INTRODUCTION
Owing to recent advancements in medicine, the life expectancy of the general population has increased. With changes in modern lifestyle, there is an increased expectation for retention of physical activity and mobility; therefore, the number of joint replacement surgeries has surged[1,2]. Around 1 million knee and hip arthroplasty procedures are performed annually in the United States, and this number is anticipated to double by 2030[3]. In addition to this increase in the number of surgeries, the incidence of PJI also continues to rise[2]. Currently PJI occurs in 1% to 2% of primary and 4% of revision arthroplasties[1,2,4,5]. Kurtz et al[1] suggest that there will be over 260,000 revision total knee arthroplasties (TKAs) performed in the United States by 2030. Compared to hip arthroplasty, the risk of PJI is higher after knee arthroplasty[6,7]. For example, the rate of PJI reported after TKA varies from 0.5%-2%, while a range of 0.5%-1.0% is reported after total hip arthroplasty. A higher risk of PJI following TKA may be attributed to less protective soft tissue coverage and higher joint mobility in the knee[8,9]. Delanois et al[10] report that PJI alone accounted for 20.4% of all revisions after TKA, and this is considered the most common etiology leading to revision surgery. A number of risk factors are associated with developing PJI including the operative setting, patient comorbidities, and implant-related factors[2]. Additionally, the longer the implanted prosthesis is expected to last, the greater the cumulative risk is for developing infection. Diagnosing PJI early can reduce the significant physical and emotional burden on the patient and the financial pressures on society. However, early diagnosis is still challenging due to the lack of tests that are highly sensitive and specific for this complication. However, early clinical suspicion in parallel to the use of existing serological markers, radiological examination, joint aspirate evaluation, and biopsy continues to be the mainstay for diagnosing PJI[11,12]. The management of PJI remains controversial and requires a complex therapeutic approach, prolonged antimicrobial therapy, and the use of a variety of surgical techniques. Selecting the optimal treatment strategy to eradicate the infection requires proper diagnosis of the infecting microorganisms and identification of their antibiotic susceptibilities. When PJI is missed or inadequately treated, the patient will likely need to endure several operations due to the persistence of infection, negatively impacting function and quality of life[13]. An interdisciplinary approach is crucial to reaching the best patient outcome, and this requires the involvement of orthopedic and plastic surgeons, infectious disease physicians, and microbiologists[2,14]. The greatest difficulty in managing PJI is the formation of the so-called biofilm, which enables the responsible pathogens to remain on the implant surface, making them resistant to most systemic intravenous antibiotics. Understanding this phenomenon helps in diagnosing and treating PJI[2]. For example, the use of modern diagnostic methods such as sonication for biofilm detection increases the sensitivity for diagnosing PJI, especially in chronic infections caused by low-virulence pathogens[2].
In this review, we provide an updated summary of the current concepts surrounding the two-stage revision procedure in the management of periprosthetic knee joint infections.
DEFINITION & CLASSIFICATION OF PJI
As there currently exists no single test that is capable of diagnosing PJI with complete accuracy, this surgical complication continues to be extremely challenging to tackle[15-17]. The Musculoskeletal Infection Society (MSIS) and the Infectious Diseases Society (IDSA) have proposed criteria to help physicians diagnose PJI[18,19]. In 2018, a second consensus meeting validated the MSIS definition of PJI, but made a few minor modifications[20]. Whilst the major criteria for PJI are the same across all definitions, the minor criteria and supporting evidence are less universally agreed upon. Lately, new tests and biomarkers have evolved and become increasingly available[21-23], including serum D-dimer[24], synovial leukocyte esterase[25], synovial alpha-defensin[26], synovial C-reactive protein (CRP)[27], and molecular techniques such as next-generation sequencing[28]. However, recent research has demonstrated the variability in the sensitivity and specificity of these tests[29]. Therefore, such advancements in PJI diagnosis demanded revision of the existing diagnostic criteria to incorporate the new testing and take into account the relative importance of the different tests included. Thus, a multi-institutional study was published in 2018 in the Journal of Arthroplasty and included new diagnostic criteria[17]. This new PJI scoring system outperformed the IDSA and MSIS criteria in terms of sensitivity and specificity.
The timing in which infection occurs can aid the identification of the infecting organism. Toms et al[30] propose a classification consisting of four modes of presentation of PJI: Stage 1 - acute infections occurring within 6 wk; Stage 2 - late onset with chronic indolent infection; Stage 3: sudden-onset in an otherwise well-functioning prosthesis with an acute presentation of infection secondary to hematogenous spread; Stage 4 ( proposed by Tsukayama, Estrada, and Gustilo[31]) - positive culture at the time of surgery without previous evidence of infection.
PATHOPHYSIOLOGY OF PERIPROSTHETIC JOINT INFECTION
Most PJI cases are iatrogenic due to inoculation of microorganisms intraoperatively[13]. Based on the virulence of the infecting microorganisms, PJI could either have an early presentation (during the 1st 4-6 wk postoperative) or be delayed (usually 3 mo to 3 years). Early infection usually presents with distinct local and systemic signs of inflammation and is typically caused by highly virulent microorganisms (e.g., Staphylococcus aureus, Streptococci spp., Enterococci spp.). On the other hand, less virulent organisms (e.g., coagulase-negative Staphylococci or Cutibacterium spp.) are the culprits of most delayed infections, which usually present with milder signs and symptoms[2,13] (Figure 1). The presence of foreign bodies such as orthopedic implants increases the infection risk, largely owing to the establishment of the so-called biofilm on prosthetic surfaces[32]. The biofilm formation process consists of several steps: (1) Adherence of the microorganisms to the implant; (2) Multiplication and elaboration of exopolysaccharides ("glycocalyx"); and (3) Coalescence of microcolonies encased in the glycocalyx to form a film[33]. Near the biofilm's surface, microorganisms are generally metabolically active and have free access to nutrients. However, deep within the biofilm, microorganisms have less nutrient access and become metabolically inactive or exist in different states of dormancy, making them more immune to host defenses[34]. Hence, the success of antimicrobial therapy may be negatively impacted by the microenvironment within a biofilm, as diffusion of drugs through the biofilm may be limited[33]. Furthermore, due to the high vascularity of periprosthetic tissue, all implants are at high risk of hematogenous seeding from a distant primary focus. While the risk of this is high during the life of the implant, the highest risk of hematogenous infection occurs in the first few years after implantation[2,35].
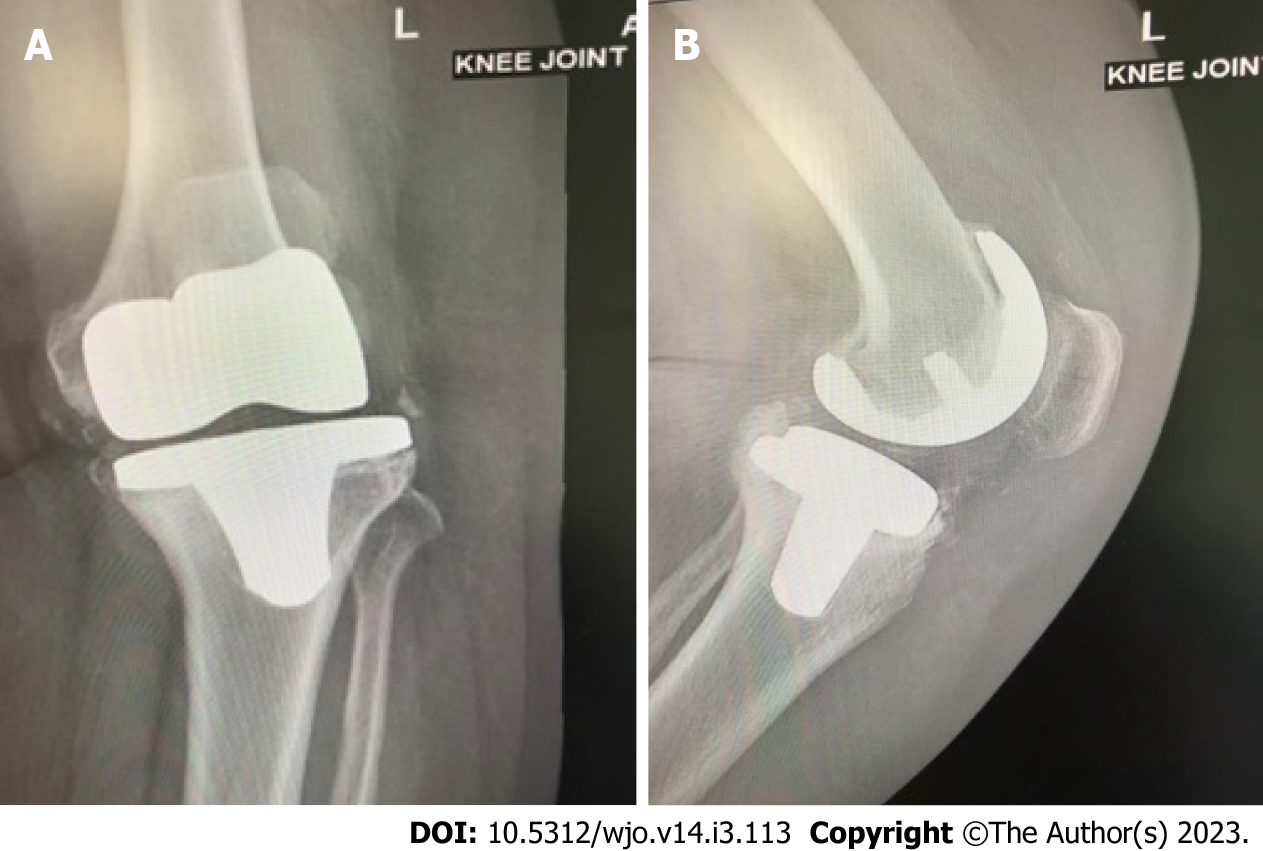
Figure 1 Anteroposterior and lateral plain radiographs showing septic loosening of a left total knee arthroplasty in a 65-year-old female patient who underwent the primary procedure 5 years previously.
A: Anteroposterior view; B: Lateral view. Preoperative aspiration confirmed Staphylococcus aureus infection and serum and synovial fluid inflammatory markers were elevated.
TREATMENT PLAN
The management of PJI remains controversial; therefore treatment plans should be tailored for each patient individually. Eradication of the infection, reduction of pain, and restoration of joint function are the primary goals of treatment[12]. In general, management of PJI consists of antimicrobial therapy alone or antimicrobial therapy combined with single or staged surgeries. The approach depends on several factors, including the timing and microbiology of infection, condition of the joint and implant, and the individual patient circumstance. Surgical options include debridement and retention of the prosthesis, resection arthroplasty with reimplantation in a single or staged procedure, resection arthroplasty alone, or in extreme cases, amputation[36]. Two-stage revision remains the favorite surgical option, with overall higher rates of eradicating PJI in comparison to single-stage revision. For example, Elson et al[37] report 3.5% failure rates with two-stage revision vs 12.4% using a single-stage strategy. Similarly, Garvin et al[38] report a failure rate of 5.6% vs 10.1%, respectively. For the purposes of this review article, we will focus mainly on the two-stage revision method.
TWO-STAGE REVISION
The two-stage revision procedure is considered to be the gold standard for the management of PJI[12]. This method was described in 1983 by Insall et al[39] and in 1995, Garvin et al[40] conducted a literature review that highlighted the success associated with this approach. The first stage of the procedure includes the removal of the in-situ prosthesis, thorough debridement of the infected bone and soft tissue, and the implantation of antibiotic-loaded cement (ALC) spacers for temporary fixation. The interim period between the two stages includes administration of intravenous antibiotics and close monitoring of the patient clinically and serologically for resolution of infection. Once the infection has resolved, the second stage, which comprises the use of antibiotic-loaded cement for reimplantation of the definitive prosthesis[12,41]. The time between stages can range from 6 wk to several months. Both stages necessitate aggressive debridement of all infected and necrotic tissue[41]. The following are indications for using a two-stage rather than a single-stage revision procedure[12]: (1) Systemic infection (sepsis) with signs of infection but an unidentified causative microorganism; (2) Antibiotic-resistant microorganisms identified by preoperative cultures; (3) Presence of a sinus tract; and (4) Insufficient soft tissue coverage to allow a single-stage procedure.
1st stage
The first stage of the procedure entails a thorough and vigorous debridement of the whole effective joint space after the removal of all implanted materials and cement[41] (Figure 2). Whenever possible, the use of antibiotics is postponed until all microbiological samples have been collected. To increase the likelihood of arriving at a conclusive diagnosis, it is recommended to obtain both aerobic and anaerobic cultures; at least three and as many as six intraoperative periprosthetic tissue samples or the explanted prosthesis itself may be sent for testing[42]. The sensitivity, specificity, positive predictive value, and negative predictive value with a minimum of two positive samples have been reported to be 94%, 97%, 77%, and 99.9%, respectively[43]. It is advised to excise the old scar and the sinus tract if present. Sending the prosthetic parts for sonification is an option, but this should be planned prior to surgery as it requires special packaging[41]. It is crucial to remove any cement, even if it is firmly affixed to the underlying bone, in addition to any soft tissue that is grossly involved by the infection[44]. Osteotomes, specialized chisels, drills, and taps, as well as various methods that make use of ultrasound-based extraction instruments, can all be used to remove the cement[45]. During this stage, the surgeon must proceed cautiously, since iatrogenic bone injury is possible[44] (Figure 3A). It is important to perform extensive lavage with a high-pressure pulsatile lavage system using at least 6 L of fluid. Normal saline is usually favored, and lavage provides a significant mechanical action that eliminates sequestra, necrotic tissue, and microorganisms. Several studies have investigated adding antibiotics to the normal saline, but no therapeutic advantage over plain lavage solution has been shown[41]. Following the removal of the implants and thorough debridement, new sterile drapes are applied followed by a spacer with ALC (Figure 3B and C). Spacers are either static or dynamic, prefabricated or handcrafted and hemiarthroplasty spacers can replace both sides of the joint. Preoperative culture and sensitivity testing of the infecting microorganisms helps to decide the best antibiotics to be added preoperatively to the cement used for construction of the spacer. A discussion with a microbiologist is also necessary to agree on the best choice of antibiotics[41].
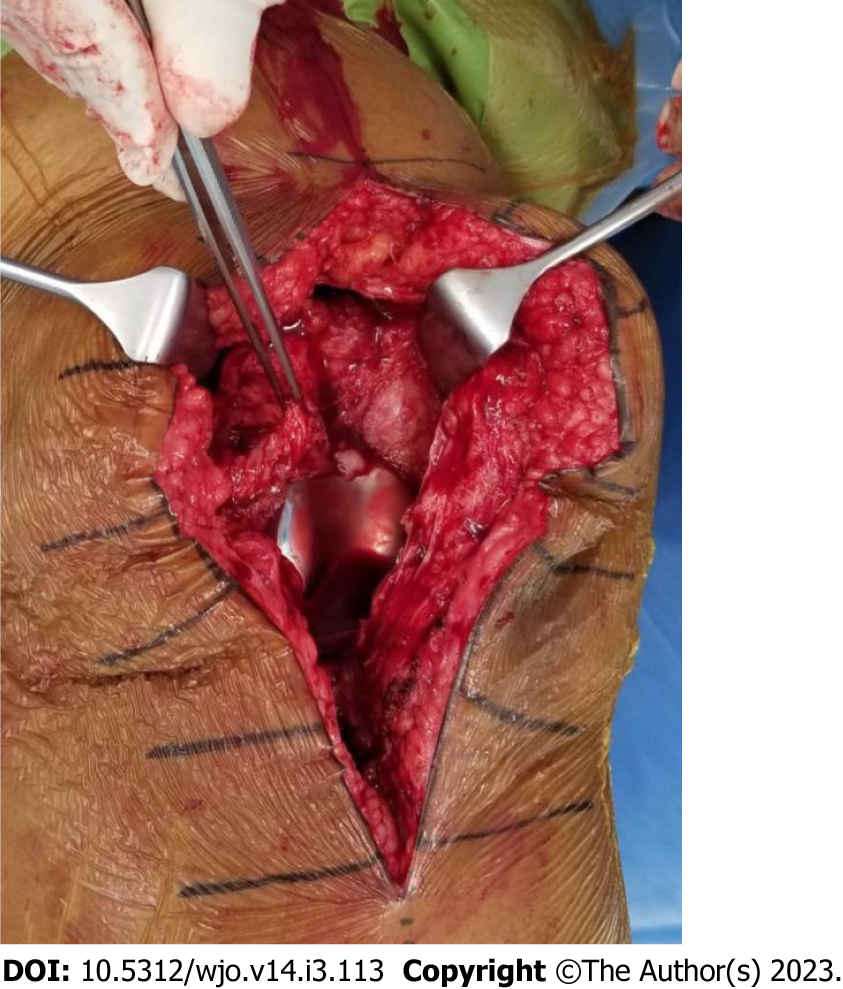
Figure 2 First stage revision of the case shown in Figure 1.
Synovial tissue surrounds the prosthesis. Total synovectomy was performed and samples sent for culture confirmed the growth of Staphylococcus aureus.
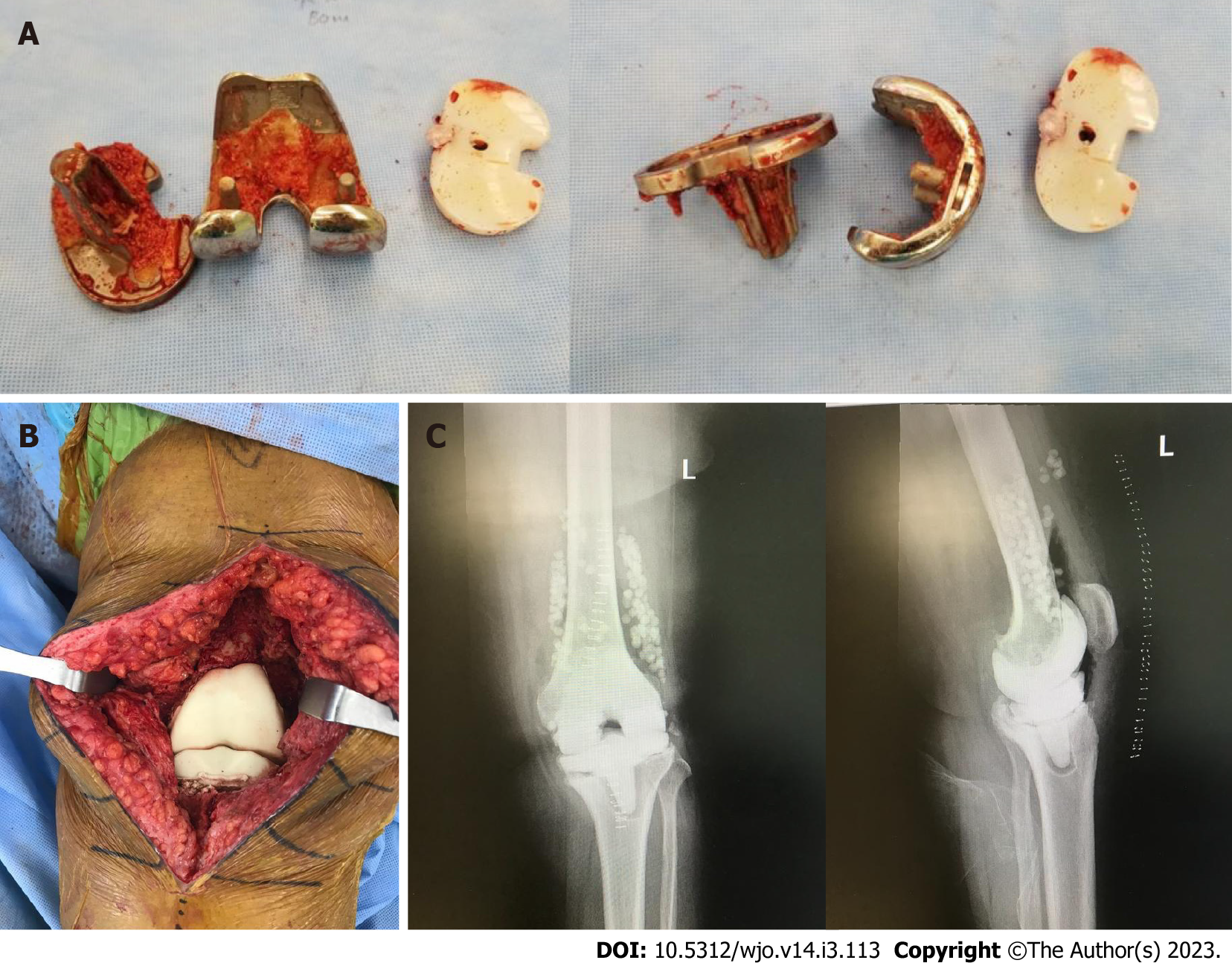
Figure 3 First stage revision.
A: Explanted prosthesis with minimal bone loss; B: Articulating spacer implantation at the end of the procedure containing vancomycin and tobramycin; C: Anteroposterior and lateral plain radiographs of the left knee after the first stage showing the spacer in situ with antibiotic beads.
Interim period
At this point, antibiotic therapy is the cornerstone and should be tailored depending on the microorganism's antimicrobial sensitivity. With the help of a microbiologist, empiric therapy should be started if the organism or sensitivities are unknown. To identify an organism, all reasonable efforts should be made[41]. The most popular regimen is intravenous antibiotics for 4-6 wk followed by discontinuation of the antibiotics for a period of 2-8 wk prior to the second stage, as this results in a high rate of infection control[46,47]. The best results are usually obtained when the infecting microorganism is sensitive and systemic antibiotics are used in the interim period[48,49]. Prolonging the interim period has been linked with suboptimal infection control rates and poor function restoration[12]. However, a single study concluded that there were no differences in functional outcome in patients who had undergone a two-stage revision with an interim period of less than 6 mo vs those with more than 6 mo between resection and reimplantation[12,19]. Deciding to move forward with prosthesis reimplantation depends on clinical, serological testing, and joint aspirate assessment. Residual infection requires further debridement and a new spacer insertion[41]. Normalization of the CRP and erythrocyte sedimentation rate (ESR) alone does not guarantee eradication of the infection, especially in coagulase-negative staphylococcal infections, as these may not trigger a significant inflammatory response[12]. Kusuma et al[50] reported that synovial white blood cell (WBC) count is the most reliable predictor of infection control, and a decision to proceed to the second stage depends on attaining a WBC count of less than 3000/µL with less than 80% polymorphonuclear cells from the joint aspirate. Negative intraoperative frozen sections and tissues grossly appearing noninfected are other criteria which are utilized in the second stage to support the decision of proceeding with reimplantation, as there is a high risk of false positive or false negative preoperative cultures. The joint aspirate is mainly used for cell count assessment[50].
Spacer
Spacers are categorized as articulating (dynamic) or non-articulating (static). Between staged procedures, dynamic spacers maintain ambulation and joint range of motion, which protect against muscle wasting; evidence has shown them to be as or more effective at eliminating infection as static spacers[51]. Being able to maintain a range of motion also prevents against the formation of soft tissue and muscle contractures, which facilitates the reimplantation procedure[12,52,53]. Brunnekreef et al[54] found a better and quicker recovery of knee function with dynamic spacers, resulting in shorter operation times. Furthermore, compared to static spacers, the use of a dynamic spacer appears to increase the rate of infection eradication (91.2% vs 87%)[55]. Moreover, using a static spacer may result in bone loss due to migration of the spacer[56,57]. Despite the above, static spacers may be preferrable in certain circumstances, such as massive bony and soft tissues loss, ligament laxity in the knee, and deficiency of the abductor muscles of the hips[41,44]. Prosthesis with Antibiotic-Loaded Acrylic Cement (PROSTALAC) is an example of an articulating spacer that delivers high concentration of broad-spectrum antibiotics locally. A common regimen used with PROSTALACs is the inclusion of 3 g of vancomycin and 2 g of gentamicin in each sachet of Palacos R cement (Schering Plough Ltd, Labo nv, Belgium). However, antibiotics in spacers may also be prepared according to the sensitivities of the infecting micro-organisms if detected preoperatively[12]. Spacers are usually augmented with a post-operative course of intravenous antibiotics until the definitive antibiotic sensitivities of the infective micro-organisms are detected from the intraoperative cultures taken at the first stage procedure[12]. However, spacers are not complication-free. Faschingbauer et al[58] reported that out of 138 patients, 27 (19.6%) developed complications, including spacer fractures in 12 cases (8.7%), dislocation in 12 cases (8.7%), 1 case of a periprosthetic femoral fracture with a spacer in situ, 1 case of a dislocation with simultaneous spacer fracture, and 1 case of protrusion into the pelvis.
2nd stage
The second stage consists of removal of the spacer, further debridement, and collection of tissue samples followed by definitive reimplantation of a new prosthesis (Figure 4). The decision to proceed with the definitive reimplantation must be made after the resolution of all infection-related symptoms and signs and improvement of laboratory results (a declining trend of CRP and ESR may be accepted as opposed to complete normalization of the values as stated earlier)[41,44]. During the second stage, the existing scar is usually utilized to approach the joint[59]. Once the joint is appropriately exposed, further samples are obtained for cultures. It is crucial to remove the cement spacer with the pseudo-synovial cavity that develops around the spacer without compromising the surrounding bone. Necrotic tissue is removed, and pulse lavage is used for extensive irrigation of the joint. This ensures the removal of any residual cement debris, which potentially can cause third body wear if left at the spacer site. If necessary, bone allografts may be utilized at this point to reconstruct any bony deficiencies followed by reimplantation of the definitive prosthesis in accordance with the preoperative plan. The use of bone allografts in revision surgery after PJI has drawn some controversy in the past[60]. Latest evidence, however, has not been able to demonstrate a substantial difference in the rate of reinfection following the use of allografts in this context. Therefore, when there is considerable bone loss, bone grafts may still be used safely[61]. Both cemented and uncemented prostheses may be utilized for the definitive implant. Modern antibiotic delivery methods like defensive antibacterial coating may also be utilized at this stage[62]. Similar reinfection rates and aseptic loosening have been reported when using cemented or uncemented prostheses in TKA revisions for infection[63]. Following surgery, antibiotics may be administered until the bacteriology results are revealed[12]. If any suspicion remains regarding infection during the second stage, a synovial leukocyte esterase strip test, synovial alpha-defensin test, or a frozen section intraoperative tissue analysis may be used to confirm. If the tests are suggestive of residual infection, aggressive debridement followed by a cemented spacer reimplantation (a repetition of the first stage) is necessary[41,44] (Figure 5).
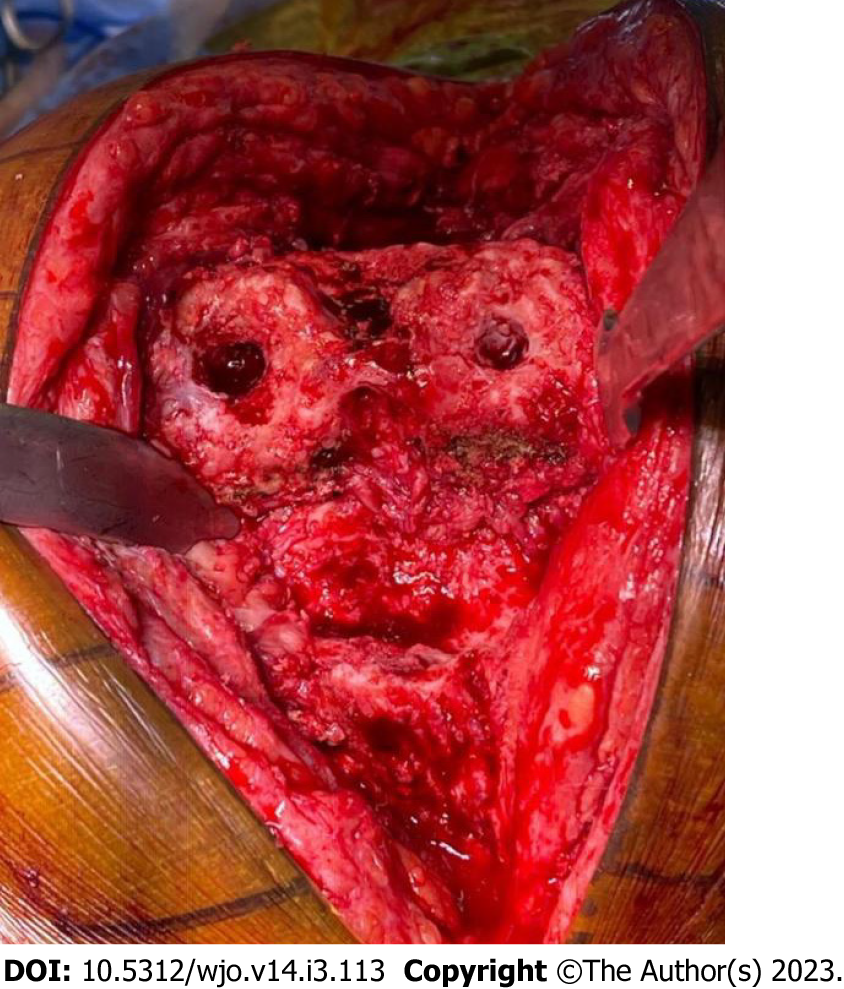
Figure 4 Second stage revision.
After the removal of the spacer, the joint is prepared for reimplantation of the prosthesis. This joint shows minimal bone loss.
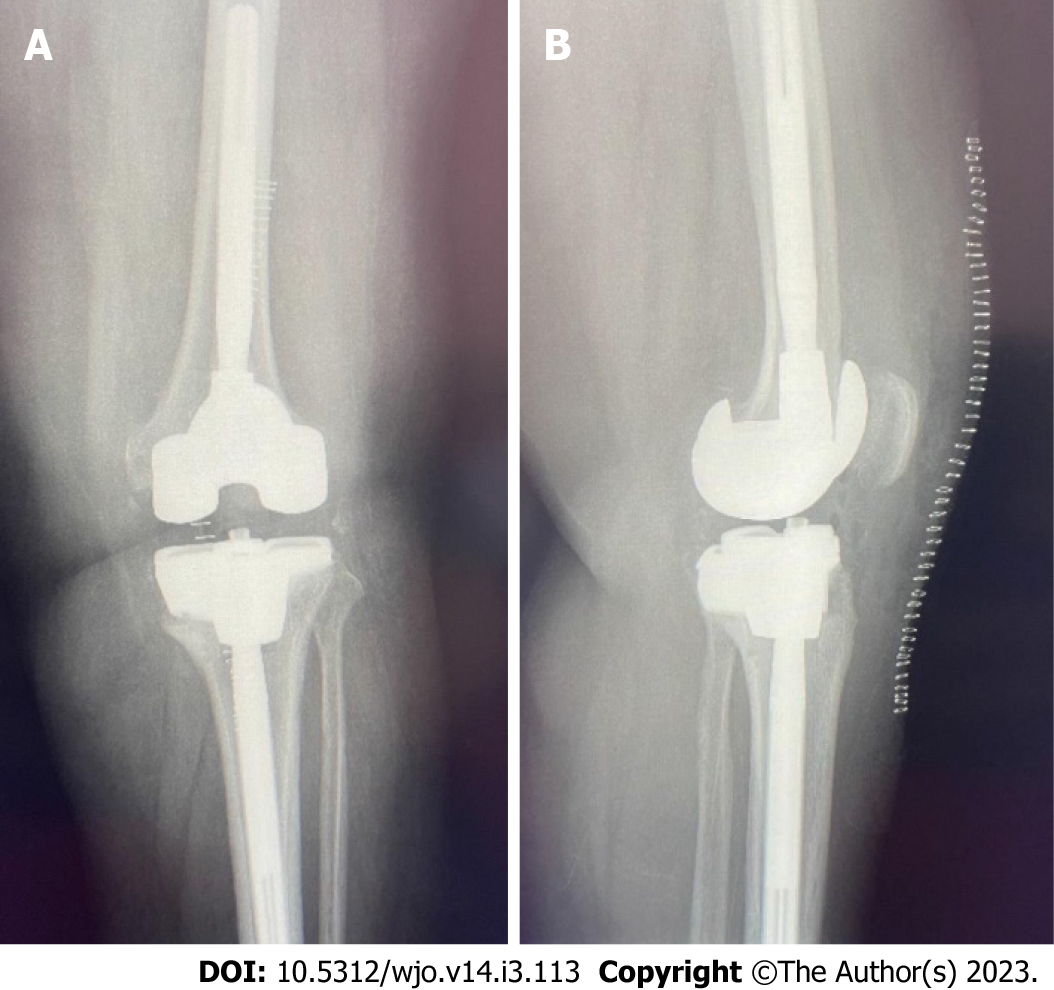
Figure 5 Postoperative anteroposterior and lateral plain radiographs.
A: Postoperative anteroposterior view of the left knee after completion of the second stage revision; B: Lateral view of the left knee after completion of the second stage revision.
CONCLUSION
PJI is challenging to manage, but recent advancements in laboratory testing have helped to facilitate early diagnosis when used collectively under the internationally agreed upon definition of this surgical complication. A multidisciplinary team approach is crucial when dealing with such cases. Efforts should be made to diagnose the causative microorganism as early as possible in order to start appropriate antimicrobial therapy and plan surgical intervention accordingly. In terms of surgical options, the two-stage revision procedure remains the gold standard approach in chronic cases and yields the highest PJI eradication rates.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Donadono C, Italy; Torres RM, Portugal S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH