Published online Mar 18, 2023. doi: 10.5312/wjo.v14.i3.90
Peer-review started: July 30, 2022
First decision: October 24, 2022
Revised: November 22, 2022
Accepted: February 13, 2023
Article in press: February 13, 2023
Published online: March 18, 2023
Processing time: 229 Days and 17.8 Hours
As the number of patients receiving total joint replacements continues to rise, considerable attention has been directed towards the early detection and prevention of postoperative complications. While D-dimer has long been studied as a diagnostic tool in venous thromboembolism (VTE), this assay has recently received considerable attention in the diagnosis of periprosthetic joint infection (PJI). D-dimer values are substantially elevated in the acute postoperative period after total joint arthroplasty, with levels often exceeding the standard institutional cutoff for VTE (500 µg/L). The utility of D-dimer in detecting VTE after total joint replacement is currently limited, and more research to assess its value in the setting of contemporary prophylaxis protocols is warranted. Recent literature supports D-dimer as a good to excellent biomarker for the diagnosis of chronic PJI, especially when using serum sample technique. Providers should exercise caution when interpreting D-dimer levels in patients with inflammatory and hypercoagulability disorders, as the diagnostic value is decreased. The updated 2018 Musculoskeletal Infection Society criteria, which includes D-dimer levels > 860 µg/L as a minor criterion, may be the most accurate for diagnosing chronic PJI to date. Larger prospective trials with transparent lab testing protocols are needed to establish best assay practices and optimal cutoff values for D-dimer in the diagnosis of PJI. This review summarizes the most current literature on the value of D-dimer in total joint arthroplasty and elucidates areas for future progress.
Core Tip: Venous thromboembolism (VTE) and periprosthetic joint infection (PJI) are potentially devastating complications after total joint arthroplasty. D-dimer has limited utility with current cutoff values in the detection of VTE in the acute postoperative period. The D-dimer assay is a valuable biomarker in the diagnosis of chronic periprosthetic joint infection, and its utility may be optimized by using serum sample technique. Larger prospective trials with transparent lab testing protocols are necessary to establish best assay practices and optimal cutoff values for D-dimer in the diagnosis of VTE and PJI in arthroplasty patients.
- Citation: Cutter B, Lum ZC, Giordani M, Meehan JP. Utility of D-dimer in total joint arthroplasty. World J Orthop 2023; 14(3): 90-102
- URL: https://www.wjgnet.com/2218-5836/full/v14/i3/90.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i3.90
Venous thromboembolism (VTE) and periprosthetic joint infection (PJI) are serious complications of total joint arthroplasty (TJA). Deep vein thrombosis (DVT) is a leading cause of morbidity and mortality during the postoperative phase[1,2]. The early diagnosis and treatment of DVT is extremely important, as delay can result in post-thrombotic syndrome and pulmonary embolism (PE). Although D-dimer has proved to be a valuable biomarker in the detection of VTE, its interpretation after total joint arthroplasty has been controversial, as postoperative levels often exceed the common institutional cutoff of 500 µg/L. Recent literature has focused on establishing new thresholds during the immediate postoperative period, in addition to using the test in new predictive models. While D-dimer has long been studied as a diagnostic tool in thromboembolism, this assay has recently received considerable attention in the evaluation of infection.
Periprosthetic joint infection continues to be a devastating complication in orthopaedic surgery, affecting roughly 2% of patients undergoing primary total joint arthroplasty[3,4]. The development of PJI dramatically decreases a patient’s quality of life and accounts for a large financial burden to the patient and national health system[5-8]. Its timely detection is important, yet establishing the diagnosis can be challenging as there is no single “gold standard” test. In 2011, the Musculoskeletal Infection Society (MSIS) introduced a diagnostic criteria (later modified by the International Consensus Meeting (ICM) in 2013) based on a combination of clinical, serum, synovial, histologic, microbial, and operative findings[9,10]. Recently, emphasis has shifted to a large number of novel hematologic and synovial biomarkers. In a 2017 study, D-dimer demonstrated excellent performance in the diagnosis of chronic PJI, with sensitivity and specificity above both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)[11]. As an inexpensive, rapid, and convenient hematologic test, it was quickly adopted into the 2018 MSIS and ICM criteria for PJI diagnosis as a minor criterion[12,13]. Although initial studies found D-dimer to exhibit excellent performance in determining PJI, other authors have published conflicting results[14-20]. Since its inclusion in the updated MSIS and ICM criteria, the utility of D-dimer as a biomarker for PJI has been intensely debated. The goal of this review is to summarize the most current literature on the value of D-dimer in total joint arthroplasty and elucidate areas for future progress.
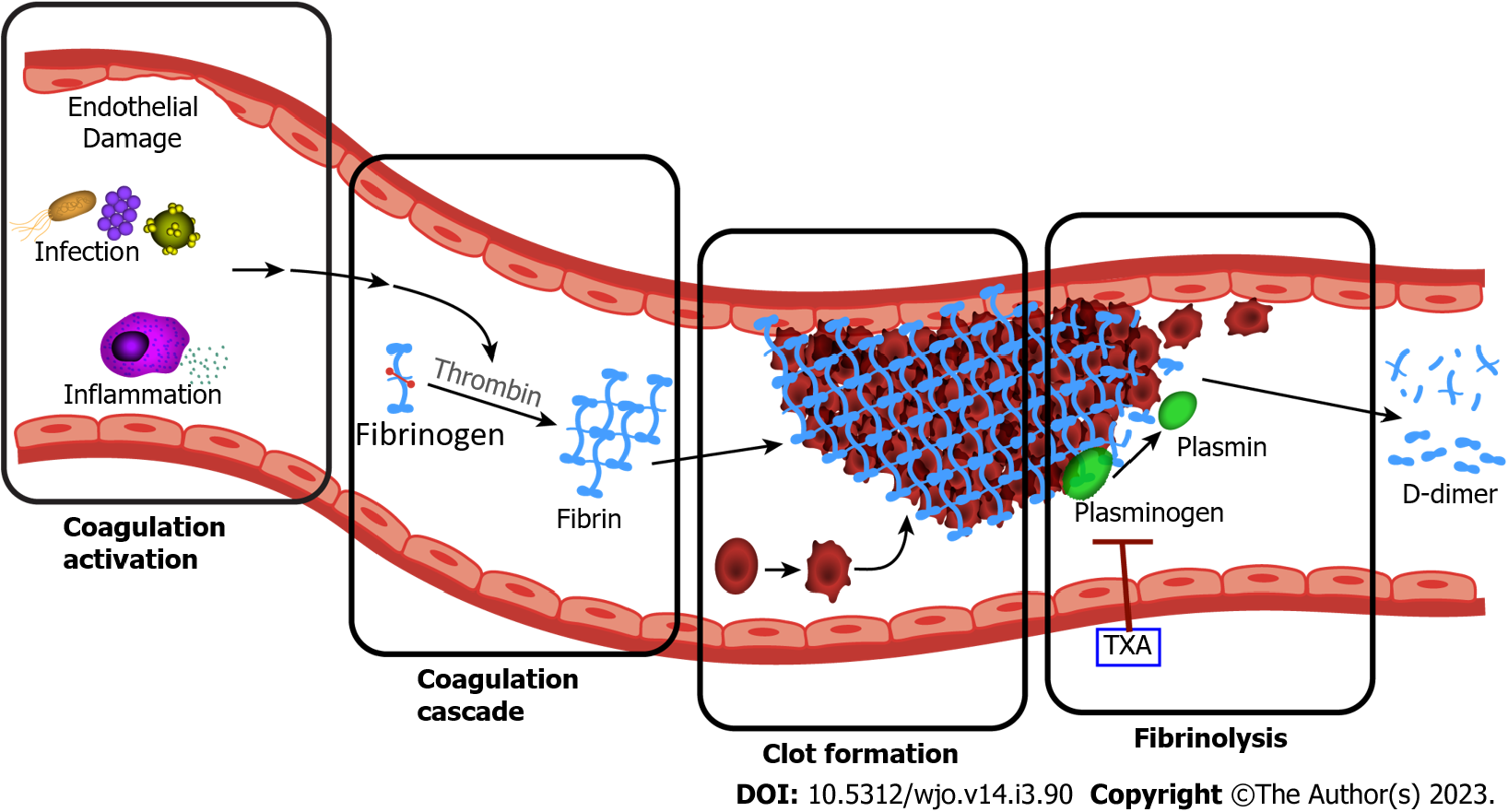
D-dimer is a small protein fragment produced by the breakdown of vascular thrombi through a process known as fibrinolysis (Figure 1). The creation of D-dimer begins with thrombus formation: thrombin is generated through the coagulation cascade, which in turn converts plasma fibrinogen into fibrin. Through multiple interactions, fibrin molecules are cross-linked to form a meshwork for the resulting blood clot. The degradation of this thrombus occurs through fibrinolysis, where plasmin (a fibrinolytic enzyme) cleaves the fibrin scaffolding, resulting in the creation of the D-dimer molecules. D-dimer is therefore a unique marker of both thrombus formation as well as subsequent thrombolytic activity[21].
Deep venous thrombosis occurs due to the creation of an intravascular clot as the result of three main mechanisms: hypercoagulability, vascular wall injury, and venous stasis[22]; all of which can be present in patients with recent surgery. In patients with infection, the initiation of the coagulation cascade by microorganisms and inflammatory mediators is a common and early event[23]. Although this hypercoagulable state can alone increase D-dimer levels, another mechanism appears to be at play. Ribera et al[24] first demonstrated significantly increased levels of synovial D-dimer within the septic joints of foals. Other studies have supported that inflamed synovium secretes large amounts of fibrin, ultimately resulting in increased intra-articular concentrations of D-dimer which can efflux out of the joint and into circulation[25].
D-dimer has been widely used as a hematogenous biomarker for the detection and exclusion of VTE, comprised of both DVT and PE, and is strongly recommended in the diagnostic algorithms of multiple medical organizations, including the American Society of Hematology[26,27]. Despite its low specificity, D-dimer has long been advocated as an effective method to screen patients for VTE, with a sensitivity up to 97%, therefore reducing expensive testing such as venography or ultrasonography. In recent years, it has also been recognized as a valuable marker for inflammation and infection. Contemporary research has found elevated D-dimer levels to be a prognostic indicator for septic shock, bacterial pneumonia, bacteremia, and COVID-19 infection[28-33]. In 2011, Saxena et al[34] first described an association between D-dimer and periprosthetic joint infection. Since that time, a considerable amount of research evaluating the relationship between D-dimer levels and total joint-related infection has been published.
Blood sample technique: There are two common and distinct methods to collect and prepare the blood sample for testing[35,36]: (1) Serum D-dimer: Serum is the liquid portion of the blood after coagulation has occurred. The sample tubes contain either coagulation enhancers or no additives and are exposed to room temperature for a defined time period (often 30-60 min). After mandatory coagulation, serum samples have significantly less fibrinogen and coagulation factors due to recent consumption; and (2) Plasma D-dimer: Plasma is the liquid portion of the blood when coagulation has been prevented. The blood collection tubes contain additives (commonly citrate), which prevent coagulation and can therefore be handled much easier than serum samples. The tubes can be immediately cooled or centrifuged in order to separate plasma from blood cells.
Assay methods: After the sample is collected and prepared, a variety of quantification methods can be utilized. D-dimer is most commonly detected and quantified using monoclonal antibodies that distinguish a specific epitope on the cross-linked D-dimer molecule, differentiating it from other coagulation related products such fibrinogen or fibrin monomers[21,37]. There are over thirty commercial D-dimer assays on the market, but these can be broadly divided into three categories: enzyme-linked immunosorbent assays (ELISA), immunofluorescent assays, and latex-agglutination assays. In general, ELISA-based assays are more sensitive (nearing 100%) than agglutination assays, however automated techniques such as immunoturbidimetric detection have narrowed the gap[37,38]. Each individual assay has its own calibration standards, cutoff values, sensitivity, and specificity for the detection of VTE[39].
Many patient conditions are known to elevate D-dimer levels, including advanced age, inflammatory disease, auto-immune disorders, cardiovascular disease, and/or a recent surgical procedure (Table 1)[39-43]. As total joint patients commonly share many of these features, surgeons have difficulty interpreting elevated D-dimer levels in this population. Age-adjusted D-dimer values have helped increase the accuracy of DVT detection in elderly patients before undergoing TJA, but spiking levels in the postoperative period pose additional challenges[43,44]. Inflammatory biomarkers such as ESR and CRP are often elevated after any recent surgical procedure, so it is not surprising that D-dimer follows this trend[45,46]. In addition, D-dimer is known to be the predominant product of extravascular fibrinolysis, a process which is emerging as an essential step in wound healing and tissue regeneration after orthopaedic surgery[47-49]. D-dimer values are substantially elevated after total joint arthroplasty, and recent investigations have discovered a consistent pattern of distribution in the postoperative phase.
| Venous thromboembolism |
| Surgery |
| Age |
| Trauma |
| Inflammation |
| Disseminated intravascular coagulation |
| Cancer |
| Infection/sepsis |
| Pregnancy |
| Cardiovascular disease |
| Liver disease |
| Renal disease |
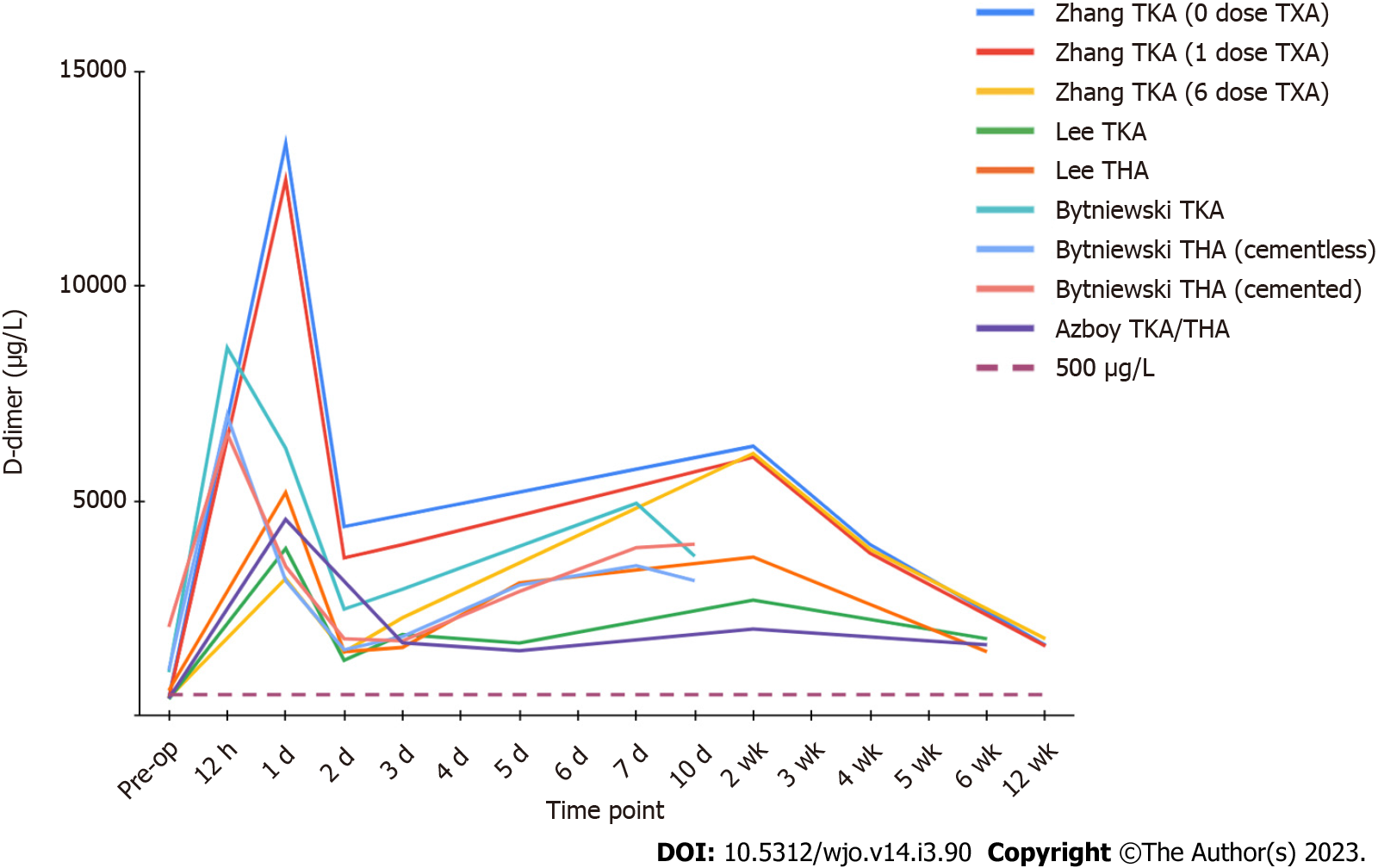
D-dimer levels appear to display a biphasic distribution after total joint replacement, with two distinct peaks (Figure 2). Levels rise sharply after the operation, peaking within the first 24 h, then sharply decrease to a trough by postoperative day 2 to 3. This is followed by a gradual increase to a second peak around the 7 to 14-d mark, with a gradual decrease thereafter[46,50-52]. Azboy et al[46] found the first peak to be almost 9-fold higher than baseline, with the mean levels of the two troughs, on day 3 and 45, still representing elevation of at least 3 times preoperative values. D-dimer appears to maintain elevation well beyond the acute post-surgical period, with Zhang et al[52] reporting persistently raised values at 3 mo. To our knowledge, there is no literature reporting D-dimer levels beyond 90 d after an uneventful joint replacement, and the time it takes to return to baseline is currently unknown.
According to the National Quality Improvement Program database, venous thromboembolism still represents one of the most common complications in patients undergoing total joint arthroplasty, affecting approximately 0.6% of patients after total hip arthroplasty (THA) and 1.4% after total knee arthroplasty (TKA)[1]. The majority of DVTs and their related complications occur within two weeks of joint replacement surgery, but can present up to 6 wk postoperatively[2,53]. In a group of 283 symptomatic PEs, Parvizi et al[54] found that 89% occurred within the first postoperative week, and 94% occurred within two weeks. As D-dimer remains considerably elevated during this period, it is clear that standard institutional cutoffs for VTE exclusion, most commonly 500 µg/L, are inappropriate in this population. At 6 wk after operation, An et al[55] found that 92% of THA patients and 100% of TKA patients had D-dimer levels above their DVT threshold for a “positive” quantitative test. The potential value of D-dimer in the detection or exclusion of DVT after total joint arthroplasty remains controversial and unclear.
Recent research has focused on establishing new D-dimer thresholds during the postoperative period after TJA. Many studies have confirmed an association between elevated D-dimer levels and the presence of DVT in total joint patients, with some establishing useful cutoffs at specific postoperative days. Shiota et al[56] reported a threshold of 10000 µg/L on postoperative day (POD) 7 to have the highest sensitivity (THA- 95.5%, TKA- 94.4%) and specificity (THA- 96.9%, TKA- 90.0%) for DVT detection. Other authors have determined cutoffs on POD1, POD3, and POD4 to be useful as well[57-60]. This data should be interpreted with caution, however, as none of these investigations used DVT chemoprophylaxis. Agents such as low molecular weight heparin, Fondaparinux, Warfarin, and factor Xa inhibitors have been shown to decrease D-dimer levels and reduce its diagnostic performance in detecting DVT[60-64]. Other authors, some of whom used chemoprophylaxis and others who did not, have determined that D-dimer has limited or no value in DVT diagnosis after a recent total joint operation[65-70].
With this conflicting evidence, the role of D-dimer in the detection of VTE after TJA is undetermined. There is a lack of research assessing the value of D-dimer when the primary prophylactic agent is aspirin, which has overwhelmingly become the most popular agent used in primary joint arthroplasty according to an American Association of Hip and Knee Surgeon survey in 2020[71]. In contrast to other contemporary anticoagulants, authors have shown that antiplatelet drugs such as aspirin and clopidogrel (Plavix) do not alter D-dimer levels, however these studies did not specifically evaluate arthroplasty patients[72,73]. Many previous investigations were also performed without the use of tranexamic acid, a known inhibitor of fibrinolysis, which has been shown to decrease D-dimer levels up to 3 days postoperatively (Figures 1 and 2)[52]. Larger trials focusing on symptomatic VTE events using contemporary prophylactic protocols are necessary. In addition, future investigations must determine when D-dimer levels finally normalize after the operation, establishing a time point for when institutional VTE cutoff values (commonly 500 µg/L) can be properly applied in this population.
Ultimately, D-dimer may be more useful as an adjunct within other diagnostic tools rather than a standalone test. In recent years, mathematical based predictive models have emerged as potentially groundbreaking tools in multiple medical fields[74]. These algorithms, which are widely used in data mining, machine learning, and artificial intelligence, can efficiently and accurately create models for the classification and prediction of adverse events based on historical case data. Chen et al[75] constructed an algorithm utilizing predictive indicators of VTE, including elevated D-dimer levels on POD 1, capable of accurately predicting the incidence of DVT after total knee arthroplasty. Although this algorithm needs validation in larger populations, the use of D-dimer in combination with other DVT indicators in computer based models will likely form the basis of future research.
D-dimer first emerged as a promising biomarker for PJI in 2017, when Shahi et al[11] demonstrated it outperformed both ESR and CRP in diagnosing chronic PJI in their cohort of 245 patients. With a cutoff of 850 µg/L, the authors found serum D-dimer to have a sensitivity of 89% and a specificity of 93% in distinguishing PJI from aseptic failure. In Parvizi et al’s 2018 evidence-based and validated criteria for the diagnosis of PJI, the authors found D-dimer, with an updated threshold of 860 µg/L, to be a valuable initial hematologic test, weighted similar to CRP and above ESR as a minor criterion in their new model[12]. This updated MSIS criteria displayed a significant increase in sensitivity compared to the prior MSIS (97.7% vs 86.9%) and ICM (97.7% vs 86.9%) criteria with similar specificity (99.5%). Furthermore, it has been validated in both American, German, and Chinese populations[12,76-77]. Since acceptance into the MSIS and ICM criteria, a growing body of literature assessing D-dimer’s value as a biomarker for PJI has emerged, with conflicting results and conclusions.
Investigations by Hu et al[78] and Qin et al[79] both supported the promising early findings, with D-dimer demonstrating better sensitivity, specificity, and diagnostic accuracy in detecting PJI when compared to ESR and CRP. Hu et al[78] found D-dimer to demonstrate a sensitivity of 87.50% and a specificity of 89.19%, superior to those of ESR (82.50% and 64.86%, respectively) and CRP (80.00% and 78.38%, respectively). Qin et al[79] determined D-dimer to have outstanding diagnostic accuracy with an area under the curve (AUC) of 0.915, far above that of ESR (0.719) and CRP (0.761). Other authors, however, have published less optimistic data. Xu et al[17] concluded that with sensitivity of 68.3% and specificity of 50.7%, D-dimer had limited value compared to traditional biomarkers. Using the previously established threshold of 850 µg/L, Pannu et al[14] demonstrated poor accuracy (61%) and low specificity (32.3%) to discriminate PJI from aseptic loosening in their population. Furthering the confusion, many studies have established different cutoffs from the recommended 860 µg/L of the new MSIS criteria, with published thresholds varying widely from 410 µg/L to 2750 µg/L[18,20].
A collection of systematic reviews and meta-analyses were recently published in an effort to eliminate confusion and draw clarity from the literature[80-87]. The overall pooled data displays that D-dimer has good diagnostic accuracy to detect PJI. Zhang et al[86] and Wang et al[84] reported D-dimer to have an overall sensitivity of 82%, a specificity of 73%, and an AUC of 0.85. However, these studies have revealed considerable heterogeneity in the current literature. Through meta-regression and subgroup analysis, this compilation of review papers published some interesting findings that illuminate possible ways to best optimize D-dimer as a biomarker for PJI. These conclusions are summarized as follows.
Serum versus plasma D-dimer: Serum D-dimer displayed better diagnostic accuracy vs plasma D-dimer: Blood sample technique was commonly found to be the number one determinant of heterogeneity among the current literature. After subgroup analysis, Li et al[81] found that serum D-dimer exhibited a superior pooled sensitivity and specificity (86% and 84%, respectively) vs plasma D-dimer (67% and 60%, respectively). Serum D-dimer demonstrated excellent diagnostic value with an AUC of 0.91, far above that of plasma D-dimer (AUC of 0.66). Other authors have further supported this finding[80,82-87]. Some studies have reported no difference in baseline D-dimer levels when using either of the two techniques, however, Boisclair et al[89] reported significant differences in sensitivity and specificity when examining serum vs plasma D-dimer in the diagnosis of disseminated intravascular coagulation, DVT, and myocardial infarction[88,89]. Large comparative trials are needed to elucidate the true value of blood sample technique in arthroplasty patients, but studies utilizing serum sampling have displayed much better accuracy in diagnosing PJI.
Inflammatory and hypercoagulability disorders: Exclusion of inflammatory and hypercoagulability disorders improved diagnostic accuracy: In their 2020 meta-analysis, Yan et al[85] found that studies which excluded patients with hypercoagulability disorders displayed higher sensitivity (85% vs 68%) and specificity (83% vs 62%) vs those that did not. Similarly, they reported D-dimer to demonstrate a higher sensitivity (81% vs 75%) when patients with inflammatory arthritis were excluded[84]. These results are not unexpected, as baseline D-dimer levels are substantially elevated in patients with inflammatory joint disease, thrombosis, malignancy, pregnancy, and heart disease vs healthy controls[41,90-93]. In addition to systemic hypercoagulation, the degradation of large quantities of fibrin deposited in the synovium of rheumatoid patients has been shown to increase D-dimer levels[94]. In patients with cardiovascular disease, autoimmune disease, and malignancy, Li et al[16] found that plasma D-dimer had no meaningful capacity to discriminate PJI from aseptic loosening (AUC of 0.50, 0.52, and 0.58, respectively). As patients with these comorbidities also display elevated inflammatory markers such as ESR and CRP, this population presents significant challenges in regard to properly establishing a diagnosis of chronic PJI.
Race and geography: White and black american populations displayed increased diagnostic accuracy vs east asian populations: In a meta-analysis of 8 studies, Lu et al[82] found geographic and racial differences to have a major impact on the diagnostic accuracy of D-dimer in PJI diagnosis. Caucasian and African American races demonstrated increased sensitivity (92%) and specificity (74%) vs those of East Asian populations (72% and 65%, respectively). Variances in study protocol and laboratory assay practices may confound these findings, however racial differences in D-dimer levels are well documented in the literature, even when controlling for social factors and comorbidities[92,95]. Providers should be mindful of demographic differences when interpreting D-dimer research, and investigators should be encouraged to disclose ethnicity to increase the external validity of future studies.
Optimal D-dimer cutoff: Current literature uses a wide range of cutoff values: There is wide variation in D-dimer threshold values used for the diagnosis of chronic PJI in the current literature. While some of the recent investigations used the previously established cutoff of 850 µg/L, others calculated their own using receiver operating characteristic curve analysis to best optimize the diagnostic value of the biomarker[11]. Furthermore, there is a scarcity of studies utilizing the cutoff of 860 µg/L, the current threshold recommended by the MSIS and ICM[12,13]. The establishment of an appropriate threshold is essential, as any change in this value can have significant impacts on diagnostic accuracy.
This wide variation is likely due to many factors, including differences in laboratory protocols and population characteristics. In addition to blood sample technique, there is potential for substantial differences in D-dimer levels depending on each laboratory’s diagnostic platform. The development of a universal reference standard for D-dimer has been infamously difficult, making standardization between assays impossible up to this point[35,38-39]. In a simulation utilizing data from 3903 Laboratories, Pearson et al[96] calculated that given identical blood samples, the mean D-dimer value varied from 540 to 880 µg/L depending on the platform utilized. In their model, a sample with a true value of 760 µg/L produced levels exceeding the 860 µg/L cutoff in 18% of their results. Likewise, a sample with a true value of 960 µg/L reported a level less than 860 µg/L in 24% of the samples. Provided the variability in D-dimer results, the authors concluded that each site should conduct their own research to determine an optimal threshold for their unique testing platform. While this may not be practical for most institutions, a surgeon’s knowledge of their center’s testing protocols combined with improved transparency in the literature will help improve the reproducibility of best cutoff values.
In summary, the inclusion of inflammatory patients, population differences, and a lack of standardization of lab protocols can all be responsible for the inconsistent results and thresholds. However, the largest reason for conflicting conclusions appears to be a difference in the type of sample technique used. With current literature in mind, we advise utilizing serum D-dimer, as opposed to plasma D-dimer, to best optimize its diagnostic value in determining chronic PJI. We conclude that serum D-dimer is an excellent serological biomarker for diagnosing chronic PJI, especially when used in combination with other infectious indicators as part of diagnostic tools such as the MSIS criteria.
Lee et al[51] displayed that D-dimer values fall more rapidly than ESR and CRP after total joint arthroplasty, leading to speculation it could be useful in the diagnosis of acute PJI. However, persistent elevation of D-dimer levels during the acute postoperative phase (up to 6 wk), poses issues with currently established cutoffs for chronic PJI. Azboy et al[46] reported that 88.7% of their uneventful TJA patients had D-dimer levels above the 860 µg/L threshold on postoperative day 15, with 77% exceeding the cutoff on day 45. As baseline D-dimer levels are already substantially inflated within the first four to six weeks due to postsurgical inflammation and fibrinolysis, D-dimer does not appear to be useful for the diagnosis of acute PJI with the currently recommended threshold. Further research is needed to determine an optimal cutoff for early PJI diagnosis, as well as establish a time point for when chronic PJI criteria can be appropriately applied.
Two-stage revision continues to be one of the most common approaches for chronic PJI treatment. There is currently no gold standard for confirmation of infection eradication prior to reimplantation, and markers such as ESR, CRP, and even alpha defensin have demonstrated limited utility in this regard[97-99]. Shahi et al[11] first predicted the utility of D-dimer in this setting. In 5 patients with “elevated” D-dimer at the time of reimplantation, 2 went on to experience septic failure. Pannu et al[100] demonstrated that D-dimer had low specificity (47%) and accuracy (AUC of 0.62) to predict persistence of infection after the second stage. However, it displayed a sensitivity of 90% and a negative predictive value of 94%, indicating promise as a biomarker to rule out residual infection and indicate safe timing for reimplantation. Furthermore, they discovered that when combined with ESR and CRP, the specificity increased to 91%. Although this study is limited by a small sample size (n = 10), it certainly sets the stage for future multicenter investigations and creates optimism that D-dimer can have an important role in this setting.
Plasma fibrinogen, the precursor to fibrin, is well known for its role in the coagulation cascade and has also been found to be a promising biomarker for the diagnosis of PJI[101]. Several recent publications have found plasma fibrinogen to exhibit significantly better diagnostic performance than plasma D-dimer in identifying chronic PJI[16,18,102]. However, all of these investigations utilized plasma sampling, and to our knowledge, there are no studies comparing serum D-dimer to plasma fibrinogen. In 2021, a meta-analysis by Xu et al[103] reported that plasma fibrinogen had better diagnostic accuracy than D-dimer when plasma and serum data was combined. However, after subgroup analysis, D-dimer actually displayed better accuracy than plasma fibrinogen when serum sample technique was utilized (AUC 0.91 vs 0.83, respectively). The authors concluded that serum D-dimer may have better diagnostic potential than plasma fibrinogen, and that plasma D-dimer has limited diagnostic value. Regardless, plasma fibrinogen appears to be a good alternative to D-dimer, especially at sites that are limited to a plasma testing protocol.
In addition to the heterogeneity of the existing literature, it is important to note additional limitations. Most studies fail to adequately describe their laboratory protocol for D-dimer testing. As Pearson et al[96] demonstrated, assay practices can have a large effect on D-dimer values. In addition, the terms “serum” and “plasma” have incorrectly been used interchangeably in the literature, promoting fear that they may be mislabeled in other investigations[55,104]. Surgeons and researchers should appreciate which type of blood sample technique is being used at their institution, and transparency of both sample technique and assay utilized is imperative for reproducibility of future research. Lastly, although pooled data seems to confirm that serum D-dimer is superior to plasma D-dimer, no comparative studies have been performed between the two sampling methods in the setting of chronic PJI. A prospective, paired trial comparing the diagnostic values of plasma and serum D-dimer for the diagnosis of PJI is necessary to provide more clarity.
D-dimer values are substantially elevated in the acute postoperative period after total joint arthroplasty, and standard institutional cutoffs for VTE (most commonly 500 µg/L) are inappropriate in these patients. The utility of D-dimer in detecting VTE after total joint arthroplasty is currently limited, and more research assessing its value in the face of contemporary DVT prophylaxis protocols is warranted. D-dimer appears to be a promising biomarker for the diagnosis of chronic PJI, especially when using serum sample technique. Providers should exercise caution when interpreting D-dimer levels in those with inflammatory and hypercoagulability disorders, as the diagnostic value is decreased in these patients. Larger prospective studies with transparent lab testing protocols are needed to establish best assay practices and optimal cutoff values. Despite the demand for further research to optimize the diagnostic performance of D-dimer, the current identification of PJI does not rely on a single test. More research assessing the value of combined biomarkers may be more useful, and the updated MSIS and ICM criteria, which include D-dimer levels > 860 µg/L as a minor criterion, may be the most accurate for diagnosing chronic PJI to date.
We thank Mary Baldwin for her contributions to the Figures/Illustrations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ding X, China; Doski JO, Iraq; Wang J, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Warren JA, Sundaram K, Anis HK, Kamath AF, Higuera CA, Piuzzi NS. Have Venous Thromboembolism Rates Decreased in Total Hip and Knee Arthroplasty? J Arthroplasty. 2020;35:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 2. | Yamaguchi T, Hasegawa M, Niimi R, Sudo A. Incidence and time course of asymptomatic deep vein thrombosis with fondaparinux in patients undergoing total joint arthroplasty. Thromb Res. 2010;126:e323-e326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Koh CK, Zeng I, Ravi S, Zhu M, Vince KG, Young SW. Periprosthetic Joint Infection Is the Main Cause of Failure for Modern Knee Arthroplasty: An Analysis of 11,134 Knees. Clin Orthop Relat Res. 2017;475:2194-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 4. | Ting NT, Della Valle CJ. Diagnosis of Periprosthetic Joint Infection-An Algorithm-Based Approach. J Arthroplasty. 2017;32:2047-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27:61-5.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1223] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 6. | Yao JJ, Hevesi M, Visscher SL, Ransom JE, Lewallen DG, Berry DJ, Maradit Kremers H. Direct Inpatient Medical Costs of Operative Treatment of Periprosthetic Hip and Knee Infections Are Twofold Higher Than Those of Aseptic Revisions. J Bone Joint Surg Am. 2021;103:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Helwig P, Morlock J, Oberst M, Hauschild O, Hübner J, Borde J, Südkamp NP, Konstantinidis L. Periprosthetic joint infection--effect on quality of life. Int Orthop. 2014;38:1077-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Walter N, Rupp M, Hierl K, Koch M, Kerschbaum M, Worlicek M, Alt V. Long-Term Patient-Related Quality of Life after Knee Periprosthetic Joint Infection. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992-2994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1212] [Cited by in RCA: 1450] [Article Influence: 103.6] [Reference Citation Analysis (1)] |
| 10. | Zmistowski B, Della Valle C, Bauer TW, Malizos KN, Alavi A, Bedair H, Booth RE, Choong P, Deirmengian C, Ehrlich GD, Gambir A, Huang R, Kissin Y, Kobayashi H, Kobayashi N, Krenn V, Drago L, Marston SB, Meermans G, Perez J, Ploegmakers JJ, Rosenberg A, Simpendorfer C, Thomas P, Tohtz S, Villafuerte JA, Wahl P, Wagenaar FC, Witzo E. Diagnosis of periprosthetic joint infection. J Arthroplasty. 2014;29:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Shahi A, Kheir MM, Tarabichi M, Hosseinzadeh HRS, Tan TL, Parvizi J. Serum D-Dimer Test Is Promising for the Diagnosis of Periprosthetic Joint Infection and Timing of Reimplantation. J Bone Joint Surg Am. 2017;99:1419-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 12. | Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty. 2018;33:1309-1314.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 885] [Cited by in RCA: 1405] [Article Influence: 200.7] [Reference Citation Analysis (0)] |
| 13. | Shohat N, Bauer T, Buttaro M, Budhiparama N, Cashman J, Della Valle CJ, Drago L, Gehrke T, Marcelino Gomes LS, Goswami K, Hailer NP, Han SB, Higuera CA, Inaba Y, Jenny JY, Kjaersgaard-Andersen P, Lee M, Llinás A, Malizos K, Mont MA, Jones RM, Parvizi J, Peel T, Rivero-Boschert S, Segreti J, Soriano A, Sousa R, Spangehl M, Tan TL, Tikhilov R, Tuncay I, Winkler H, Witso E, Wouthuyzen-Bakker M, Young S, Zhang X, Zhou Y, Zimmerli W. Hip and Knee Section, What is the Definition of a Periprosthetic Joint Infection (PJI) of the Knee and the Hip? Can the Same Criteria be Used for Both Joints? Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34:S325-S327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 14. | Pannu TS, Villa JM, Patel PD, Riesgo AM, Barsoum WK, Higuera CA. The Utility of Serum d-Dimer for the Diagnosis of Periprosthetic Joint Infection in Revision Total Hip and Knee Arthroplasty. J Arthroplasty. 2020;35:1692-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Huang J, Zhang Y, Wang Z, Dong Y, Zhao Y, Zheng J, Lian H, Jin Y. The serum level of D-Dimer is not suitable for distinguishing between prosthetic joint infection and aseptic loosening. J Orthop Surg Res. 2019;14:407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Li R, Shao HY, Hao LB, Yu BZ, Qu PF, Zhou YX, Chen JY. Plasma Fibrinogen Exhibits Better Performance Than Plasma D-Dimer in the Diagnosis of Periprosthetic Joint Infection: A Multicenter Retrospective Study. J Bone Joint Surg Am. 2019;101:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 17. | Xu H, Xie J, Huang Q, Lei Y, Zhang S, Pei F. Plasma Fibrin Degradation Product and D-Dimer Are of Limited Value for Diagnosing Periprosthetic Joint Infection. J Arthroplasty. 2019;34:2454-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Wu H, Meng Z, Pan L, Liu H, Yang X, Yongping C. Plasma Fibrinogen Performs Better Than Plasma d-Dimer and Fibrin Degradation Product in the Diagnosis of Periprosthetic Joint Infection and Determination of Reimplantation Timing. J Arthroplasty. 2020;35:2230-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Fu J, Ni M, Chai W, Li X, Hao L, Chen J. Synovial Fluid Viscosity Test is Promising for the Diagnosis of Periprosthetic Joint Infection. J Arthroplasty. 2019;34:1197-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Ackmann T, Möllenbeck B, Gosheger G, Schwarze J, Schmidt-Braekling T, Schneider KN, Frommer A, Dieckmann R, Theil C. Comparing the Diagnostic Value of Serum D-Dimer to CRP and IL-6 in the Diagnosis of Chronic Prosthetic Joint Infection. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Johnson ED, Schell JC, Rodgers GM. The D-dimer assay. Am J Hematol. 2019;94:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 22. | Lurie JM, Png CYM, Subramaniam S, Chen S, Chapman E, Aboubakr A, Marin M, Faries P, Ting W. Virchow's triad in "silent" deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2019;7:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Hansrani V, Khanbhai M, McCollum C. The Diagnosis and Management of Early Deep Vein Thrombosis. Adv Exp Med Biol. 2017;906:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Ribera T, Monreal L, Armengou L, Ríos J, Prades M. Synovial fluid D-dimer concentration in foals with septic joint disease. J Vet Intern Med. 2011;25:1113-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Hügle T, Nasi S, Ehirchiou D, Omoumi P, So A, Busso N. Fibrin deposition associates with cartilage degeneration in arthritis. EBioMedicine. 2022;81:104081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 26. | Lim W, Le Gal G, Bates SM, Righini M, Haramati LB, Lang E, Kline JA, Chasteen S, Snyder M, Patel P, Bhatt M, Braun C, Begum H, Wiercioch W, Schünemann HJ, Mustafa RA. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2018;2:3226-3256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 276] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 27. | Cicala C, Cirino G. Linkage between inflammation and coagulation: an update on the molecular basis of the crosstalk. Life Sci. 1998;62:1817-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Rodelo JR, De la Rosa G, Valencia ML, Ospina S, Arango CM, Gómez CI, García A, Nuñez E, Jaimes FA. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30:1991-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Gris JC, Bouvier S, Cochery-Nouvellon E, Faillie JL, Lissalde-Lavigne G, Lefrant JY. Fibrin-related markers in patients with septic shock: individual comparison of D-dimers and fibrin monomers impacts on prognosis. Thromb Haemost. 2011;106:1228-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Ge YL, Liu CH, Wang N, Xu J, Zhu XY, Su CS, Li HL, Zhang HF, Li ZZ, Zhang X, Chen H, Yu HL, Fu AS, Wang HY. Elevated Plasma D-Dimer in Adult Community-Acquired Pneumonia Patients is Associated with an Increased Inflammatory Reaction and Lower Survival. Clin Lab. 2019;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Schwameis M, Steiner MM, Schoergenhofer C, Lagler H, Buchtele N, Jilma-Stohlawetz P, Boehm T, Jilma B. D-dimer and histamine in early stage bacteremia: A prospective controlled cohort study. Eur J Intern Med. 2015;26:782-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Ullah W, Thalambedu N, Haq S, Saeed R, Khanal S, Tariq S, Roomi S, Madara J, Boigon M, Haas DC, Fischman DL. Predictability of CRP and D-Dimer levels for in-hospital outcomes and mortality of COVID-19. J Community Hosp Intern Med Perspect. 2020;10:402-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Kaftan AN, Hussain MK, Algenabi AA, Naser FH, Enaya MA. Predictive Value of C-reactive Protein, Lactate Dehydrogenase, Ferritin and D-dimer Levels in Diagnosing COVID-19 Patients: a Retrospective Study. Acta Inform Med. 2021;29:45-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Saxena A, Baratz M, Austin MS, Purtill JJ, Parvizi J. Periprosthetic joint infection can cause abnormal systemic coagulation. J Arthroplasty. 2011;26:50-57, 57.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Lima-Oliveira G, Monneret D, Guerber F, Guidi GC. Sample management for clinical biochemistry assays: Are serum and plasma interchangeable specimens? Crit Rev Clin Lab Sci. 2018;55:480-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Liu X, Hoene M, Wang X, Yin P, Häring HU, Xu G, Lehmann R. Serum or plasma, what is the difference? Anal Chim Acta. 2018;1037:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Olson JD. D-dimer: An Overview of Hemostasis and Fibrinolysis, Assays, and Clinical Applications. Adv Clin Chem. 2015;69:1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 38. | Tripodi A. D-dimer testing in laboratory practice. Clin Chem. 2011;57:1256-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 39. | Thachil J, Lippi G, Favaloro EJ. D-Dimer Testing: Laboratory Aspects and Current Issues. Methods Mol Biol. 2017;1646:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Prochaska JH, Frank B, Nagler M, Lamparter H, Weißer G, Schulz A, Eggebrecht L, Göbel S, Arnold N, Panova-Noeva M, Hermanns I, Pinto A, Konstantinides S, Ten Cate H, Lackner KJ, Münzel T, Espinola-Klein C, Wild PS. Age-related diagnostic value of D-dimer testing and the role of inflammation in patients with suspected deep vein thrombosis. Sci Rep. 2017;7:4591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Simes J, Robledo KP, White HD, Espinoza D, Stewart RA, Sullivan DR, Zeller T, Hague W, Nestel PJ, Glasziou PP, Keech AC, Elliott J, Blankenberg S, Tonkin AM; LIPID Study Investigators. D-Dimer Predicts Long-Term Cause-Specific Mortality, Cardiovascular Events, and Cancer in Patients With Stable Coronary Heart Disease: LIPID Study. Circulation. 2018;138:712-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 42. | Haase C, Joergensen M, Ellervik C, Joergensen MK, Bathum L. Age- and sex-dependent reference intervals for D-dimer: evidence for a marked increase by age. Thromb Res. 2013;132:676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Wu JX, Qing JH, Yao Y, Chen DY, Jiang Q. Performance of age-adjusted D-dimer values for predicting DVT before the knee and hip arthroplasty. J Orthop Surg Res. 2021;16:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Imai N, Miyasaka D, Shimada H, Suda K, Ito T, Endo N. Usefulness of a novel method for the screening of deep vein thrombosis by using a combined D-dimer- and age-based index before total hip arthroplasty. PLoS One. 2017;12:e0172849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | De Maio F, Fidone G, Caterini A, Gorgolini G, Petrungaro L, Farsetti P. Monitoring of C-reactive protein level (CRP) and Erythrocyte sedimentation rate (ESR) after total hip and knee arthroplasty. J Biol Regul Homeost Agents. 2020;34:63-68. IORS Special Issue on Orthopedics. [PubMed] |
| 46. | Azboy I, Çatal B, Başarır K, Mutlu M, Bilgen ÖF, Parvizi J. The Natural Course of Serum D-Dimer, C-Reactive Protein, and Erythrocyte Sedimentation Rate Levels After Uneventful Primary Total Joint Arthroplasty. J Arthroplasty. 2021;36:3118-3122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Opneja A, Kapoor S, Stavrou EX. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb Res. 2019;179:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 48. | Wong RMY, Choy VMH, Li J, Li TK, Chim YN, Li MCM, Cheng JCY, Leung KS, Chow SK, Cheung WH. Fibrinolysis as a target to enhance osteoporotic fracture healing by vibration therapy in a metaphyseal fracture model. Bone Joint Res. 2021;10:41-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | O'Keefe RJ. Fibrinolysis as a Target to Enhance Fracture Healing. N Engl J Med. 2015;373:1776-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Bytniewski P, Machała W, Romanowski L, Wiśniewski W, Kosowski K. The dynamics of D-dimer level fluctuation in patients after the cemented and cementless total hip and total knee replacement. J Orthop Surg Res. 2014;9:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Lee YS, Lee YK, Han SB, Nam CH, Parvizi J, Koo KH. Natural progress of D-dimer following total joint arthroplasty: a baseline for the diagnosis of the early postoperative infection. J Orthop Surg Res. 2018;13:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 52. | Zhang S, Xie J, Cao G, Lei Y, Huang Q, Pei F. Six-Dose Intravenous Tranexamic Acid Regimen Further Inhibits Postoperative Fibrinolysis and Reduces Hidden Blood Loss following Total Knee Arthroplasty. J Knee Surg. 2021;34:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Hu C, Liu C, Wang Y, Ding T, Sun K, Tian S. The Timing of Symptomatic Pulmonary Embolism in Patients With Nonwarfarin Anticoagulants Following Elective Primary Total Joint Arthroplasty. J Arthroplasty. 2020;35:1703-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Parvizi J, Huang R, Raphael IJ, Maltenfort MG, Arnold WV, Rothman RH. Timing of Symptomatic Pulmonary Embolism with Warfarin Following Arthroplasty. J Arthroplasty. 2015;30:1050-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | An TJ, Engstrom SM, Oelsner WK, Benvenuti MA, Polkowski GG, Schoenecker JG. Elevated d-Dimer Is Not Predictive of Symptomatic Deep Venous Thrombosis After Total Joint Arthroplasty. J Arthroplasty. 2016;31:2269-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Shiota N, Sato T, Nishida K, Matsuo M, Takahara Y, Mitani S, Murakami T, Inoue H. Changes in LPIA D-dimer levels after total hip or knee arthroplasty relevant to deep-vein thrombosis diagnosed by bilateral ascending venography. J Orthop Sci. 2002;7:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Niimi R, Hasegawa M, Sudo A, Shi D, Yamada T, Uchida A. Evaluation of soluble fibrin and D-dimer in the diagnosis of postoperative deep vein thrombosis. Biomarkers. 2010;15:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Yoo MC, Cho YJ, Ghanem E, Ramteke A, Kim KI. Deep vein thrombosis after total hip arthroplasty in Korean patients and D-dimer as a screening tool. Arch Orthop Trauma Surg. 2009;129:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Yoshitaka T, Abe N, Minagawa H, Date H, Sakoma Y, Nishida K, Ozaki T. Disease-specific screening for deep venous thrombosis and pulmonary thromboembolism using plasma D-dimer values after total knee arthroplasty. Mod Rheumatol. 2008;18:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 60. | Niimi R, Hasegawa M, Shi DQ, Sudo A. The influence of fondaparinux on the diagnosis of postoperative deep vein thrombosis by soluble fibrin and D-dimer. Thromb Res. 2012;130:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Aguilar C, Del Villar V. Diagnostic value of D-dimer in outpatients with suspected deep venous thrombosis receiving oral anticoagulation. Blood Coagul Fibrinolysis. 2007;18:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Sasaki H, Ishida K, Shibanuma N, Tei K, Tateishi H, Toda A, Yamashiro Y, Matsumoto T, Kuroda R, Kurosaka M. Retrospective comparison of three thromboprophylaxis agents, edoxaban, fondaparinux, and enoxaparin, for preventing venous thromboembolism in total knee arthroplasty. Int Orthop. 2014;38:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Zhou J, Fang R, Yan Q, Li C, Zhou Y, Nur AA, Liu T, Wang W. Low-molecular-weight heparin followed by rivaroxaban or not for the prevention of deep venous thromboembolism after total knee arthroplasty. Blood Coagul Fibrinolysis. 2019;30:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Arnesen H, Dahl OE, Aspelin T, Seljeflot I, Kierulf P, Lyberg T. Sustained prothrombotic profile after hip replacement surgery: the influence of prolonged prophylaxis with dalteparin. J Thromb Haemost. 2003;1:971-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Chen CJ, Wang CJ, Huang CC. The value of D-dimer in the detection of early deep-vein thrombosis after total knee arthroplasty in Asian patients: a cohort study. Thromb J. 2008;6:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Bounameaux H, Miron MJ, Blanchard J, de Moerloose P, Hoffmeyer P, Leyvraz PF. Measurement of plasma D-dimer is not useful in the prediction or diagnosis of postoperative deep vein thrombosis in patients undergoing total knee arthroplasty. Blood Coagul Fibrinolysis. 1998;9:749-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Kim KI, Cho KY, Jin W, Khurana SS, Bae DK. Recent Korean perspective of deep vein thrombosis after total knee arthroplasty. J Arthroplasty. 2011;26:1112-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Mitani G, Takagaki T, Hamahashi K, Serigano K, Nakamura Y, Sato M, Mochida J. Associations between venous thromboembolism onset, D-dimer, and soluble fibrin monomer complex after total knee arthroplasty. J Orthop Surg Res. 2015;10:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Rafee A, Herlikar D, Gilbert R, Stockwell RC, McLauchlan GJ. D-Dimer in the diagnosis of deep vein thrombosis following total hip and knee replacement: a prospective study. Ann R Coll Surg Engl. 2008;90:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Wu CT, Chen B, Wang JW, Yen SH, Huang CC. Plasma D-dimer is not useful in the prediction of deep vein thrombosis after total knee arthroplasty in patients using rivaroxaban for thromboprophylaxis. J Orthop Surg Res. 2018;13:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Abdel MP, Meneghini RM, Berry DJ. Current Practice Trends in Primary Hip and Knee Arthroplasties Among Members of the American Association of Hip and Knee Surgeons: An Update During the COVID-19 Pandemic. J Arthroplasty. 2021;36:S40-S44.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 72. | Schol-Gelok S, van der Hulle T, Biedermann JS, van Gelder T, Klok FA, van der Pol LM, Versmissen J, Huisman MV, Kruip MJHA. Clinical effects of antiplatelet drugs and statins on D-dimer levels. Eur J Clin Invest. 2018;48:e12944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Cassar K, Bachoo P, Ford I, Greaves M, Brittenden J. Clopidogrel has no effect on D-dimer and thrombin-antithrombin III levels in patients with peripheral arterial disease undergoing peripheral percutaneous transluminal angioplasty. J Vasc Surg. 2005;42:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Davagdorj K, Pham VH, Theera-Umpon N, Ryu KH. XGBoost-Based Framework for Smoking-Induced Noncommunicable Disease Prediction. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 75. | Chen Y, Jiang Y. Construction of Prediction Model of Deep Vein Thrombosis Risk after Total Knee Arthroplasty Based on XGBoost Algorithm. Comput Math Methods Med. 2022;2022:3452348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 76. | Abdelaziz H, Rademacher K, Suero EM, Gehrke T, Lausmann C, Salber J, Citak M. The 2018 International Consensus Meeting Minor Criteria for Chronic Hip and Knee Periprosthetic Joint Infection: Validation From a Single Center. J Arthroplasty. 2020;35:2200-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 77. | Guan H, Fu J, Li X, Chai W, Hao L, Li R, Zhao J, Chen J. The 2018 new definition of periprosthetic joint infection improves the diagnostic efficiency in the Chinese population. J Orthop Surg Res. 2019;14:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Hu Q, Fu Y, Tang L. Serum D-dimer as a diagnostic index of PJI and retrospective analysis of etiology in patients with PJI. Clin Chim Acta. 2020;506:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | Qin L, Li F, Gong X, Wang J, Huang W, Hu N. Combined Measurement of D-Dimer and C-Reactive Protein Levels: Highly Accurate for Diagnosing Chronic Periprosthetic Joint Infection. J Arthroplasty. 2020;35:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 80. | Chen X, Li H, Zhu S, Wang Y, Qian W. Is D-dimer a reliable biomarker compared to ESR and CRP in the diagnosis of periprosthetic joint infection? Bone Joint Res. 2020;9:701-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Li C, Margaryan D, Ojeda-Thies C, Perka C, Trampuz A. Meta-analysis of serum and/or plasma D-dimer in the diagnosis of periprosthetic joint infection. J Orthop Surg Res. 2020;15:298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Lu G, Li T, Ye H, Liu S, Zhang P, Wang W. D-dimer in the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Orthop Surg Res. 2020;15:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Tian B, Cui L, Jiang W. The diagnostic effect of α-defensin, D-dimer, and IL-6 in periprosthetic joint infection: A systematic review and diagnostic meta-analysis. J Orthop Surg (Hong Kong). 2020;28:2309499020971861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Wang Y, Man Z, Yuan T, Cao H, Sun S. Reliability of d-Dimer Determination in Diagnosis of Peri-Prosthetic Joint Infection: A Systematic Review and Meta-Analysis. Surg Infect (Larchmt). 2021;22:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 85. | Yan J, Xie K, Jiang X, Han X, Wang L, Yan M. D-dimer for diagnosis of periprosthetic joint infection: A meta-analysis. J Orthop Sci. 2021;26:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Zhang H, Sun X, Xin P, Zhu X, Jie K, Cao H, Feng W, Zeng Y, Lv Y, Chen J, Li J, Zeng J. Diagnostic accuracy of D-dimer in periprosthetic joint infection: a diagnostic meta-analysis. J Orthop Surg Res. 2020;15:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Balato G, De Franco C, Balboni F, De Matteo V, Ascione T, Baldini A, Lippi G. The role of D-dimer in periprosthetic joint infection: a systematic review and meta-analysis. Diagnosis (Berl). 2021;9:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Korte W, Riesen W. Latex-enhanced immunoturbidimetry allows D-dimer determination in plasma and serum samples. Clin Chem. 2000;46:871-872. [PubMed] |
| 89. | Boisclair MD, Lane DA, Wilde JT, Ireland H, Preston FE, Ofosu FA. A comparative evaluation of assays for markers of activated coagulation and/or fibrinolysis: thrombin-antithrombin complex, D-dimer and fibrinogen/fibrin fragment E antigen. Br J Haematol. 1990;74:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 90. | Beckham JC, Caldwell DS, Peterson BL, Pippen AM, Currie MS, Keefe FJ, Weinberg JB. Disease severity in rheumatoid arthritis: relationships of plasma tumor necrosis factor-alpha, soluble interleukin 2-receptor, soluble CD4/CD8 ratio, neopterin, and fibrin D-dimer to traditional severity and functional measures. J Clin Immunol. 1992;12:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 91. | O'Neal WT, Soliman EZ, Howard G, Howard VJ, Safford MM, Cushman M, Zakai NA. Inflammation and hemostasis in atrial fibrillation and coronary heart disease: The REasons for Geographic And Racial Differences in Stroke study. Atherosclerosis. 2015;243:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Pieper CF, Rao KM, Currie MS, Harris TB, Cohen HJ. Age, functional status, and racial differences in plasma D-dimer levels in community-dwelling elderly persons. J Gerontol A Biol Sci Med Sci. 2000;55:M649-M657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Zakai NA, McClure LA, Judd SE, Kissela B, Howard G, Safford M, Cushman M. D-dimer and the Risk of Stroke and Coronary Heart Disease. The REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Thromb Haemost. 2017;117:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | Busso N, Hamilton JA. Extravascular coagulation and the plasminogen activator/plasmin system in rheumatoid arthritis. Arthritis Rheum. 2002;46:2268-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 95. | Hackler E 3rd, Lew J, Gore MO, Ayers CR, Atzler D, Khera A, Rohatgi A, Lewis A, Neeland I, Omland T, de Lemos JA. Racial Differences in Cardiovascular Biomarkers in the General Population. J Am Heart Assoc. 2019;8:e012729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 96. | Pearson LN, Moser KA, Schmidt RL. D-Dimer Varies Widely Across Instrument Platforms and is Not a Reliable Indicator of Periprosthetic Joint Infections. Arthroplast Today. 2020;6:686-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | Lee YS, Fernando N, Koo KH, Kim HJ, Vahedi H, Chen AF. What Markers Best Guide the Timing of Reimplantation in Two-stage Exchange Arthroplasty for PJI? Clin Orthop Relat Res. 2018;476:1972-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 98. | Fu J, Ni M, Li H, Li X, Chai W, Zhou Y, Hao L, Chen J. The proper timing of second-stage revision in treating periprosthetic knee infection: reliable indicators and risk factors. J Orthop Surg Res. 2018;13:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 99. | Samuel LT, Sultan AA, Kheir M, Villa J, Patel P, Parvizi J, Higuera CA. Positive Alpha-defensin at Reimplantation of a Two-stage Revision Arthroplasty Is Not Associated with Infection at 1 Year. Clin Orthop Relat Res. 2019;477:1615-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 100. | Pannu TS, Villa JM, Engh C 3rd, Patel A, Levine BR, Piuzzi NS, Higuera CA, Riesgo AM. Plasma D-dimer Does Not Anticipate the Fate of Reimplantation in Two-stage Exchange Arthroplasty for Periprosthetic Joint Infection: A Preliminary Investigation. Clin Orthop Relat Res. 2021;479:1458-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 101. | Klim SM, Amerstorfer F, Gruber G, Bernhardt GA, Radl R, Leitner L, Leithner A, Glehr M. Fibrinogen - A Practical and Cost Efficient Biomarker for Detecting Periprosthetic Joint Infection. Sci Rep. 2018;8:8802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 102. | Wang Y, Li Y, Qiao L, Sun S. Comparison of a Comprehensive Set of Fibrinolytic Markers With C-Reactive Protein and Erythrocyte Sedimentation Rate for the Diagnosis of Periprosthetic Joint Infection. J Arthroplasty. 2020;35:2613-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Xu H, Xie JW, Yang JL, Huang ZY, Pei FX. Role of D-dimer and Fibrinogen in the Diagnosis of Periprosthetic Joint Infection: A Systematic Review and Meta-Analysis. Orthop Surg. 2021;13:692-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | Pannu TS, Villa JM, Riesgo AM, Higuera CA. Letter to the Editor on "Combined Measurement of D-Dimer and C-Reactive Protein Levels: Highly Accurate for Diagnosing Chronic Periprosthetic Joint Infection". J Arthroplasty. 2021;36:e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |