Published online Jan 18, 2023. doi: 10.5312/wjo.v14.i1.23
Peer-review started: September 14, 2022
First decision: October 17, 2022
Revised: October 20, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: January 18, 2023
Processing time: 121 Days and 2.4 Hours
Osteoarthritis (OA) is the most common joint disorder, is associated with an increasing socioeconomic impact owing to the ageing population.
To analyze and compare the efficacy and safety of bone-marrow-derived mesenchymal stromal cells (BM-MSCs) and adipose tissue-derived MSCs (AD-MSCs) in knee OA management from published randomized controlled trials (RCTs).
Independent and duplicate electronic database searches were performed, including PubMed, EMBASE, Web of Science, and Cochrane Library, until August 2021 for RCTs that analyzed the efficacy and safety of AD-MSCs and BM-MSCs in the management of knee OA. The visual analog scale (VAS) score for pain, Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), Lysholm score, Tegner score, magnetic resonance observation of cartilage repair tissue score, knee osteoarthritis outcome score (KOOS), and adverse events were analyzed. Analysis was performed on the R-platform using OpenMeta (Analyst) software. Twenty-one studies, involving 936 patients, were included. Only one study compared the two MSC sources without patient randomization; hence, the results of all included studies from both sources were pooled, and a comparative critical analysis was performed.
At six months, both AD-MSCs and BM-MSCs showed significant VAS improvement (P = 0.015, P = 0.012); this was inconsistent at 1 year for BM-MSCs (P < 0.001, P = 0.539), and AD-MSCs outperformed BM-MSCs compared to controls in measures such as WOMAC (P < 0.001, P = 0.541), Lysholm scores (P = 0.006; P = 0.933), and KOOS (P = 0.002; P = 0.012). BM-MSC-related procedures caused significant adverse events (P = 0.003) compared to AD-MSCs (P = 0.673).
Adipose tissue is superior to bone marrow because of its safety and consistent efficacy in improving pain and functional outcomes. Future trials are urgently warranted to validate our findings and reach a consensus on the ideal source of MSCs for managing knee OA.
Core Tip: With the ongoing rise in the exploration of the clinical efficacy of mesenchymal stromal cells (MSCs) in the management of osteoarthritis (OA), there is an imminent need to identify the ideal source of MSCs to be utilized. Our meta-analysis has brought out the lacunae in the literature for studies to evaluate the impact of the source of MSCs in the management of OA. From a single-arm meta-analysis of available studies on the two commonly used sources such as bone marrow (BM) and adipose tissue, we found the adipose tissue to be superior to BM concerning the safety and consistent efficacy in improving pain and functional outcomes. However, considering the paucity of evidence, we recommend future trials to validate our findings and reach a consensus on the ideal source of MSCs for managing knee OA.
- Citation: Muthu S, Patil SC, Jeyaraman N, Jeyaraman M, Gangadaran P, Rajendran RL, Oh EJ, Khanna M, Chung HY, Ahn BC. Comparative effectiveness of adipose-derived mesenchymal stromal cells in the management of knee osteoarthritis: A meta-analysis. World J Orthop 2023; 14(1): 23-41
- URL: https://www.wjgnet.com/2218-5836/full/v14/i1/23.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i1.23
Osteoarthritis (OA) of the knee is the world’s leading cause of degenerative joint disease leading to articular cartilage damage resulting in pain, stiffness, and loss of joint mobility[1]. Owing to the hypovascular and aneural nature, the articular cartilage has a decreased integrity for intrinsic repair mechanisms[2]. The management of OA knee aims to provide painless functional joint with a full range of motion. To minimize the morbidity in the surgical management of OA knee, regenerative and translational medicine has paved a way to manage the articular cartilage defects with orthobiological products due to the limited potential for redifferentiation of chondrocytes[3,4].
Cell-based therapy has revolutionized its usage in the area where disease-modifying pharmacological agents or biological therapies are unavailable to treat the disorders. Mesenchymal stromal cells (MSCs) have proven the benefits in the formation of articular cartilage in the OA knee[5,6]. There are various sources of MSCs available namely bone marrow (BM), adipose tissue, synovium, peripheral blood, placenta, menstrual fluid, and amniotic fluid where the regenerative potential of all these sources of MSCs varies[7]. Out of all these sources of MSCs, the most commonly used sources are BM and adipose tissue for cartilage regeneration.
Adipose tissue possesses higher stem cell yield than BM[8]. One gram of adipose tissue yields approximately 0.35-1 million MSCs whereas one gram of BM yields 500-50000 MSCs[9]. BM-derived MSCs (BM-MSCs) show early senescence during expansion than adipose-derived MSCs (AD-MSCs)[10]. Mohamed-Ahmed et al[11] have demonstrated that AD-MSCs continued to proliferate up to 21 d than BM-MSCs and AD-MSCs showed considerable chondrogenic capacity, but less than BM-MSCs. Im et al[12] stated that osteogenic and chondrogenic potentials of BM-MSCs and AD-MSCs differ. The difference in potentiality exponentiated when an equal amount of bioactive factors are seeded and AD-MSCs demonstrated inferior regenerative potential to differentiate into bone and cartilage when compared with BM-MSCs[12]. However, Jeyaraman et al[13] demonstrated the efficacy, safety, and superiority of AD-MSCs transplantation when compared to BM-MSCs in OA knee management. With the conflicting evidence in literature[14-16], we aim to critically analyze the clinical efficacy and patient safety in the use of BM-MSCs and AD-MSCs in the management of OA of the knee.
We conducted this meta-analysis in accordance with the guidelines from the Back Review Group of Cochrane Collaboration[17] and we followed the reporting guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[18].
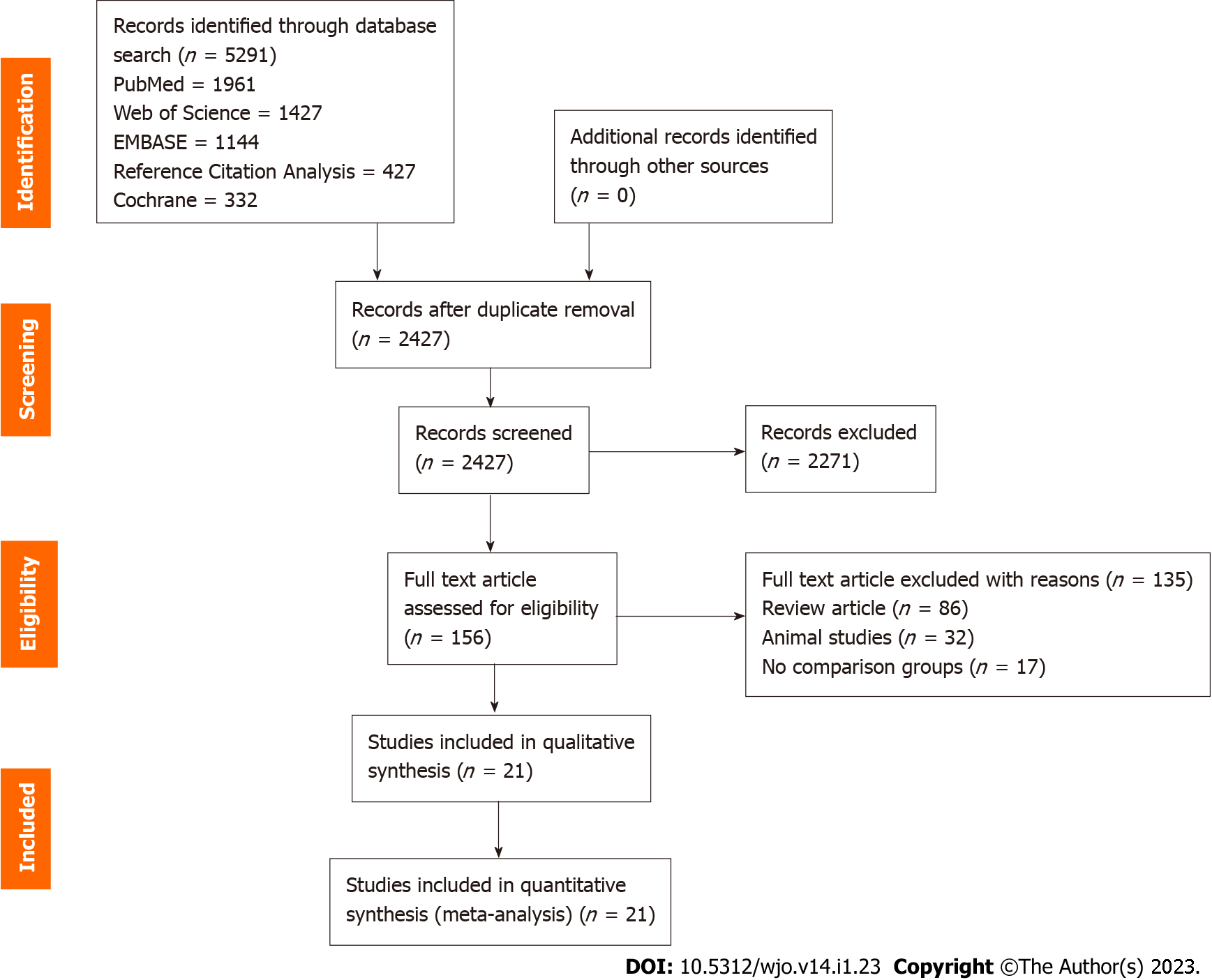
We conducted an independent and duplicate electronic literature search for studies evaluating the ideal source of MSC therapy for knee OA. The literature databased searched the relevant studies include: PubMed, EMBASE, Web of Science, Reference Citation Analysis, and the Cochrane Library up to August 2021. We did not apply any language or date restrictions to the search query. We used the following keywords in the search strategy “Knee Osteoarthritis”, “Knee Degeneration”, “Stem Cell Therapy” and “Mesenchymal Stromal Cells”, “Bone marrow”, “Adipose”. We have presented a sample search strategy utilized for retrieving the relevant studies from one of the included databases in Supplementary Table 1. Apart from the above databases, we also searched to identify studies not identified in the primary search from the reference list of potential articles shortlisted. Based on the criteria identified as a priori for inclusion and exclusion of studies, eligible studies were identified and included for meta-analysis. In case of discrepancy among the reviewers in study selection, discussion was made until a consensus was obtained. PRISMA flow diagram of the selection of the studies included in the analysis is given in Figure 1.
Studies were included for quantitative review if they met the following PICOS criteria: Population: Patients with OA of knee. Intervention: AD-MSC therapy. Comparator: BM-MSC therapy. Outcomes: Visual analog score (VAS) for Pain, Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), Lysholm Knee Scale (Lysholm), Magnetic resonance observation of cartilage repair tissue (MOCART) Score, knee osteoarthritis outcome score (KOOS), Tegner Activity Score (TAS) and reported adverse events. Study design: Randomized controlled trials.
We excluded studies from analysis if they were of the following characteristics: (1) In-vitro studies involving stem cell therapy; (2) Studies of observational nature and interventional studies without appropriate comparison group; (3) Studies conduction animal models of knee OA investigating stem cell therapy; and (4) Review articles and in-vitro studies involving stem cell therapy.
We made an independent and duplication extraction of the following data from the included studies: (1) Study characteristics: Name of the author, publication year, country, total number of patients enrolled in the study and level of evidence of the study; (2) Baseline characteristics: Age (mean with standard deviations), gender proportions of the individual groups, Kellgren Lawrence grades of OA, type of MSC source used in them, protocol of intervention utilised for both the groups, mean duration of follow-up of the study population and parameters used for assessment of clinical measures. We grouped studies utilizing BM based therapies involving BM concentrates and isolated expanded BM-MSCs into one group and another group involving studies using stromal vascular fraction (SVF) and isolated expanded AD-MSCs; (3) Efficacy outcomes: Pain outcomes using VAS, functional outcomes using WOMAC score, Lysholm score, KOOS, TAS, and radiological outcomes like MOCART score; (4) Safety outcomes: Reported adverse events; and (5) In case of any disagreement in data collection, discussion was made until a consensus was attained.
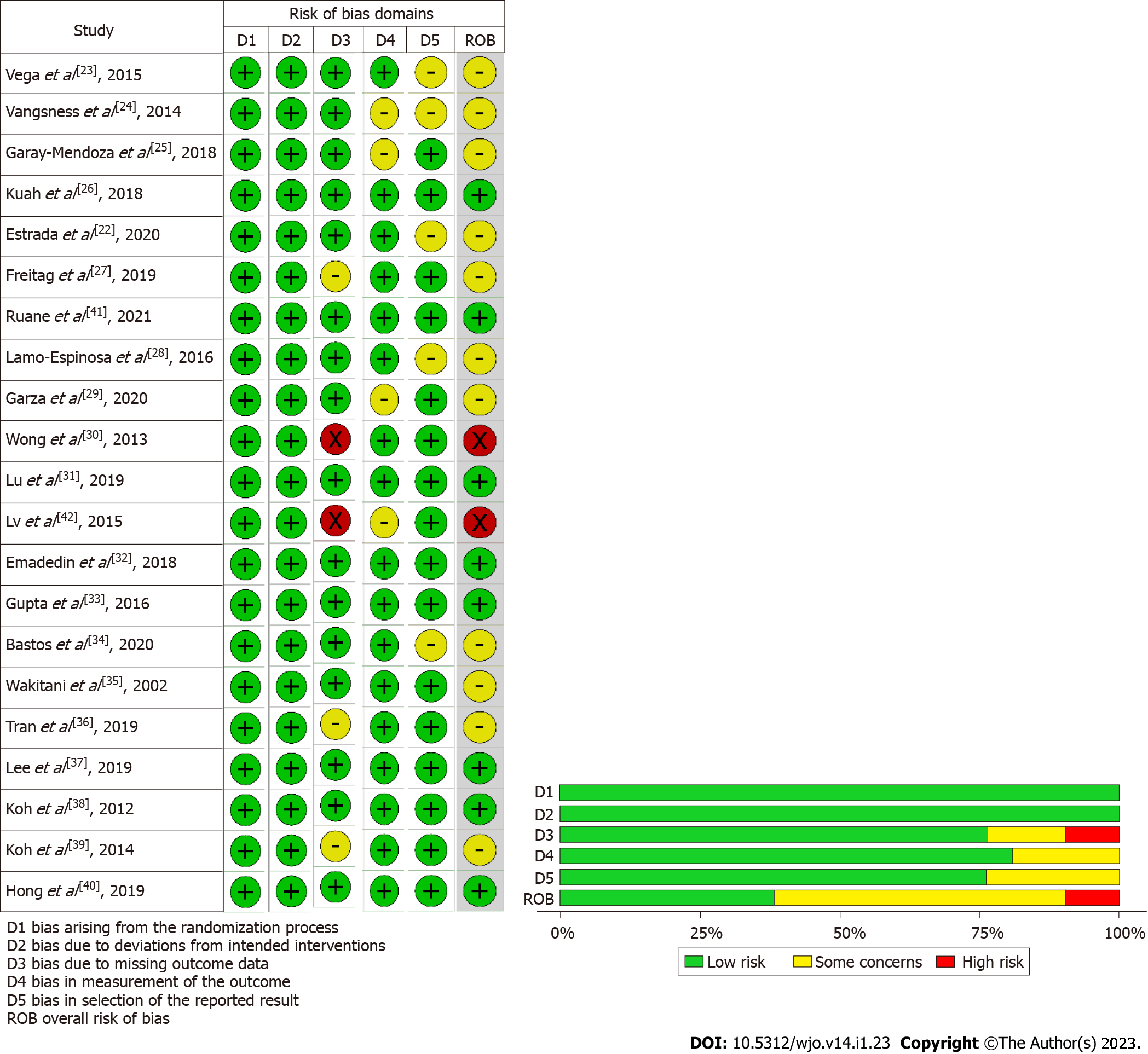
We performed an independent and duplicate analysis of the methodological quality of the included studies by two reviewers based on the ROB2 tool of Cochrane Collaboration for randomized studies. The tool has five domains of bias assessment including randomization process followed in the studies, bias in application of the intended intervention, bias in the presentation of the study outcome data, bias in the measurement of measured outcome, and bias in reporting of results of the study[19].
We performed the analysis in the R platform using OpenMeta(Analyst) software[20]. We used risk ratio (RR) with 95% confidence interval (CI) for analysing dichotomous variable outcomes and weighted mean difference (WMD) with 95%CI for continuous variable outcomes. We analysed the heterogeneity observed in the results analysed using the I2 test [21]. We used fixed-effects model to evaluate the outcomes if the value of I2 < 50% and P > 0.1. We used random-effects model if the value of I2 > 50% and P < 0.1. We considered a P-value < 0.05 to be significant. We performed sensitivity analyses in case of heterogeneity among the reported results from the studies included for analysis. We used Funnel plot, Egger regression test, and normal quantile plot to analyse the publication bias for the outcomes in the included studies.
Our initial electronic database screening yielded 4864 articles, which upon removal of the duplicate articles resulted in 2427 articles. We then performed title and abstract screening and shortlisted 156 eligible articles and excluded 2271 articles. We made a full-text review of the 156 articles qualified articles and excluded 135 of them for the reasons listed in the PRISMA flow diagram for study selection (Figure 1). Among the included studies, we found only one study by Estrada et al[22] to make a direct comparison of the adipose tissue and BM as a source of MSC and found no significant difference among the groups compared despite observing a significant improvement from the baseline. The study had a selective allocation of the subjects based on the stage of the disease and utilized adipose tissue-based cellular therapy for high-grade disease and BM-based therapy for intermediate grade disease and platelet-based therapy for early disease. To objectively evaluate the results of the study across all the grades of disease, we pooled the results of all the included studies of both sources and made a combined comparative quantitative analysis of all 21 included studies[22-42] with 936 patients. 9/21 studies[22,26,27,29,31,36-40] utilized MSC of adipogenic origin, of which 1 study utilized AD-MSC of allogenic source while rest 8 studies utilized AD-MSCs of autogenous source. 12/21 studies[22-25,28,30,32-35,41,42] utilized MSC of BM origin, of which 2 studies utilized BM-MSCs of allogenic sources, and the rest 10 studies utilized autogenous sources of BM-MSC. We did not note a standardised utilization of the dose of the MSCs transplanted in the included studies. We did not note uniformity among the included studies for the measures of outcomes assessment employed. We presented the general characteristics of the included studies in Table 1. The protocol of intervention used in the case and control groups along with the measures of outcome assessment were given in Table 2.
| Sl. No | Ref. | Country | Nature of study | Kellgren Lawrence Grade | Sample size | Treatment/ | Mean age (SD) | Male/female | MSC type | MSC source | Follow-up (mo) | ||
| Treatment group | Control group | Treatment group | Control group | ||||||||||
| 1 | Vega et al[23], 2015 | Spain | RCT | II, III, IV | 30 | 15/15 | 56.6 ± 9.24 | 57.3 ± 9.09 | 06/09 | 05/10 | BM | Allo | 12 |
| 2 | Vangsness et al[24], 2014 | United States | RCT | NR | 55 | 36/19 | 44.6 ± 9.82 | 47.8 ± 8 | 25/11 | 13/06 | BM | Allo | 24 |
| 3 | Garay-Mendoza et al[25], 2018 | Mexico | RCT | NR | 61 | 30/31 | 55.57 ± 12.02 | 59.32 ± 10.85 | 07/23 | 09/22 | BM | Auto | 6 |
| 4 | Kuah et al[26], 2018 | Australia | RCT | I, II, III | 20 | 16/4 | 50.8 ± 7.29 | 55.0 ± 10.42 | 11/05 | 01/03 | AD | Allo | 12 |
| 5 | Estrada et al[22], 2020 | Argentina | RCT | I, II, III | 89 | 60/29 | 61 ± 12 | 61 ± 12 | NR | NR | BM / AD | Auto | 12 |
| 6 | Freitag et al[27], 2019 | Australia | RCT | II, III | 30 | 20/10 | 54.6 ± 6.3 | 51.5 ± 6.1 | 11/09 | 01/09 | AD | Auto | 12 |
| 7 | Ruane et al[41], 2021 | United States | RCT | I, II, III | 32 | 17/15 | 58.06 ± 9.14 | 58.6 ± 8.05 | 09/08 | 10/05 | BM | Auto | 12 |
| 8 | Lamo-Espinosa et al[28], 2016 | Spain | RCT | II, III, IV | 30 | 20/10 | 65.9 | 60.3 | 12/08 | 07/03 | BM | Auto | 12 |
| 9 | Garza et al[29], 2020 | United States | RCT | II, III | 39 | 26/13 | 60.5 ± 7.9 | 57.1 ± 9.1 | 15/11 | 7/6 | AD | Auto | 12 |
| 10 | Wong et al[30], 2013 | Singapore | RCT | NR | 56 | 28/28 | 53 | 49 | 15/13 | 14/14 | BM | Auto | 24 |
| 11 | Lu et al[31], 2019 | China | RCT | I, II, III | 53 | 27/26 | 55.03 ± 9.19 | 59.64 ± 5.97 | 03/24 | 03/23 | AD | Auto | 12 |
| 12 | Lv et al[42], 2015 | Huang | RCT | I, II | 80 | 40/40 | 55.9 ± 8.1 | 55.1 ± 6.8 | 14/26 | 13/27 | BM | Auto | 12 |
| 13 | Emadedin et al[32], 2018 | Iran | RCT | II, III, IV | 43 | 19/24 | 51.7 ± 9.2 | 54.7 ± 5.3 | 12/07 | 15/09 | BM | Auto | 6 |
| 14 | Gupta et al[33], 2016 | India | RCT | II, III | 60 | 40/20 | 58.10 ± 8.23 | 54.90 ± 8.27 | 12/28 | 4/16 | BM | Allo | 12 |
| 15 | Bastos et al[34], 2020 | Brazil | RCT | I, II, III, IV | 47 | 30/17 | 55.7 ± 7.8 | 55.9 ± 13.4 | 15/15 | 09/08 | BM | Auto | 12 |
| 16 | Wakitani et al[35], 2002 | Japan | I, II | 24 | 12/12 | NR | NR | NR | NR | BM | Auto | 16 | |
| 17 | Tran et al[36], 2019 | Taiwan | RCT | II, III | 33 | 15/18 | 58.2 ± 5.70 | 59.0 ± 6.04 | 03/12 | 05/13 | AD | Auto | 24 |
| 18 | Lee et al[37], 2019 | South Korea | RCT | II, III, IV | 24 | 12/12 | 62.2 ± 6.5 | 63.2 ± 4.2 | 03/09 | 03/09 | AD | Auto | 6 |
| 19 | Koh et al[38], 2012 | South Korea | RCT | IV | 50 | 25/25 | 54.2 ± 9.3 | 54.4 ± 11.3 | 08/17 | 08/17 | AD | Auto | 16 |
| 20 | Koh et al[39], 2014 | South Korea | RCT | I, II, III | 44 | 23/21 | 52.3 ± 4.9 | 54.2 ± 2.9 | 06/17 | 05/16 | AD | Auto | 24 |
| 21 | Hong et al[40], 2019 | China | RCT | II, III | 32 | 16/16 | 51 ± 5.95 | 53 ± 10.97 | 03/13 | 03/13 | AD | Auto | 12 |
| Ref. | MSC type | MSC source | MSC preparation | MSC count (107 cells) | Treatment group intervention | Control group intervention | Outcome measures |
| Vega et al[23], 2015 | BM | Allo | CE-BMMSC | 4 | sIA injection of MSC | sIA Injection of 60 mg HA | VAS, WOMAC |
| Vangsness et al[24], 2014 | BM | Allo | CE-BMMSC | 5/15 | sIA injection of MSC + 20 mg HA | sIA Injection of 20 mg HA | VAS, Lysholm Score |
| Garay-Mendoza et al[25], 2018 | BM | Auto | BMC | NA | 600 μg/d G-CSF for 3 consecutive days before the procedure + sIA injection of MSC | Oral acetaminophen, 500 mg every 8 h for 6 mo | VAS, WOMAC |
| Kuah et al[26], 2018 | AD | Allo | CE-ADMSC | 0.39-0.67 | sIA injection of MSC | Placebo sIA injection of cell culture media and cryopreservative | VAS, WOMAC, MRI assessment |
| Estrada et al[22], 2020 | AD | Auto | BMC | NA | sIA injection of BM concentrate | sIA injection of PRP | IKDC, Lysholm Score, KOOS |
| Estrada et al[22], 2020 | BM | Auto | SVF | NA | sIA injection of lipoaspirate | sIA injection of PRP | |
| Freitag et al[27], 2019 | AD | Auto | CE-ADMSC | 10 | sIA injection of MSC ± 2nd injection at 6 mo | Conservative management | VAS, WOMAC, KOOS, MRI assessment |
| Ruane et al[41], 2021 | BM | Auto | BMC | NA | sIA injection of BM concentrate + PRP | Gel-One® Cross-Linked hyaluronate injection | VAS, KOOS |
| Lamo-Espinosa et al[28], 2016 | BM | Auto | CE-BMMSC | 1 | sIA injection of MSC + 60 mg HA | sIA injection of 60 mg HA | VAS, WOMAC, MRI assessment |
| Garza et al[29], 2020 | AD | Auto | SVF | NA | sIA injection of MSC | Placebo injection without cells | WOMAC, MRI assessment |
| Wong et al[30], 2013 | BM | Auto | CE-BMMSC | 1.46 | HTO + microfracture + sIA injection of MSC + 20 mg HA | HTO + microfracture + sIA injection of 20 mg HA | Tegner Score, Lysholm Score |
| Lu et al[31], 2019 | AD | Auto | CE-ADMSC | 5 | 2 IA injection of MSC at 0, 3 wk and sham injection at 1, 2 wk | 4 IA injection of 25 mg HA at 0, 1, 2, 3 wk | VAS, WOMAC |
| Lv et al[42], 2015 | BM | Auto | CE-BMMSC | 3.82 | 3 × monthly IA injection of MSC + 20 mg HA | sIA injection of 20 mg HA | Tegner Score, Lysholm Score |
| Emadedin et al[32], 2018 | BM | Auto | CE-BMMSC | 4 | sIA injection of MSC | Placebo sIA injection of normal saline | VAS, WOMAC |
| Gupta et al[33], 2016 | BM | Allo | CE-BMMSC | 2.5-15 | sIA injection of MSC + 20 mg HA | Placebo sIA injection of 20 mg HA | VAS, WOMAC, MRI assessment |
| Bastos et al[34], 2020 | BM | Auto | CE-BMMSC | 4 | sIA injection of MSC in 10 mL of PRP | sIA injection of 4 mg dexamethasone | KOOS, MRI assessment |
| Wakitani et al[35], 2002 | BM | Auto | CE-BMMSC | 1 | HTO + microfracture + sIA injection of MSC | HTO + microfracture + placebo injection | MRI assessment, HSS knee rating scale |
| Tran et al[36], 2019 | AD | Auto | SVF | NA | Arthroscopic micro fracture + sIA injection of MSC | Arthroscopic micro fracture | WOMAC, MRI assessment |
| Lee et al[37], 2019 | AD | Auto | CE-ADMSC | 10 | sIA injection of MSC | Placebo injection with normal saline | WOMAC, MRI assessment |
| Koh et al[38], 2012 | AD | Auto | SVF | 0.189 | Arthroscopic debridement + sIA injection of MSC + PRP | Arthroscopic debridement + PRP | VAS, Tegner Score, Lysholm Score |
| Koh et al[39], 2014 | AD | Auto | CE-ADMSC | 0.411 | HTO + sIA injection of MSC + PRP | HTO + PRP | VAS, Lysholm Score |
| Hong et al[40], 2019 | AD | Auto | SVF | 0.745 | sIA injection of MSC | sIA injection of 40 mg HA | VAS, WOMAC, MRI assessment |
We utilised RoB2 tool for the evaluation of the methodological quality of the included studies and presented in Figure 2. We did not note the included studies to have high risk of bias to warrant exclusion from the analysis.
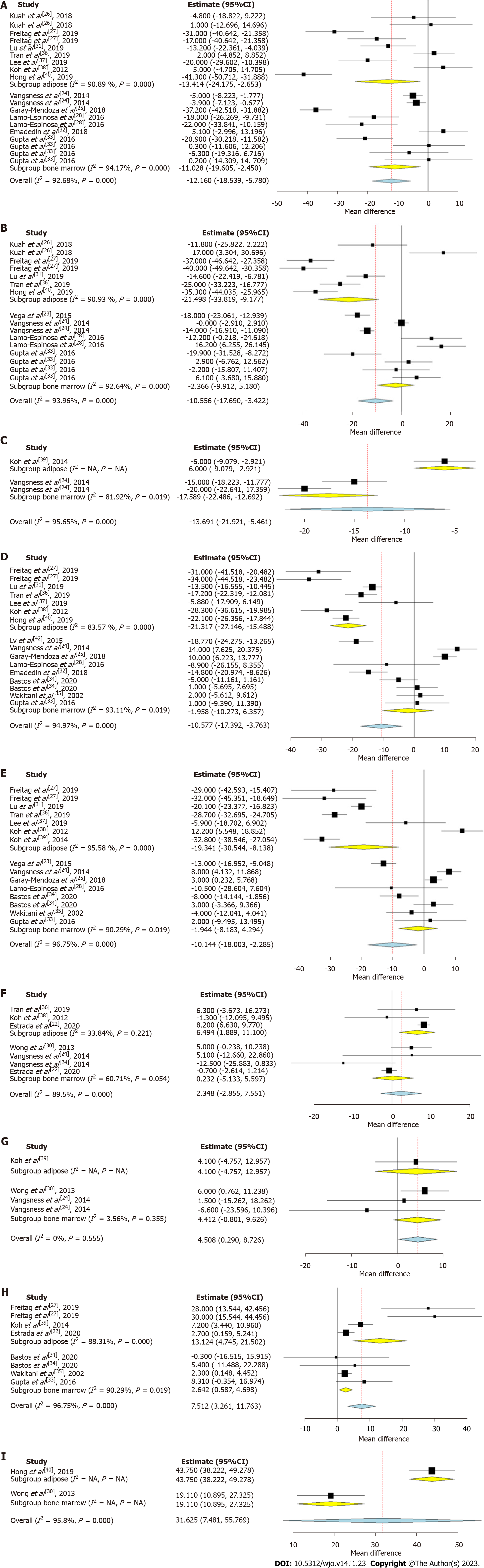
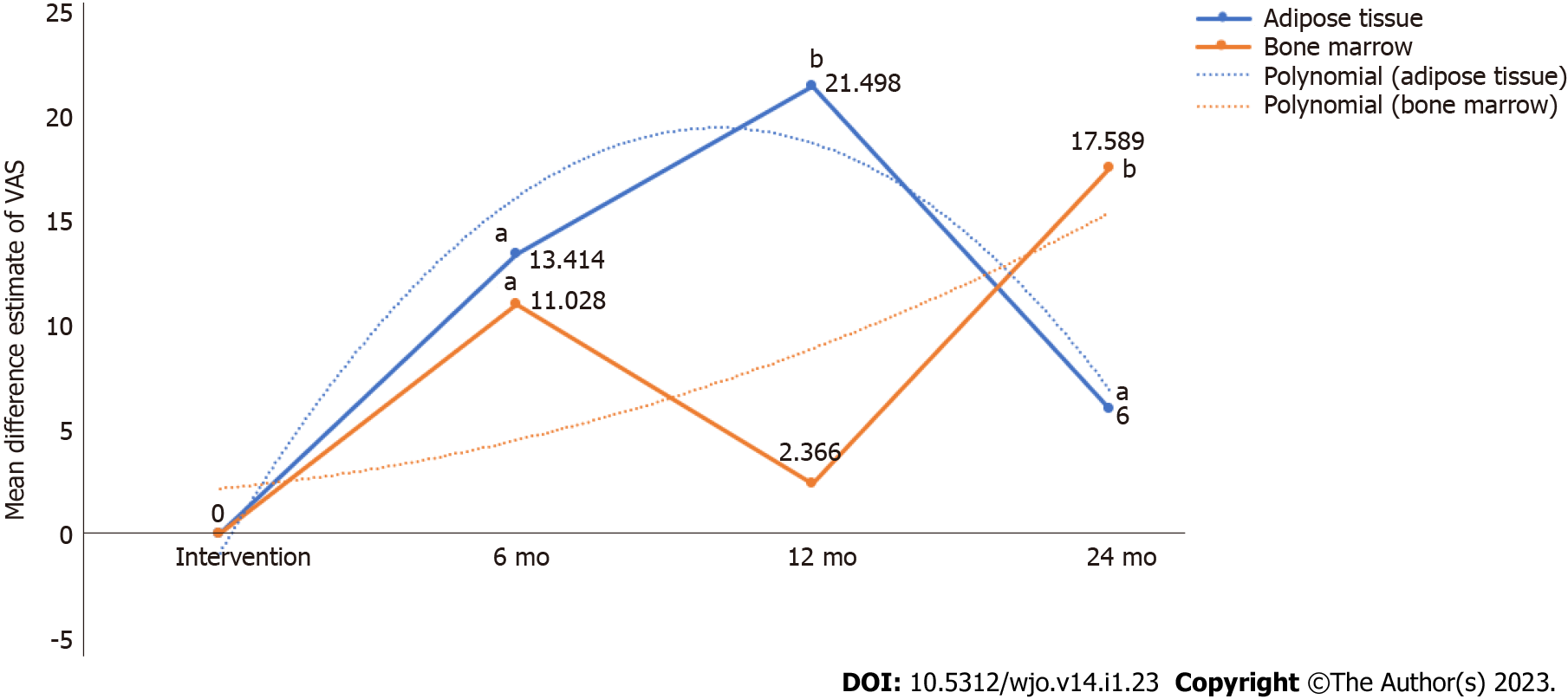
Visual analog scale for pain: We analysed 7 studies[16,17,21,26-28,30], 5 studies[26,27,31,36,40], and 1 study[39] reporting the VAS outcome at 6, 12, and 24 mo respectively using adipose tissue as the source of MSCs. There was a significant heterogeneity observed between the included studies. (I2 > 80%, P < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, significant reduction in VAS score was noted compared to their controls at 6 mo [WMD = -13.414, 95%CI: (-24.175)-(-2.653), P < 0.015; Figure 3A], 12 mo [WMD = -21.498, 95%CI: (-33.819)-(-9.177), P < 0.001; Figure 3B), and 24 mo [WMD = -6.000, 95%CI: (-9.079)-(-2.921), P < 0.05; Figure 3C] compared to their controls as shown in Figure 3. Similarly, we analysed 5 studies[24,25,28,32,33], 4 studies[23,24,28,33], and 1 study[24] reporting the VAS outcome at 6, 12, and 24 mo respectively using BM as the source of MSCs. There was a significant heterogeneity observed between the included studies. (I2 > 80%, P < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, significant reduction in VAS score was noted compared to their controls at 6 mo [WMD = -11.028, 95%CI: (-19.605)-(-2.450), P < 0.012; Figure 3A), and 24 mo [WMD = -17.589, 95%CI: (-22.486)-(-12.692), P < 0.001; Figure 3C), with a drop in the pain control at 12 mo [WMD = -2.366, 95%CI: (-9.912)-5.180, P = 0.539; Figure 3B], period compared to their controls.
On critical analysis of the pain reduction potential of both the sources, it is noted as shown in Figure 4 that despite the inconsistency in the pain reduction at 12 mo with BM, we noted a rising trend curve in pain reduction which favors the therapy. Although both the sources were capable of significant pain reduction compared to their controls, adipose tissue demonstrated consistent results across all the time points. However, the inconsistencies in the results of BM could also be accounted to the heterogeneity in the studies included for analysis.
WOMAC score: We analyzed 6 studies[27,31,36,37,39,40], and 6 studies[27,31,36-39] reporting the WOMAC scores at 6, and 12 mo respectively using adipose tissue as the source of MSCs. There was a significant heterogeneity observed between the included studies. (I2 > 80%, P < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, significant reduction in WOMAC scores were noted compared to their controls at 6 mo [WMD = -21.317, 95%CI: (-27.146)-(-15.488), P < 0.001; Figure 3D], and 12 mo [WMD = -19.341, 95%CI: (-30.544)-(-8.138), P < 0.001; Figure 3E] compared to their controls as shown in Figure 3. Similarly, we analyzed 7 studies[24,25,28,32-35], and 6 studies[24,25,28,33-35] reporting the WOMAC outcome at 6, and 12 mo respectively using BM as the source of MSCs. There was a significant heterogeneity observed between the included studies (I2 > 80%, P < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, we did not note any significant reduction in WOMAC scores compared to their controls at 6 mo [WMD = -1.958, 95%CI: (-10.273)- 6.357, P = 0.644; Figure 3D], and 12 mo [WMD = -1.944, 95%CI: (-8.183)-4.294, P = 0.541; Figure 3E] compared to their controls.
On critical analysis of the WOMAC score reduction potential of both the sources, it is noted as shown in Figure 3 that most of the studies that utilized BM did not report any significant improvement compared to their controls, despite their heterogeneity in results at both 6 mo and 12 mo. Since the WOMAC score concentrates more on the functional efficiency of the intervention apart from pain reduction, adipose tissue stands superior to BM as a dependable source of MSC to give better functional results consistently across both time points.
Lysholm knee score: We analyzed 3 studies[36,36,38], and one study[39] reporting the lysholm score at 12, and 24 mo respectively using adipose tissue as the source of MSCs. There was a significant heterogeneity observed between the included studies. (I2 > 80%, P < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, significant improvement in scores was noted compared to their controls at 12 mo (WMD = 6.494, 95%CI: 1.889-11.100, P = 0.006; Figure 3F). However, at 24 mo, the improvement in scores was not sustained [WMD = 4.100, 95%CI: (-4.757)-12.9557, P = 0.757; Figure 3G] compared to their controls as shown in Figure 3. Similarly, we analyzed 3 studies[22,24,30], and 2 studies[24,30] reporting Lysholm scores outcome at 12 and 24 mo respectively using BM as the source of MSCs. There was a significant heterogeneity observed between the included studies. (I2 > 80%, P < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, we did not note any significant improvement in Lysholm score compared to their controls at both 12 mo [WMD = 0.232, 95%CI: (-5.133)-5.597, P = 0.933; Figure 3F], and 24 mo [WMD = 4.412, 95%CI: (-0.801)-9.626, P = 0.097; Figure 3G] respectively. On critical analysis of the improvement of the Lysholm score of both the sources, it is noted only in studies utilizing adipose tissue as the source of MSC significant improvement in the functional outcomes is noted which is in corroboration with the WOMAC score results.
We analyzed the quality of life outcomes such as KOOS reported in 3 studies[22,27,39] using adipose tissue and 3 studies[22,34,41] utilizing BM as the source of MSCs. There was a significant heterogeneity observed between the included studies (I2 > 80%, P < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, significant improvement in scores was noted in both adipose tissue (WMD = 13.124, 95%CI: 4.745-21.502, P = 0.002; Figure 3H) and BM (WMD = 2.642, 95%CI: 0.587-4.698, P = 0.012; Figure 3H) as the sources compared to their controls, despite the inconsistencies noted earlier in the functional outcomes such as WOMAC or Lysholm scores.
Similarly, we analyzed 2 studies that objectively analyzed the regenerate cartilage tissue using magnetic resonance imaging with MOCART score between the two sources[30,40]. There was a significant heterogeneity observed between the included studies (I2 > 80%, P < 0.001). Hence, the random-effects model was used for analysis. We noted significant improvement in the MOCART scores at 12 mo in both the sources (WMD = 31.625, 95%CI: 7.481-55.769, P = 0.010; Figure 3I) compared to their controls. As shown in Figure 3, although both the sources had significantly improved KOOS and MOCART scores at 12 mo, the improvement noted with adipose tissues stands relatively high compared to the BM.
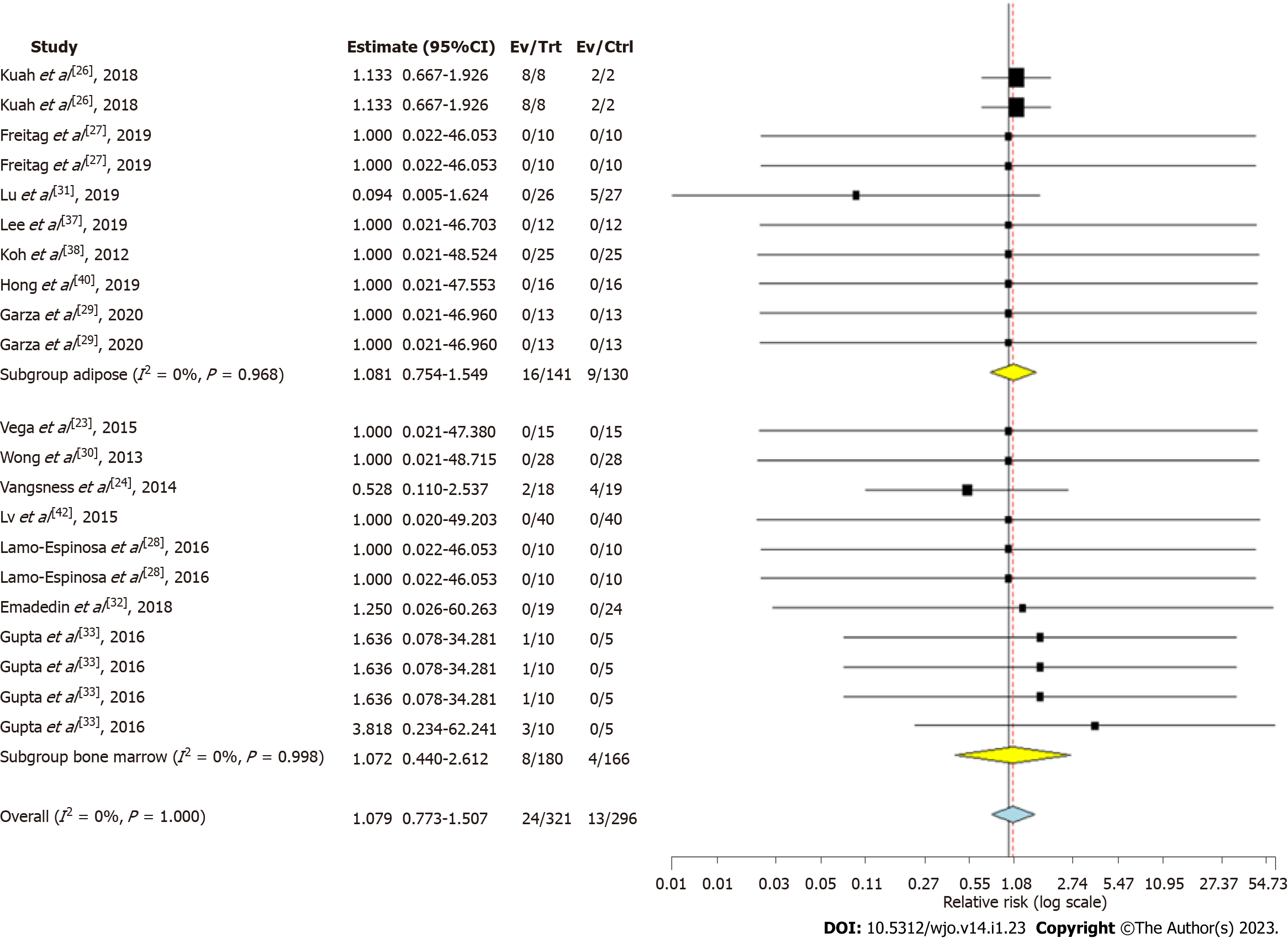
Seven studies involving 141 patients reported adverse effects with low heterogeneity among the included studies using adipose tissue as the source of MSC for knee OA. (I2 = 0.0%, P = 0.968). Hence, a fixed-effects model was used for analysis. There was no significant increase in the adverse events compared to the controls (RR = 1.081, 95%CI: 0.754-1.549, P = 0.673; Figure 5).
Seven studies involving 180 patients reported adverse effects with low heterogeneity among the included studies with AD-MSC (I2 = 0.0%, P = 0.996). Hence, a fixed-effects model was used for analysis. There was no significant increase in the adverse events compared to the controls (RR = 1.072, 95%CI: 0.440-2.612, P = 0.876; Figure 5). No major serious adverse events with permanent effects such as death, tumor, or immune reaction to the intervention were noted during follow-up in either of the sources of MSCs.
We conducted sensitivity analysis whenever heterogeneity was noted in the outcomes analysed. The results of the outcomes analysed such as VAS for pain, WOMAC, Lysholm, KOOS, MOCART, and adverse events were not significantly altered by sequentially omitting each study in the meta-analysis. We also did not note a change in the consistency of the results for the outcomes analysed upon changing the analysis to the random-effects model.
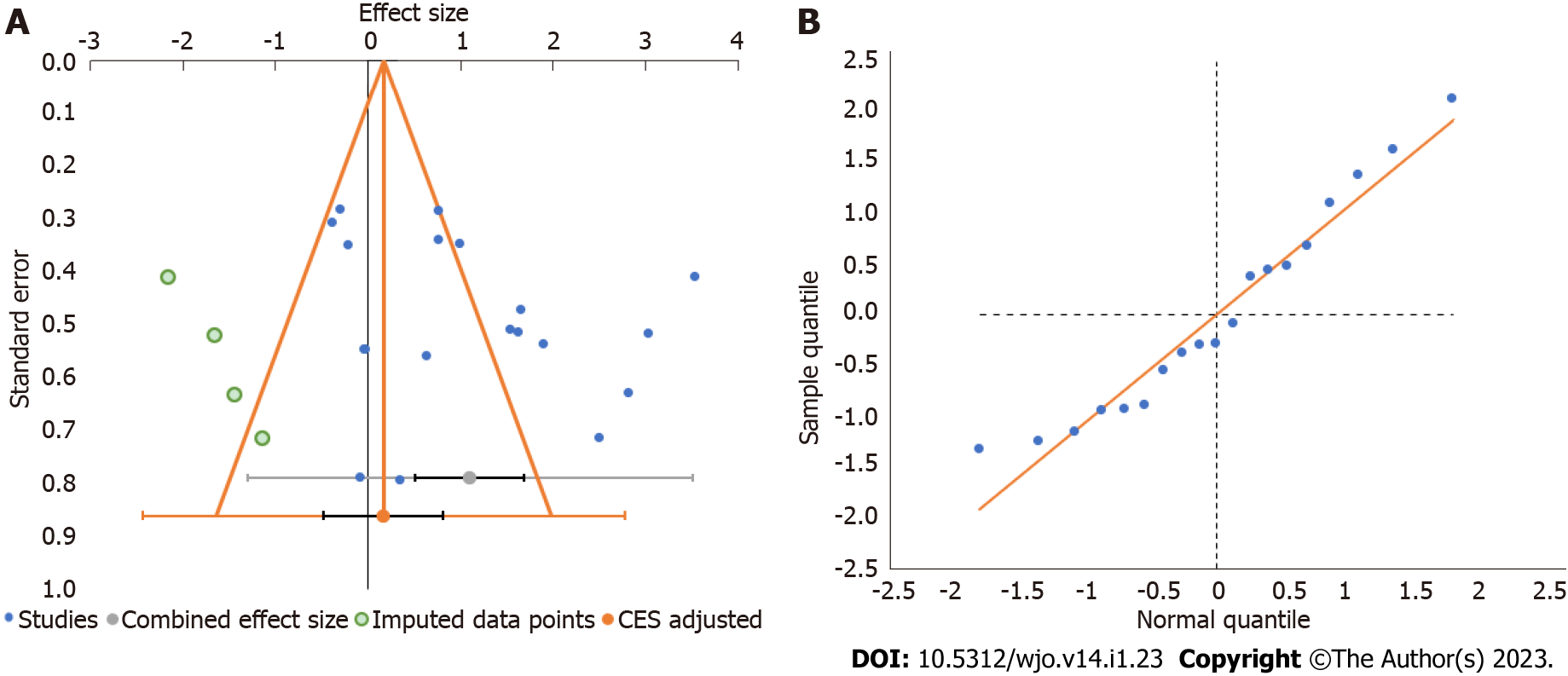
Publication bias was analyzed utilizing the funnel plot, normal quantile plot, and Egger’s regression test for the meta-analysis performed. There was no evidence of publication bias by funnel plot and normal quantile plot as shown in Figure 6 or by Egger’s regression test (P = 0.519). We noted symmetrical distribution of studies in the funnel plot and studies were found to lie close to the 95%CI and no significant heterogeneity was noted in the distribution of the studies about the axes, suggestive of minimal publication bias.
In the era of regenerative medicine, MSCs serve the ideal cell-based resort for treating cartilage disorders and provide a platform for regeneration. Various animal models have demonstrated the safety and efficacy of MSCs in cartilage regeneration. MSCs bridge a gap between pharmacological and surgical management of OA of the knee. MSCs offer a balanced equilibrium between pro-and anti-apoptotic, pro-and anti-inflammatory cytokines, and pro-and anti-angiogenic factors to maintain joint homeostasis which is required for cartilage regeneration. Though the reliability of cellular therapy for OA knee has been tested in various preclinical and clinical trials, they provide the readers with conflicting results in the source of MSCs to be used for cartilage regeneration. In literature, the ideal source of MSCs for cartilage regeneration is still under debate. The chondrogenesis among the available sources of MSCs is demonstrated in all the sources of MSCs. The most ideal chondrogenic MSC is still under question.
The efficacy of MSC in cartilage regeneration should withstand the biomechanical stress which has to be evaluated according to regulatory guidelines to demonstrate the role of cellular therapy for adoption across an expanding patient population. The reasons behind the less exploration of other sources of MSCs for chondrogenesis are inadequate standardization of isolation protocols to retrieve MSC from that particular source and the strict regulatory guidelines laid by the governing bodies. In this analysis, we tried to analyze whether BM-MSCs or AD-MSCs are the ideal sources for chondrogenesis. Among all the available sources of MSCs, extraction of MSCs from BM and adipose tissue pose minimal morbidity to the donor site while compared with other sources of MSCs. BM-MSC is the most popular source and widely used MSC for osseous and cartilage regeneration. The MSC count in BM appears to be less when compared with the MSC count in adipose tissue. Hence the source of MSC from where it is retrieved plays a major role in cartilage regeneration.
Although Estrada et al[22] in their study compared the two sources, they did not randomize the study participants to the interventions analyzed. Instead, they categorized the patients with severe disease to be allotted to adipose-based therapy while mild and moderate diseases to platelet- and BM-based therapy respectively. Hence one cannot objectively compare the efficacy of the two different sources, which necessitated us to undergo a pooled analysis of the studies using adipose tissue and BM as the source of MSCs in the management of knee OA and compared their results using minimum clinical importance difference (MCID) for the parameter concerned.
We comprehensively and critically reviewed all available literature to identify the ideal source of MSCs for knee OA and found that: AD-MSCs showed a statistically significant and consistent improvement in all functional outcome measures, such as the VAS score for pain, WOMAC, Lysholm, KOOS, and radiological outcome parameters such as MOCART at varied time intervals compared to their corresponding controls. In contrast, despite better improvement in the VAS score for pain in the long term (24 mo), BM, as a source of MSCs, did not show functional benefits when evaluated using the WOMAC and Lysholm. However, objective measures of quality of life using KOOS and radiological outcome parameters, such as MOCART, showed significant benefits compared to their corresponding controls.
On comparing the relative improvement in various analyzed parameters, such as the VAS score, WOMAC, Lysholm, KOOS, and MOCART, between the two sources adipose tissue outperformed BM, with the difference in their outcome parameters more than the MCID for the concerned parameter. The MCID used were 15 for VAS score, 10 for WOMAC, 25 for Lysholm, 15 for KOOS[43,44]. There were no significant adverse events with either MSC compared to their controls.
The source of MSC harvesting is an important factor in stem cell research. Although the BM-MSC harvesting method has been the most commonly used method of MSC harvesting, recent studies have pointed towards AD-MSC owing to their ease of extraction and lack of procedure-related morbidity[45]. Isolation of AD-MSCs from adipose tissue blocks is superior to liposuction[11]. There is a well-documented procedure for harvesting a larger number of AD-MSCs under local anesthesia with minimal procedure-related patient morbidity[46]. Although there have been reports of fat embolism during AD-MSC harvesting, its incidence is very low. With appropriate techniques and skill, the incidence can be further reduced. The ease of access to fat sources and its minimally invasive approach, unlike access to BM, is sufficient to compel researchers to further explore AD-MSC harvesting techniques.
Pendleton et al[46] reported that AD-MSCs had a higher yield than BM-MSCs. Furthermore, a higher seeding density is necessary for the successful growth and expansion of BM-MSCs. Luna et al[47] recovered 1 × 106 adipocytes, 1 × 106 ASCs, 1 × 106 vascular endothelial cells, and 1 × 106 other cells from 1 g of adipose tissue. Adipose tissue contains up to 3% stem and progenitor cells in the uncultured SVF, containing 2500 times more stem cells than the BM source[48,49]. SVF mixture, a derivative of adipose tissue, contains 30% MSCs, 3% endothelial cells, and 14% endothelial precursor cells[50], whereas BM-MSCs contain 0.001% MSCs, 0.1% endothelial cells, and 2% endothelial precursor cells[51].
AD-MSCs demonstrate a consistently faster proliferation rate across multiple passages[46]. While the proliferation rate of MSCs from both sources was comparable on days 3 and 7, AD-MSCs continued to proliferate significantly up to day 21, and BM-MSCs attained a plateau from day 14. Similarly, significantly higher cellular metabolic activity was noted in AD-MSCs than in BM-MSCs on days 14 and 21, indicating a higher cellular yield of MSCs[46].
Although AD-MSCs are harvested with minimal morbidity and provide a better yield than BM-MSCs, the ultimate target of these MSCs in orthopedic research is their differentiation potential in chondrogenic and osteogenic lineages. Chondrogenic differentiation at the gene level, determined by real-time quantitative polymerase chain reaction, showed that the expression of the chondrogenic gene aggrecan varied in AD-MSCs and BM-MSCs from different donors. However, overall, the expression was significantly higher in BM-MSCs than in AD-MSCs. There was no remarkable difference in cartilaginous proteoglycan matrix formation between AD-MSCs and BM-MSCs[11]. The expression of Runx2, collagen type I, and alkaline phosphatase increases from day 7 to day 14 in both AD-MSCs and BM-MSCs, with significantly higher expression in BM-MSCs than in AD-MSCs[11].
Despite easier harvest and superior yield from adipose tissue, AD-MSCs fall short in terms of differentiation potential in chondrogenesis or osteogenesis compared to BM-MSCs. Therefore, research to enhance the necessary lineage differentiation characteristics of AD-MSCs is ongoing to reap the full benefits of its abundant availability and ease of harvesting because AD-MSCs have a more grounded immunomodulatory impact than BM-MSCs in altering the pathological milieu of the target site[52-54].
Short- and long-term storage of AD-MSCs was investigated. The storage of AD-MSCs decreases their cellular proliferative capacity over time[55]. Hence, it must be supplemented with 10% human serum or PRP in 0.9% saline solution at 4 °C for the first 2 h and not more than 4 h[56,57]. For long-term storage, AD-MSCs can be stored at -80 °C in liquid nitrogen for up to 6 mo[58,59]. In contrast, BM-MSCs have been stored for more than 10 years without losing their multipotency[60].
With the evolution in the understanding of the biology of MSCs, there is a corresponding expanding horizon of their therapeutic possibilities with their properties towards induction of angiogenesis; regulation of immune response and inflammation; modulation of cell differentiation and proliferation; extracellular matrix formation; neuroprotective and neurotrophic effects; and anti-apoptotic, anti-tumor, and anti-microbial activities[61]. Apart from identifying the ideal source of MSCs for a particular scenario, the development of methods to identify their potency is needed for objective assessment of the individual MSCs concerned to account for individual variability, which might affect the therapeutic response[62]. The future of MSC-based therapies is driving towards a cell-free secretome-based therapy using MSC-derived exosomal vesicles that exert the necessary functional activities of MSCs, where the ideal required cellular characteristics of MSCs from multiple sources could be combined to obtain the maximum benefits of the individual MSC source[63].
Our study had certain limitations. First, we could not find data on the blinding of the intervention to the participants in most of the included studies, which could invite room for bias on the part of patients or observers. Second, we noted heterogeneity among the majority of the analyzed outcomes, which could be due to the variability in the protocols followed for intervention in the included studies, as shown in Table 2. The heterogeneity could also be attributed to the inclusion of patients with a different spectrum of disease processes or difference in the control interventions utilized across the included studies. Therefore, we recommend a large multicenter trial with a standardized dosage and intervention protocol, evaluated using established outcome measures both in the short and long term, without any adjuvant procedures to further confirm our analysis results.
Our critical analysis of the literature showed that adipose tissue is superior to BM as a source of MSC because of its safety and consistent efficacy concerning improvement in pain and functional outcomes in managing knee OA. However, future trials of sufficient quality are warranted to validate our findings to arrive at a consensus on the ideal source of MSC for use in cellular therapy for knee OA.
Mesenchymal stromal cell (MSC)-based therapies are being commonly utilized in the context of knee osteoarthritis (OA) with promising results. The commonly used sources of the MSC remain in the bone marrow (BM) and the adipose derived (AD).
Despite the prevalence of the use of MSCs of varying origins in the management of knee OA, the literature is not clear on the ideal source to focus on for future research.
In this study, we aim to compare the efficacy and safety of the two commonly used sources of MSCs namely BM and adipose tissue in the management of knee OA.
We conducted a systematic review and meta-analysis of the randomized controlled trials (RCTs) in the literature identified from databases such as PubMed, EMBASE, Web of Science, and Cochrane Library until August 2021 that analyzed the efficacy and safety of AD and BM-MSCs in the management of knee OA. we used outcome parameters such as the visual analog scale (VAS) score for pain, Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), Lysholm score, Tegner score, magnetic resonance observation of cartilage repair tissue (MOCART) score, knee osteoarthritis outcome score (KOOS), and adverse events.
We identified twenty-one studies including 936 patients. Of all the studies included, only one study compared the two MSC sources without patient randomization; hence, the results of all included studies from both sources were pooled, and a comparative critical analysis was performed. At six months, both AD-MSCs and BM-MSCs showed significant VAS improvement (P = 0.015, P = 0.012); this was inconsistent at 1 year for BM-MSCs (P < 0.001, P = 0.539), and AD-MSCs outperformed BM-MSCs compared to controls in measures such as WOMAC (P < 0.001, P = 0.541), Lysholm scores (P = 0.006; P = 0.933), and KOOS (P = 0.002; P = 0.012). BM-MSC-related procedures caused significant adverse events (P = 0.003) compared to AD-MSCs (P = 0.673).
Our study identified adipose tissue to be superior to BM in terms of its safety and consistent efficacy in improving the pain and functional outcome parameters analyzed.
We suggest for future RCTs be conducted to make a direct comparison of the two sources considering the paucity of the literature identified in this study and also to validate the findings arrived in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang YC, China; Yu FY, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian J Intern Med. 2011;2:205-212. [PubMed] |
| 2. | Karuppal R. Current concepts in the articular cartilage repair and regeneration. J Orthop. 2017;14:A1-A3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Goldberg A, Mitchell K, Soans J, Kim L, Zaidi R. The use of mesenchymal stem cells for cartilage repair and regeneration: a systematic review. J Orthop Surg Res. 2017;12:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 4. | Le H, Xu W, Zhuang X, Chang F, Wang Y, Ding J. Mesenchymal stem cells for cartilage regeneration. J Tissue Eng. 2020;11:2041731420943839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 5. | Davatchi F, Sadeghi Abdollahi B, Mohyeddin M, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis. 2016;19:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Reissis D, Tang QO, Cooper NC, Carasco CF, Gamie Z, Mantalaris A, Tsiridis E. Current clinical evidence for the use of mesenchymal stem cells in articular cartilage repair. Expert Opin Biol Ther. 2016;16:535-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Muthu S, Jeyaraman M, Jain R, Gulati A, Jeyaraman N, Prajwal GS, Mishra PC. Accentuating the sources of mesenchymal stem cells as cellular therapy for osteoarthritis knees-a panoramic review. Stem Cell Investig. 2021;8:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells. 2014;6:312-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 269] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 9. | De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 852] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 10. | Dmitrieva RI, Minullina IR, Bilibina AA, Tarasova OV, Anisimov SV, Zaritskey AY. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: differences and similarities. Cell Cycle. 2012;11:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, Idris SB. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. 2018;9:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 335] [Article Influence: 47.9] [Reference Citation Analysis (1)] |
| 12. | Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 627] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 13. | Jeyaraman M, Muthu S, Ganie PA. Does the Source of Mesenchymal Stem Cell Have an Effect in the Management of Osteoarthritis of the Knee? Cartilage. 2021;13:1532S-1547S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 14. | Freitag J, Wickham J, Shah K, Tenen A. Real-world evidence of mesenchymal stem cell therapy in knee osteoarthritis: a large prospective two-year case series. Regen Med. 2022;17:355-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 15. | Zhu C, Wu W, Qu X. Mesenchymal stem cells in osteoarthritis therapy: a review. Am J Transl Res. 2021;13:448-461. [PubMed] |
| 16. | Shariatzadeh M, Song J, Wilson SL. The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019;378:399-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, Bronfort G, van Tulder MW; Editorial Board of the Cochrane Back, Neck Group. 2015 Updated Method Guideline for Systematic Reviews in the Cochrane Back and Neck Group. Spine (Phila Pa 1976). 2015;40:1660-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 527] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 18. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47111] [Article Influence: 2944.4] [Reference Citation Analysis (0)] |
| 19. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15131] [Article Influence: 2521.8] [Reference Citation Analysis (0)] |
| 20. | Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J Stat Softw. 2012;49:1-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 511] [Article Influence: 39.3] [Reference Citation Analysis (1)] |
| 21. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46435] [Article Influence: 2110.7] [Reference Citation Analysis (3)] |
| 22. | Estrada E, Décima JL, Rodríguez M, Di Tomaso M, Roberti J. Patient-Reported Outcomes After Platelet-Rich Plasma, Bone Marrow Aspirate, and Adipose-Derived Mesenchymal Stem Cell Injections for Symptomatic Knee Osteoarthritis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120931086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, Sánchez A, García-Sancho J. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation. 2015;99:1681-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 448] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 24. | Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 292] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 25. | Garay-Mendoza D, Villarreal-Martínez L, Garza-Bedolla A, Pérez-Garza DM, Acosta-Olivo C, Vilchez-Cavazos F, Diaz-Hutchinson C, Gómez-Almaguer D, Jaime-Pérez JC, Mancías-Guerra C. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int J Rheum Dis. 2018;21:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Kuah D, Sivell S, Longworth T, James K, Guermazi A, Cicuttini F, Wang Y, Craig S, Comin G, Robinson D, Wilson J. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: a randomized double-blind placebo-controlled single ascending dose study. J Transl Med. 2018;16:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 27. | Freitag J, Bates D, Wickham J, Shah K, Huguenin L, Tenen A, Paterson K, Boyd R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14:213-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (2)] |
| 28. | Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Nuñez-Córdoba JM, Sánchez-Echenique C, Bondía JM, Aquerreta JD, Andreu EJ, Ornilla E, Villarón EM, Valentí-Azcárate A, Sánchez-Guijo F, Del Cañizo MC, Valentí-Nin JR, Prósper F. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2016;14:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 29. | Garza JR, Campbell RE, Tjoumakaris FP, Freedman KB, Miller LS, Santa Maria D, Tucker BS. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am J Sports Med. 2020;48:588-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 30. | Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years' follow-up. Arthroscopy. 2013;29:2020-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 31. | Lu L, Dai C, Zhang Z, Du H, Li S, Ye P, Fu Q, Zhang L, Wu X, Dong Y, Song Y, Zhao D, Pang Y, Bao C. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther. 2019;10:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 32. | Emadedin M, Labibzadeh N, Liastani MG, Karimi A, Jaroughi N, Bolurieh T, Hosseini SE, Baharvand H, Aghdami N. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 33. | Gupta PK, Chullikana A, Rengasamy M, Shetty N, Pandey V, Agarwal V, Wagh SY, Vellotare PK, Damodaran D, Viswanathan P, Thej C, Balasubramanian S, Majumdar AS. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 34. | Bastos R, Mathias M, Andrade R, Amaral RJFC, Schott V, Balduino A, Bastos R, Miguel Oliveira J, Reis RL, Rodeo S, Espregueira-Mendes J. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: a controlled, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc. 2020;28:1989-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 35. | Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 648] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 36. | Tran TDX, Wu CM, Dubey NK, Deng YH, Su CW, Pham TT, Thi Le PB, Sestili P, Deng WP. Time- and Kellgren⁻Lawrence Grade-Dependent Changes in Intra-Articularly Transplanted Stromal Vascular Fraction in Osteoarthritic Patients. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl Med. 2019;8:504-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 38. | Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19:902-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 299] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 39. | Koh YG, Kwon OR, Kim YS, Choi YJ. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy. 2014;30:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 40. | Hong Z, Chen J, Zhang S, Zhao C, Bi M, Chen X, Bi Q. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop. 2019;43:1123-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 41. | Ruane JJ, Ross A, Zigmont V, McClure D, Gascon G. A Single-Blinded Randomized Controlled Trial of Mesenchymal Stem Cell Therapy for the Treatment of Osteoarthritis of the Knee with Active Control. J Stem Cells Regen Med. 2021;17:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Lv X, Huang C, Yin Z, Hong B, Jiang H, Huang X. Effectiveness of Autologous Bone Marrow Mesenchymal Stem Cell Transplant for Knee Osteoarthritis. Chin J Cell Stem Cell. 2015;5:28-32. |
| 43. | Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, Bombardier C, Felson D, Hochberg M, van der Heijde D, Dougados M. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 804] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 44. | Ogura T, Merkely G, Bryant T, Winalski CS, Minas T. Autologous Chondrocyte Implantation "Segmental-Sandwich" Technique for Deep Osteochondral Defects in the Knee: Clinical Outcomes and Correlation With Magnetic Resonance Imaging Findings. Orthop J Sports Med. 2019;7:2325967119847173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 1247] [Article Influence: 207.8] [Reference Citation Analysis (0)] |
| 46. | Pendleton C, Li Q, Chesler DA, Yuan K, Guerrero-Cazares H, Quinones-Hinojosa A. Mesenchymal stem cells derived from adipose tissue vs bone marrow: in vitro comparison of their tropism towards gliomas. PLoS One. 2013;8:e58198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 47. | Luna AC, Madeira ME, Conceição TO, Moreira JA, Laiso RA, Maria DA. Characterization of adipose-derived stem cells of anatomical region from mice. BMC Res Notes. 2014;7:552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Baptista LS. Adipose stromal/stem cells in regenerative medicine: Potentials and limitations. World J Stem Cells. 2020;12:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012:812693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 50. | Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 51. | Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, Yi T, Mirensky TL, Nalbandian A, Udelsman B, Hibino N, Shinoka T, Saltzman WM, Snyder E, Kyriakides TR, Pober JS, Breuer CK. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107:4669-4674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 417] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 52. | Leto Barone AA, Khalifian S, Lee WP, Brandacher G. Immunomodulatory effects of adipose-derived stem cells: fact or fiction? Biomed Res Int. 2013;2013:383685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5012] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 54. | Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, Lee JE, Kim YJ, Yang SK, Jung HL, Sung KW, Kim CW, Koo HH. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 55. | Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev Rep. 2011;7:269-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 324] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 56. | Chu DT, Nguyen Thi Phuong T, Tien NLB, Tran DK, Minh LB, Thanh VV, Gia Anh P, Pham VH, Thi Nga V. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 57. | Wu YD, Li M, Liao X, Li SH, Yan JX, Fan L, She WL, Song JX, Liu HW. Effects of storage culture media, temperature and duration on human adiposederived stem cell viability for clinical use. Mol Med Rep. 2019;19:2189-2201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Shaik S, Wu X, Gimble J, Devireddy R. Effects of Decade Long Freezing Storage on Adipose Derived Stem Cells Functionality. Sci Rep. 2018;8:8162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 59. | De Rosa A, De Francesco F, Tirino V, Ferraro GA, Desiderio V, Paino F, Pirozzi G, D'Andrea F, Papaccio G. A new method for cryopreserving adipose-derived stem cells: an attractive and suitable large-scale and long-term cell banking technology. Tissue Eng Part C Methods. 2009;15:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 60. | Kumazawa K, Sugimoto T, Yamazaki Y, Takeda A, Uchinuma E. Osteogenic Potential, Multipotency, and Cytogenetic Safety of Human Bone Tissue-Derived Mesenchymal Stromal Cells (HBT-MSCs) after Long-Term Cryopreservation. Kitasato Med J. 2014;44:95-103. |
| 61. | Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, Gurtner GC. Stem Cells in Wound Healing: The Future of Regenerative Medicine? Gerontology. 2016;62:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 62. | Lechanteur C, Briquet A, Giet O, Delloye O, Baudoux E, Beguin Y. Clinical-scale expansion of mesenchymal stromal cells: a large banking experience. J Transl Med. 2016;14:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 63. | Jeyaraman M, Muthu S, Gulati A, Jeyaraman N, G S P, Jain R. Mesenchymal Stem Cell-Derived Exosomes: A Potential Therapeutic Avenue in Knee Osteoarthritis. Cartilage. 2021;13:1572S-1585S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |