 wk. Patients visited clinical sites up to 4 study visits. During all visits data was collected by Investigators within the routine clinical practice. In addition, during each visit patient reported outcomes were generated, assessing Knee or Hip OA outcome, as well as a simple patient satisfaction questionnaire. Depending on the target joint, one of the questionnaires, either the Knee injury and Osteoarthritis Outcome Score (KOOS) or the Hip Osteoarthritis Outcome Score (HOOS) was used.
Mean improvement on Pain subscale in patients with knee and hip OA was 22.87 and 22.81 respectively, on the Symptoms subscales – 20.78 and 19.93, on the Physical Function subscale – 16.60 and 18.77, and on the Quality-of-Life subscale – 24.87 and 22.71 from baseline to the end of observation (up to 64 wk). Number of patients using any NSAIDs decreased from 43.1% to 13.5% by the end of the observation period. Treatment-related AEs were reported for 2.8% of patients and mainly included gastrointestinal disorders [25 AEs in 24 (2.2%) patients]. Most patients (78.1%) were satisfied with the treatment. Most patients (91.3%) generally complied with the recommended duration of treatment (not less than 3 mo per year).
This observational study showed that long-term oral treatment with GA + CS is associated with decrease of pain, improvements of joint function, quality of life and decrease of concomitant usage of NSAIDs in patients with knee and hip OA in routine clinical practice. Treatment with GA + CS combination showed low incidence of drug-related AEs, and high level of patients’ satisfaction with the treatment along with high compliance with long duration of the treatment.
In the long term perspective, this study will contribute to the enhancement of guidelines for the treatment of OA and improve the long-term outcomes for patients with OA. Authors believe, that application of the results of this study will help to reduce the medicinal load on the patient with analgesics and NSAIDs, which ultimately can reduce the number of concomitant adverse events and increase the safety profile of the therapy.
The authors are grateful to Kirill Sokolov, Julia Kalentyeva, and Irina Orlovskaya, Bayer Russia employees, for ensuring that the study procedures were in full compliance with international requirements for good clinical practice; Andreas Ehret, Head of Clinical Operations, Bayer AG, for responsible oversight and advice; and Evgenia Radkova, OCT Rus company, for assistance in technical preparation of medical texts (study protocol and study report) under the guidance of the authors. Authors are thankful to the Contract Research Organization (OCT Rus company, Russia), which handled all operational procedures of the study and ensured data management. Our study team is also thankful to Sciencefiles (Russia) for providing technical support during the preparation of this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rheumatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Omari B, United Arab Emirates; Muthu S, India S-Editor: Liu JH L-Editor: A P-Editor: Ji MX
| 1. | Sabha M, Hochberg MC. Non-surgical management of hip and knee osteoarthritis; comparison of ACR/AF and OARSI 2019 and VA/DoD 2020 guidelines. Osteoarthr Cartil Open. 2022;4:100232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9354] [Cited by in RCA: 8390] [Article Influence: 1198.6] [Reference Citation Analysis (4)] |
| 3. | Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 2640] [Article Influence: 440.0] [Reference Citation Analysis (0)] |
| 4. | Galushko EA, Bolshakova TY, Vinogradova IB, Ivanova ON, Lesnyak OM, Menshikova LV, Petrachkova TN, Erdes SF. Structure of rheumatic diseases among adult population of Russia according to data of an epidemiological study (preliminary results). Rheumatology Science and Practice. 2009;11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Alekseeva LI, Taskina EA, Kashevarova NG. Osteoarthritis: epidemiology, classification, risk factors, and progression, clinical presentation, diagnosis, and treatment. Modern Rheumatology Journal. 2019;13: 9-21 (In Russ.). [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, Espinosa R, Haugen IK, Lin J, Mandl LA, Moilanen E, Nakamura N, Snyder-Mackler L, Trojian T, Underwood M, McAlindon TE. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1958] [Article Influence: 326.3] [Reference Citation Analysis (0)] |
| 7. | Bruyère O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM, Al-Daghri NM, Herrero-Beaumont G, Martel-Pelletier J, Pelletier JP, Rannou F, Rizzoli R, Roth R, Uebelhart D, Cooper C, Reginster JY. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum. 2019;49:337-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 343] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 8. | Association of Rheumatologists of Russia; Russian Association of Traumatologists and Orthopedists. Gonarthrotic. Clinical Guidelines of Russian Federation. ID 667. Cited 06 September 2021. Available from: https://cr.minzdrav.gov.ru/recomend/667_1. |
| 9. | Association of Rheumatologists of Russia; Russian Association of Traumatologists and Orthopedists. Coxarthrosis. Clinical Guidelines of Russian Federation, ID 666. Cited 03 September 2021. Available from: https://cr.minzdrav.gov.ru/recomend/666_1. |
| 10. | Alexander LAM, Ln D, Eg Z, Is D, Ay K, Ss R, Ea T, Sp Y, Ez Y, L G. Pharmacological Management of Osteoarthritis With a Focus on Symptomatic Slow-Acting Drugs: Recommendations From Leading Russian Experts. J Clin Rheumatol. 2021;27:e533-e539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | du Souich P. Absorption, distribution and mechanism of action of SYSADOAS. Pharmacol Ther. 2014;142:362-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Zeng C, Wei J, Li H, Wang YL, Xie DX, Yang T, Gao SG, Li YS, Luo W, Lei GH. Effectiveness and safety of Glucosamine, chondroitin, the two in combination, or celecoxib in the treatment of osteoarthritis of the knee. Sci Rep. 2015;5:16827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Zhu X, Wu D, Sang L, Wang Y, Shen Y, Zhuang X, Chu M, Jiang L. Comparative effectiveness of glucosamine, chondroitin, acetaminophen or celecoxib for the treatment of knee and/or hip osteoarthritis: a network meta-analysis. Clin Exp Rheumatol. 2018;36:595-602. [PubMed] |
| 14. | Hochberg MC, Martel-Pelletier J, Monfort J, Möller I, Castillo JR, Arden N, Berenbaum F, Blanco FJ, Conaghan PG, Doménech G, Henrotin Y, Pap T, Richette P, Sawitzke A, du Souich P, Pelletier JP; MOVES Investigation Group. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2016;75:37-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 15. | Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G; CS/GS Combined Therapy Study Group. Combined Treatment With Chondroitin Sulfate and Glucosamine Sulfate Shows No Superiority Over Placebo for Reduction of Joint Pain and Functional Impairment in Patients With Knee Osteoarthritis: A Six-Month Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Arthritis Rheumatol. 2017;69:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Singh JA, Noorbaloochi S, MacDonald R, Maxwell LJ. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;1:CD005614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Fransen M, Agaliotis M, Nairn L, Votrubec M, Bridgett L, Su S, Jan S, March L, Edmonds J, Norton R, Woodward M, Day R; LEGS study collaborative group. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis. 2015;74:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Wandel S, Jüni P, Tendal B, Nüesch E, Villiger PM, Welton NJ, Reichenbach S, Trelle S. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 352] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 19. | Towheed TE, Maxwell L, Anastassiades TP, Shea B, Houpt J, Robinson V, Hochberg MC, Wells G. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;2005:CD002946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 20. | Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 1965] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 21. | Vasiliadis HS, Tsikopoulos K. Glucosamine and chondroitin for the treatment of osteoarthritis. World J Orthop. 2017;8:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (5)] |
| 22. | Atinga RA, Akosen G, Bawontuo V. Perceived characteristics of outpatient appointment scheduling association with patient satisfaction and treatment adherence: An innovation theory application. Hosp Pract (1995). 2021;49:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Telyshev K, Alekseeva L, Lila A, Baranov A, Trofimov E. Ab0885 Effectiveness and Safety of Glucosamine and Chondroitin Combination in Patients with Knee and Hip Osteoarthritis: Interim Analysis Results of an Observational Study. Ann Rheum Dis. 2020;79:1747-1747. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 24. | Lila АМ, Alekseeva LI, Telyshev КА, Baranov АА, Trofimov EА. The efficiency of treatment with a combined chondroitin sulfate and glucosamine hydrochloride drug for knee and hip osteoarthritis: intermediate results of a Russian observational study. Modern Rheumatology Journal. 2020;14:71-78. [DOI] [Full Text] |
| 25. | Measures of hip function and symptoms: Harris Hip Score (HHS), Hip Disability and Osteoarthritis Outcome Score (HOOS), Oxford Hip Score (OHS), Lequesne Index of Severity for Osteoarthritis of the Hip (LISOH), and American Academy of Orthopedic Surgeons (AAOS) Hip and Knee Questionnaire . [RCA] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 339] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 26. | Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S208-S228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 872] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 27. | Bayer, Prospective Multicenter Non-interventional Study in Patients With Knee or Hip Osteoarthritis Having a Theraflex® Treatment to Evaluate Changes in Pain, Functions in Daily Living, and Quality of Life for an Observation Period up to 64 Weeks. clinicaltrials.gov Available from: https://clinicaltrials.gov/ct2/show/NCT03330288. |
| 28. | Al-Omari B, McMeekin P. Patients' Preferences Regarding Osteoarthritis Medications: An Adaptive Choice-Based Conjoint Analysis Study. Patient Prefer Adherence. 2020;14:2501-2515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Al-Omari B, McMeekin P, Bate A. Systematic Review of Studies Using Conjoint Analysis Techniques to Investigate Patients' Preferences Regarding Osteoarthritis Treatment. Patient Prefer Adherence. 2021;15:197-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1893] [Cited by in RCA: 1860] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 31. | Qiu GX, Weng XS, Zhang K, Zhou YX, Lou SQ, Wang YP, Li W, Zhang H, Liu Y. [A multi-central, randomized, controlled clinical trial of glucosamine hydrochloride/sulfate in the treatment of knee osteoarthritis]. Zhonghua Yi Xue Za Zhi. 2005;85:3067-3070. [PubMed] |
| 32. | Zegels B, Crozes P, Uebelhart D, Bruyère O, Reginster JY. Equivalence of a single dose (1200 mg) compared to a three-time a day dose (400 mg) of chondroitin 4&6 sulfate in patients with knee osteoarthritis. Results of a randomized double blind placebo controlled study. Osteoarthritis Cartilage. 2013;21:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Lippiello L, Woodward J, Karpman R, Hammad TA. In vivo chondroprotection and metabolic synergy of glucosamine and chondroitin sulfate. Clin Orthop Relat Res 2000: 229-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 34. | Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, Bradley JD, Bingham CO 3rd, Weisman MH, Jackson CG, Lane NE, Cush JJ, Moreland LW, Schumacher HR Jr, Oddis CV, Wolfe F, Molitor JA, Yocum DE, Schnitzer TJ, Furst DE, Sawitzke AD, Shi H, Brandt KD, Moskowitz RW, Williams HJ. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 770] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 35. | Honvo G, Reginster JY, Rabenda V, Geerinck A, Mkinsi O, Charles A, Rizzoli R, Cooper C, Avouac B, Bruyère O. Safety of Symptomatic Slow-Acting Drugs for Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis. Drugs Aging. 2019;36:65-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1182] [Cited by in RCA: 1671] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 37. | Lyman S, Lee YY, McLawhorn AS, Islam W, MacLean CH. What Are the Minimal and Substantial Improvements in the HOOS and KOOS and JR Versions After Total Joint Replacement? Clin Orthop Relat Res. 2018;476:2432-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 38. | Kemp JL, Collins NJ, Roos EM, Crossley KM. Psychometric properties of patient-reported outcome measures for hip arthroscopic surgery. Am J Sports Med. 2013;41:2065-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 389] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 39. | Rationale and impact-Osteoarthritis in over 16s: diagnosis and management-Guidance -NICE. 2022. Available from: https://www.nice.org.uk/guidance/ng226/chapter/Rationale-and-impact#information-and-support-2. |