Published online May 18, 2022. doi: 10.5312/wjo.v13.i5.411
Peer-review started: February 25, 2021
First decision: October 17, 2021
Revised: October 31, 2021
Accepted: April 24, 2022
Article in press: April 24, 2022
Published online: May 18, 2022
Processing time: 441 Days and 11.6 Hours
Combined musculoskeletal and vascular injuries of the extremities are conditions in which a multidisciplinary approach is a sine qua non to ensure life initially and limb viability secondarily. Vascular injuries as part of musculoskeletal trauma are usually the result of the release of a high energy load in the wound site so that the prognosis is determined by the degree of soft-tissue damage, duration of limb ischemia, patient’s medical status and presence of associated injuries. The management of these injuries is challenging and requires a specific algorithm of action, because they are usually characterized by increased morbidity, amputation rate, infection, neurological and functional deficits, and they could be life threatening. Although vascular injuries are rare and occur either isolated or in the context of major combined musculoskeletal trauma, the high index of suspicion, imaging control, and timely referral of the patient to organized trauma centers ensure the best functional outcome of the extremity in such challenging cases. Even after a successful initial treatment of a combined trauma pattern, long-term follow-up is crucial to prevent and detect early possible complications. The purpose of this manuscript is to provide an update on diagnosis and treatment of combined musculoskeletal and vascular injuries of the extremities, from an orthopedic point of view.
Core Tip: A complex extremity injury that, in addition to a fracture, dislocation or even crush, involves a vascular injury represents a rare trauma pattern. Historically, control of hemorrhage following trauma has been of interest as far as vascular injury is a leading cause of death and disability. We here discuss the epidemiology, diagnosis and team management of combined musculoskeletal and vascular injuries of the extremities.
- Citation: Stefanou N, Arnaoutoglou C, Papageorgiou F, Matsagkas M, Varitimidis SE, Dailiana ZH. Update in combined musculoskeletal and vascular injuries of the extremities. World J Orthop 2022; 13(5): 411-426
- URL: https://www.wjgnet.com/2218-5836/full/v13/i5/411.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i5.411
A complex extremity injury that, in addition to a fracture, dislocation or even crush, involves a vascular injury represents a rare trauma pattern. It is a part of combined extremity injury or mangled extremity, and an emergency condition in which multidisciplinary approach is a sine qua non to ensure life initially and limb viability secondarily[1]. Historically, control of hemorrhage following trauma has been of interest as vascular injury is a leading cause of death and disability. Moreover, limb salvage in patients with combined musculoskeletal and vascular injuries is distinctly dependent on the severity of soft-tissue injury, duration of limb ischemia and early, accurate diagnosis and treatment of vascular damage[2]. The management of these injuries is challenging and requires a specific algorithm of action, because they are usually characterized by increased morbidity, amputation rate, infection, neurological and functional deficits, and they could be life threatening.
The purpose of this review is to provide an update on diagnosis and treatment of combined musculoskeletal and vascular extremity injuries, from an orthopedic point of view. Combined injuries of the hand, despite possible vascular implication, are not included to this update, due to unique characteristics requiring special treatment and reconstructive pathway.
For many centuries, the manipulation of any hemorrhagic scenario, especially on the battlefield, was controlled by compressive dressings, styptics, hot iron, raw ligature of vessels and improvised tourniquets[3]. The first arterial repair was performed by Dr. Hallowell in 1759 at Newcastle upon Tyne in England and the case was reported by Dr. Richard Lambert in 1761[4]. At the end of the 19th century, in 1896, John B. Murphy carried out an end-to-end anastomosis at Mercy Hospital in Chicago, followed in only one decade by Alexis Carrel and Charles C. Guthrie who established other vascular operative techniques, thus providing a more refined approach in the management of vascular trauma[5-7]. Since a dry and distinct surgical field is necessary for the management of a vascular injury, the gradual widespread use of the tourniquet has greatly helped in the development of surgical techniques. Johannes Friedrich August von Esmarch who was one of the Bernhard von Langenbeck’s assistants, developed in 1873 the famous apparatus widely known as the Esmarch bandage[8,9]. Ligations and amputations have been common practice during the Balkan Wars (1911–1913), the First World War (1914–1918) and until the end of World War II (1939–1945), when the use of effective antibiotics and the presence of blood banking improved significantly vascular injury treatment options[10-12].
The time for the predominance of vascular repair over amputation was initially the Korean War (1950–1953), in which amputation rate was about 13% compared to approximately 49% that followed arterial ligation in World War II, and then the Vietnam War (1955–1975)[8,13-15]. Over the years and reaching the Iraq war, the early amputation rate was about 5%-10%[16,17]. All the techniques of vascular rehabilitation and the management algorithms of multiple-trauma patients have been further improved until early 21st century (expanded Advanced Trauma Life Support protocol, utilization of tourniquet, temporary intraluminal shunts, medical air evacuation, high quality intensive care units, sophisticated pharmaceutical agents) and extended recording, statistical analysis, long-term follow-up and publishing of data in recent decades both from the battlefields (Iraq and Afghanistan) and the major urban centers provided significant help in that direction[18-22].
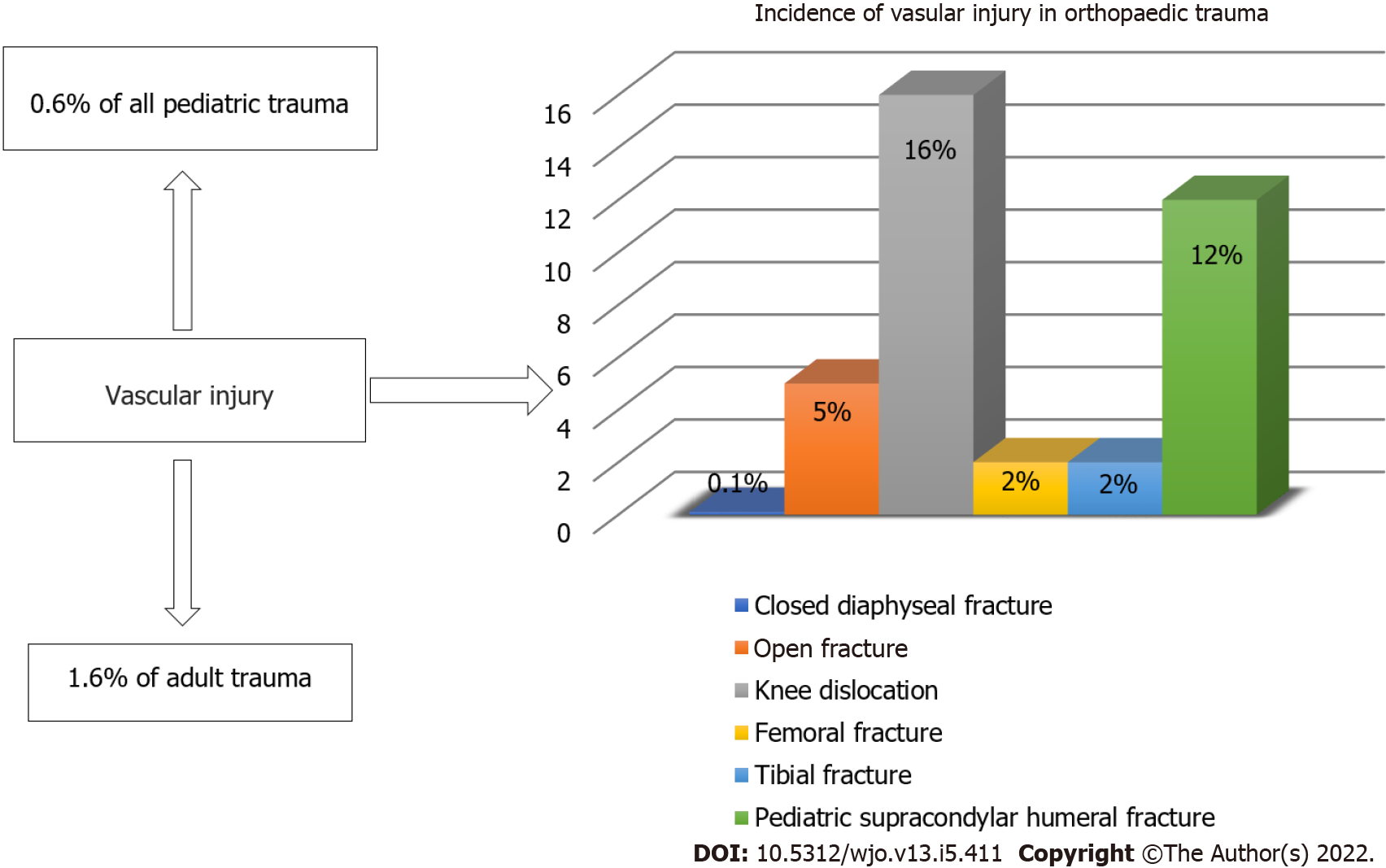
Trauma patterns and the incidence of vascular complications differ greatly between urban centers and war zones, while the existing geographical distribution reflects the impact of high velocity weapons, motor vehicle accidents, industrialization, socioeconomic status and increasing criminality[22-24]. Vascular injuries of the upper and lower extremities following trauma are relatively uncommon and represent almost 2%-3% of all civilian trauma including blunt, penetrating and gunshot injuries[24]. According to Barmparas et al[25] vascular trauma occurs in only 0.6% of all pediatric trauma patients and in 1.6% of adults, predominantly young men (Figure 1).
In general, the amount of energy transferred on tissues during an injury increases proportionally the possibility of vascular insult[26]. In patients with severe crush injuries associated with extensive bone (segmental shaft fracture, floating joint) and soft-tissue damage, the index of suspicion for associated arterial injury must be high. Closed diaphyseal long bone fractures carry a reported risk of vascular injury of 0.1% but open fractures have a 3.6-fold increase in the chance of vascular injury[27]. Five percent of open fractures present with a vascular injury requiring repair (type IIIc), with an amputation rate of about 16%[28]. Severe open tibial fractures are associated with a 9% incidence of vascular injury, whereas knee dislocations, especially posterior, have been associated with vascular injury in up to 16% (KD-IV > KD-V > KD-III according to Schenck classification). Femoral and popliteal artery injuries are associated with a fracture in the adjacent bone structures in a percentage of about 2% and injury of the brachial artery can occur in 8%-12% of children suffering from a supracondylar humeral fracture, usually a Gartland III fracture[21,24,26,29].
Clinical diagnosis of combined vascular injuries in musculoskeletal trauma patients represents the first challenging step in an overall demanding treatment pathway. Physical examination is not always able to detect a limb-threatening injury in the absence of obvious signs of vascular impairment[26].
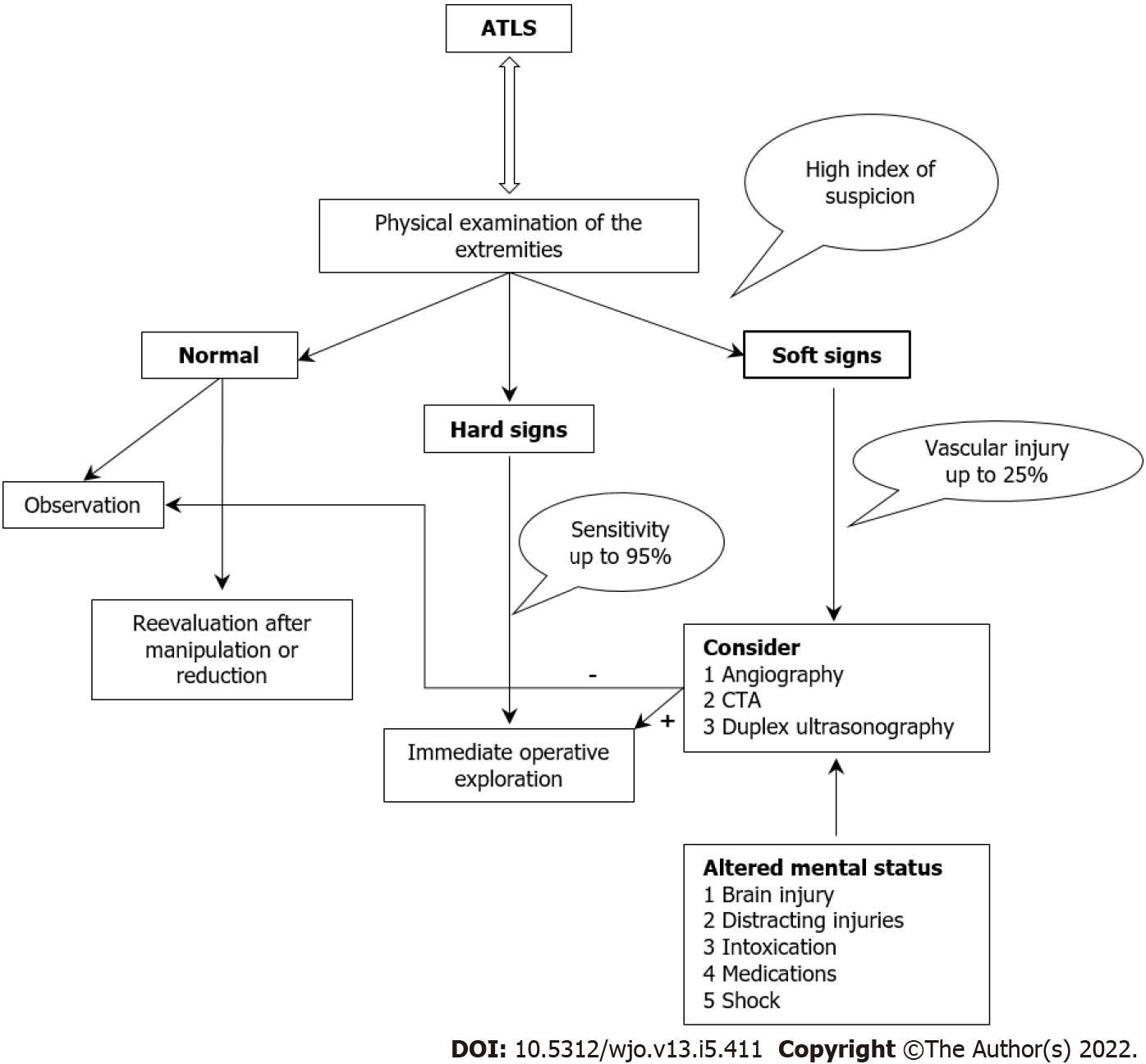
Given the fact that these injuries are in the context of major trauma, in polytrauma patients, the initial approach is performed via the well-established Advanced Trauma Life Support (ATLS) protocols[30]. In case of major bleeding, direct pressure, potential tourniquet application and resuscitation are the initial actions according to ATLS principles. After initial resuscitation and primary evaluation, a detailed clinical examination with neurological and vascular status documentation, is performed during the patient’s secondary evaluation. In this step, palpation of distal pulses, extremity color and temperature, time required for skin capillary refill in the distal digits, and sensory and motor deficits are assessed, compared with the contralateral limb, and recorded. Reassessment is required after any intervention, manipulation, or reduction maneuver to exclude secondary provoked vascular injury[31]. However, high index of suspicion and low threshold for vascular imaging is required because vascular injury might be present in the first place, with normal pulse palpation in a rate of 5%-15%[32].
To establish an objective approach and clinical criteria for further treatment, findings of the physical examination are classified into hard and soft signs of extremity for vascular injury (Table 1). Hard signs include massive bleeding, presence of rapidly expanding hematoma, detection of one classic sign of arterial occlusion (6Ps: Pulselessness, pallor, paresthesia, pain, paralysis and poikilothermia), palpable thrill or audible bruit[33]. The presence of hard signs has 92%-95% sensitivity for incidence of vascular injuries requiring surgical intervention with a positive predictive value of 95%[34,35]. In these cases, immediate operative intervention – injury site exploration in the presence of any hard sign – is strongly suggested by the literature without further delay for additional advanced vascular imaging [computed tomography (CT) or conventional angiography] (Figure 2)[36,37]. Soft signs for vascular injury include history of arterial bleeding at the accident scene or during transit to the hospital, proximity of penetrating or blunt injury to an artery of the limb, small, no pulsatile hematoma over a named artery, and presence of neurological deficit affecting nerves with proximity to a named artery. In these cases, incidence of vascular injuries ranges from 3% to 25%, depending on which one or which soft sign combination exists (Figure 2)[38,39].
| Hard signs | Soft signs (or subtle signs) |
| Active pulsatile or massive bleeding | History of arterial bleeding at the trauma scene |
| Rapidly expanding or pulsatile hematoma | Proximity-related injury |
| Bruit/thrill | Neurologic findings from nerve adjacent to a named artery |
| Evidence of arterial insufficiency and ischemia (6P: Pallor; pain; paresthesia; paralysis; pulseless; poikilothermia) | Hematoma over a named artery |
| Diminished unilateral distal pulse |
Besides physical examination, an arterial pressure index (API) or injured extremity index (IEI) (analogous to the ankle–brachial index – ABI) should be performed. The API/IEI is the ratio of the highest systolic occlusion pressure in the injured extremity at the level of the dorsalis pedis, posterior tibial or radial/ulnar arteries divided by the systolic pressure in a proximal vessel in an uninjured extremity (most often the brachial artery)[40,41]. Normal API values (> 0.9) are highly specific for exclusion of vascular injury in cases of blunt and penetrating injuries, making further vascular imaging unnecessary[42,43]. In cases with diminished peripheral pulses or API < 0.9 an imaging study, typically arteriography, should be performed.
Special consideration is required in cases of older diabetic patients with pre-existing peripheral vascular compromise, due to possible Monckeberg’s medial calcific sclerosis with clinically false normal API index of ≥ 1[44]. Recent literature, however, demonstrates high accuracy and good agreement of ankle–brachial index duplex ultrasonography in patients with type 2 diabetes and peripheral artery disease[45].
A specific, well known, clinical scenario is the knee dislocation and its association with popliteal artery injury. In these cases, clinical examination should include assessment of the stability of the knee joint, as laxity may suggest presence of a knee dislocation with spontaneous reduction. For further advanced imaging, current literature data are conflicting, with many authors advocating low threshold for vascular imaging in such an injury pattern and others being against routine imaging after spontaneous or performed reduction of a knee dislocation if normal pulses are present[38,46].
Supracondylar fractures represent another well-known clinical scenario. These fractures are of the most common traumatic fractures seen in children aged 5-7 years, with the urgency depending on whether the hand remains perfused or not, as rich collateral circulation may be sufficient despite vascular injury. In the case of absent distal pulses in supracondylar fractures of the humerus in children, the decision to explore is based on quality of extremity perfusion rather than absence of pulse. Generally, a well-perfused extremity is warm and pink in contrast to a poorly perfused extremity that is cold and pale with an arterial capillary refill > 2 s. Some studies recommend early aggressive surgical intervention for both patterns of vascular compromise in order to prevent limb loss and serious long-term complications such as Volkmann’s ischemic contracture[21,24,25,29].
Imaging modalities used in the diagnosis of vascular injuries in musculoskeletal trauma include conventional angiography, computed tomography angiography (CTA) and duplex ultrasound. Conventional catheter-based angiography provides accurate diagnosis of arterial injuries, even in minor injuries such as intimal tears, after intra-arterial or intravenous contrast agent injection[47,48]. As conventional angiography has several disadvantages such as cost, delay, need of specialized team, and possible renal toxicity, it is currently preserved for intraoperative use and not for routine imaging at an Emergency Department basis[49].
Duplex ultrasound scanning (DUS) represents a combination of B-mode real-time ultrasound and pulsed Doppler flow. DUS is accurate in assessing vascular injury with reported sensitivity from 50% to 100% and specificity exceeding 95%[50,51]. It is also noninvasive and cost-effective but can be time-consuming and significantly operator-dependent. Therefore, many surgeons consider it unreliable for the detection of vascular injuries in extremity trauma. In contrast, it may have a role in the surveillance of known vascular injuries in which nonoperative treatment has been selected[52].
CTA is established as the gold standard imaging method when dealing with vascular injuries in the extremity trauma[53-55]. CTA provides a rapid confirmation of the presence and location of vascular injury with simultaneous detection of bone and soft-tissue injuries/defects, without being an interventional examination and with high cost-effectiveness index[56]. It can highlight radiological findings like hematoma, active bleeding (contrast medium extravasation), arterial dissection, transection or occlusion, pseudoaneurysm, arteriovenous fistulas, focal narrowing or spasm, as well as anatomical variants of vascular origin like bifurcation with an hypoplastic posterior tibial artery or popliteal artery trifurcation in the popliteal fossa[53]. The study has limitations in cases with CT artifacts in the presence of foreign body such as metallic fragments at the study field, suboptimal accuracy in poor timing of intravenous contrast agent administration and the potential risk for renal damage from contrast medium[57]. As mentioned above, presence of obvious hard signs dictates operative treatment on an emergency basis without further imaging. However, trauma teams can consider the execution of CTA in the case of hemodynamically stable patients despite hard signs for vascular injury, for preoperative planning so as to avoid extended exploration intraoperatively and to decrease surgical time[58].
Magnetic resonance angiography seems to have limited role in the detection of vascular compromise in trauma emergencies. CTA provides better spatial resolution and faster isotropic image acquisition, is more readily available and avoids potential hazards of prolonged imaging[59].
As mentioned above, patients with combined musculoskeletal and vascular injuries are approached in the first place according to ATLS principles, having in mind the “life over limb” quote. Current universal guidelines for severely multiple injured patients agree on the early administration of tranexamic acid followed by Massive Transfusion Protocol with packed red blood cells, fresh frozen plasma and platelets, as well as an antifibrinolytic agent, to treat/prevent massive hemorrhage and trauma-induced coagulopathy[60-62]. Bleeding can be controlled by direct digital or dressing compression and proximal tourniquet application, for a limited time, which is preserved for special circumstances in hospital environment, where local hemorrhage cessation cannot be achieved with other manipulations[63]. Patients with diminished peripheral pulses and associated obvious gross limb deformity, such as displaced long bone fracture or joint dislocation, should undergo immediate manipulation to restore limb axis realignment and joint reduction respectively. The neurovascular status is consequently reassessed[64]. Reassessment is also required in cases of initial shock or hypothermia, after patient’s resuscitation and rewarming, because vascular status under these circumstances can be misleading[65].
A crucial question to be answered early after patient presentation and initial resuscitation, is the one of limb salvage versus primary amputation, and in case of amputation, the timing and level of amputation. This decision is usually hard for patients, families and trauma surgeons. Patients in most cases are young and active with long remaining working lifespan, not willing to integrate a major, permanent physical modification. Surgical teams, however, usually experience amputation as a therapeutic failure and sometimes multiple, complex salvage procedures are guided, with uncertain outcome but with preservation of the patient’s body image, at least initially. Progress and ongoing experience in microsurgical and reconstructive techniques provides wide range of surgical options, but limb salvage choice with extended indications, has often remarkable functional and emotional cost for the patients[66].
The Lower Extremity Assessment Project, a prospective multicenter study of patients with severe lower extremity injuries, stated that these limb-threatening injuries are likely associated with poor functional outcomes regardless of the choice between limb salvage and amputation[67]. Analogous results were presented by MacKenzie et al[68], in a series of 397 patients with severe lower limb trauma. The functional results were equally poor after amputation and limb reconstruction, and the outcome scores indicated substantial disability in 50% of patients.
Predictive factors for complications and poor outcomes in patients with combined musculoskeletal and vascular injuries are evaluated by variable injury scoring systems (MESS, NISSSA, LSI, PSI and HFS) including extensive soft-tissue injury, presence of open fracture, ischemic period exceeding 6 h, neurological deficit and other associated major injuries[69,70]. MESS (Mangled Extremity Severity Score) is predictive of amputation but its clinical validity is still in debate. MESS has been devised as a treatment decision aid; a score of ≥ 7 points usually indicates the need for primary amputation[71]. In 1994, Shanmuganathan[72] proposed the NISSSA score (Nerve Injury, Ischemia, Soft tissue injury, Skeletal injury, Shock and Age of the patient), which had the additional component of nerve injury – especially the loss of plantar sensation – apart of the bone and soft tissue injury components. Both scores were highly predictive of amputation, but the NISSSA had better sensitivity (81.8% vs 63.6%) and specificity (92.3% vs 69.2%) compared to MESS[72]. The Predictive Salvage Index (PSI) was proposed by Bosse et al[67] in 1987, with a reported sensitivity and specificity of 78% and 100%, respectively. The limb salvage index (LSI) was proposed by Russell in 1991 and although not utilized widely, the score was found more predictable than the MESS, PSI, NISSSA, and HFS-97 when assessing type III tibial fractures. Primary amputation is recommended after significant distal trauma, extensive crush, severe nerve dysfunction, an ischemic limb with duration exceeding 4 h of warm ischemia, segmental bone loss over one third of the length of the tibia, muscle loss in more than two compartments, and severe open foot injuries[73]. Besides injury characteristics, factors like non-Caucasian race, older age, female sex, lower educational level, lower economic status, smoking, and poor reported pre-injury general health condition, seem to play an important role in patients with less-than-optimal results[68].
Limb amputation is a realistic solution in an emergency and could be life-saving in the setting of sepsis or ongoing bleeding in a hemodynamically unstable trauma patient, with the advantages of shorter hospitalization, fewer secondary procedures, and faster rehabilitation period. Α published case series of nonsalvageable limb injuries reported that a delay of up to 5 d can be tolerated without negative impact on stump infection risk, giving the time for patient preparation and procedure planning on a semi-elective basis[74]. It must be underlined that long-term costs of prosthetics could likely make amputation an ultimately more expensive procedure[75].
Amputation level, determined by the nature of injury, is another crucial point of interest related with long-term functional outcome. It is well recognized that preservation of the knee joint provides a significant functional advantage. With a stump of sufficient length, the prosthesis has better support and less energy for movement is demanded[76]. Transtibial amputees perform better than transfemoral or through-knee ones, with decreased rehabilitation time[77,78]. However, the tissue condition after traumatic injuries requiring below-knee amputation is not always adequate to provide a lengthy and durable stump able to support a prosthesis, given the fact that at least 10 cm from medial tibial plateau is recommended[76,79]. In such cases, composite flaps harvested from the amputated limb and spare-part surgery concept may provide stump lengthening, thus avoiding a more proximal amputation or the use of flap[80,81].
Preoperative planning: Patient’s clinical situation, other major trauma (trunk, visceral or craniocerebral) than extremity injury requiring surgical treatment or nonoperative surveillance and determination of the type of vascular injury, are factors considered when proceeding to the operating theater. Α necessary condition for fast and safe surgical management includes grouping of injured tissues, planning of the procedure and defining the appropriate equipment for successful cooperation between different specialties. Furthermore, possible required equipment and devises should be confirmed, including magnifying loupes and vascular surgery – microsurgical instrumentation, external and internal fixation systems, and shunts.
In the presence of acute life-threatening injuries, like intracranial hematoma, thoracic, abdominal or pelvic bleeding, team management with two operating teams should be considered to manage the life-threatening injury, and control bleeding and re-establish perfusion at the injured extremity. In such cases, temporary measures, such as external fixation systems and intraluminal shunts, are the chosen treatment options following the Damage Control Surgery principles[82-84].
Types of vascular injury: Vascular injuries include a variety of lesions from arterial spasm to complete transection. Pre- or intraoperative accurate diagnosis of injury type is crucial as it guides the treatment modality. Overall, vascular injuries include complete wall defects with hemorrhage or pseudoaneurysm, intimal tears like disruption, flap or subintimal hematomas, complete transections with hemorrhage/ occlusion, arteriovenous fistulas and vascular spasm[21,64].
Regarding the injury mechanism, penetrating trauma is most associated with complete transections, wall defects or secondary development of arteriovenous fistulas. Blunt trauma, however, is usually associated with intimal defects or subintimal hematomas. Arterial spasm can be a result of either blunt or penetrating injury, more commonly in young patients[65].
Vascular injuries that are nonocclusive, like arterial spasm, intramural hematomas, or intimal flaps have a satisfactory healing potential with nonoperative treatment, with reported rates of 87%-95%[85,86]. Single vessel occlusion located peripherally – distal to knee/elbow – does not constitute risk for the viability of the limb. In these cases, in the absence of mangled extremity or severe soft tissue damage, observation with close clinical and imaging surveillance is a viable option. Other treatment options, besides open vascular repair, have a role in selected, isolated traumatic aneurysms where embolization can be performed[64]. Moreover, small pseudoaneurysms and intimal flaps or tears can be amenable to endovascular treatments, placing endovascular stents or stent grafts[87,88].
Operative treatment: Care should be taken from the operative team to establish the best conditions required for major surgical intervention. An operative tourniquet can replace the bleeding-control apparatus used at the emergency department. Skin aseptic preparation and draping should encompass potential areas of distal and proximal vascular control, fasciotomy sites and ipsilateral limb from groin to toenails for possible greater or lesser saphenous vein retrieval[52,64]. Adequate surgical approach is essential, with proximal and distal extension of the injury zone, to control hemorrhage and determine type and extend of arterial injury.
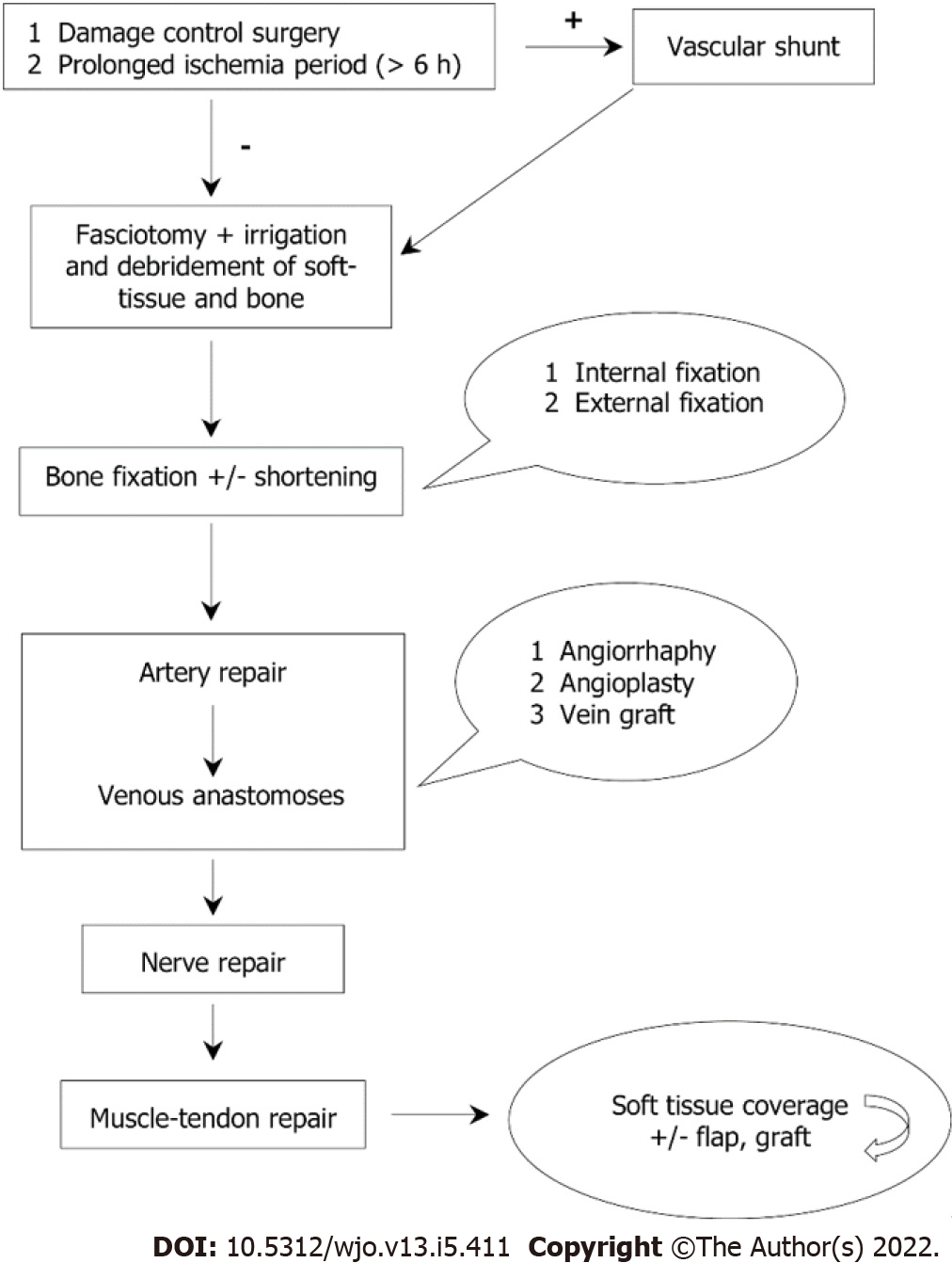
Sequence of intervention when orthopedic and vascular surgeons are implicated, remains an area of relative debate, despite being reported that this surgical sequence probably does not affect lower limb amputations rates[89]. Fracture reduction and fixation offers a stable skeletal “scaffold” for subsequent vascular repair (Figure 3). Vascular repair decreases warm ischemia time of the affected extremity[26,90]. Ischemia is crucial and determines the sequence of surgical intervention, with 6 h generally being the cutoff time interval for higher limb salvage rates[89,91]. It must be underlined that ischemia time is never accurate and in most cases is underestimated by patient, relatives, ambulance crew or other healthcare professional. As a general rule, in cases with cold ischemia (pulseless limb with no capillary refill) and in patients with prolonged period of warm ischemia (present capillary refill), vascular intervention should be performed first so as to restore perfusion as soon as possible via temporary intraluminal vascular shunt insertion[64,92,93]. Fracture fixation can be performed first when there is no evidence of cold or prolonged warm ischemia, especially in concomitant presence of unstable, comminuted fracture pattern.
Concerning operative treatment of coexisting fracture, the dilemma between temporary versus definitive fracture fixation turns towards temporary external fixation by most authors, in order to offer prospective for bone defect management and avoid infection, “second hit” phenomenon related to coagulopathy, systematic inflammatory immune system response and fat/pulmonary embolism[94-96]. Bone transport as well as induced membrane or Masquelet technique are options for large, segmental bone defects[97-99].
Regarding operative options for vascular injury manipulation, they include direct repair – anastomosis, vein patch angioplasty, bypass anastomosis with interposition graft placement, endovascular stents, and temporary intraluminal vascular shunts.
In cases of small lacerations, lateral angiorrhaphy with or without vein patch angioplasty is adequate as repair option. Injuries involving complete artery transection are addressed with central and peripheral debridement till healthy intima followed by end-to-end anastomosis[100,101]. If the anastomosis is not free of tension, a substitute conduit must be interposed. Conduit of choice for the most cases of peripheral vascular injuries is the autogenous reversed saphenous vein graft from the contralateral, uninjured lower limb with the alternatives of cephalic or basilic upper limb veins and synthetic grafts[100,102].
A keynote step in the operative treatment pathway of vascular injuries is the early recognition of patients indicated for temporary intraluminal shunt insertion, rather than definitive vascular repair and prior to bone stabilization. These indications include critically injured patients as part of damage control surgery, Gustilo IIIC open fractures, prolonged ischemia period, need for distal perfusion while another complex revascularization procedure like extra-anatomical bypass is performed and prior to replantation perfusion of an amputated upper extremity part[103,104].
Vein injuries and possible repair are usually addressed with less caution, in the presence of arterial injuries that are limb threatening. Peripheral vein ligation rarely consists a threat for limb salvage, but increases postoperative swelling and venous soft-tissue hemorrhage, having transient adverse effects on arterial inflow and leading to increased morbidity and infection risk. Operative options for peripheral venous repair include venous patch angioplasty, thoracostomy tubes as intraluminal venous shunts, or interposition graft placement from contralateral greater saphenous vein, common femoral vein, or axillary vein[105].
Injured structures are identified from superficial to deep layer, based on the knowledge of the cross-sectional anatomy, and reconstruction starts from the deeper layer. Nerves are repaired after bone and vascular structures followed by muscle and tendons, which are the last to be reapproximated. Careful documentation by photos and/or video is mandatory, not only to accurately define the postoperative rehabilitation protocol, but for medicolegal purposes too. Tension-free nerve restoration is advocated, as neuroma formation is a common complication when nerve repair is performed incorrectly or under tension. Soft tissue coverage is the last stage that will ensure the rapid healing of the tissues. Decision for coverage should be taken in conjunction with reconstruction surgeon, including muscle flap transportation or extra-anatomical bypass graft fashioning[101,105,106].
The use of negative pressure wound therapy as an alternative treatment of soft tissue defects, seems to improve angiogenesis, regulate perfusion, and promote local microvascular maturation. However, its use is limited and its efficacy and superiority over conventional dressing is questionable according to current multicenter randomized control trials and to Wound management of open lower limb fractures (WOLLF) study[107-109].
Secondary procedures: Cases with combined musculoskeletal and vascular injuries almost never have a unique surgical intervention. Second look for surgical site infection, soft-tissue viability and confirmation of vascular perfusion is routinely performed 24-48 h after initial surgery. The fasciotomy incision closure is performed after 7-10 d in case of optimal postoperative findings. At the same time, definite skeletal stabilization should be performed (external to internal fixation modification or unilateral external fixation system conversion to circular frame/hybrid fixation system) to minimize the risk of surgical site infection after the definite treatment, in case of preceding pin track infection.
Compartment syndrome is defined as the increased pressure within a closed fascial space, leading to reduction of capillary perfusion to less than required level for tissue viability, well recognized as cause for poor limb functional outcome[110,111]. It is the result of external compression or fascia closure and after increased compartment pressure in cases of ischemia–reperfusion, fractures, soft-tissue contusion, and venous outflow obstruction. In this concept, situations like crush injury, significant limb swelling, combined arterial and venous injuries requiring simultaneous clamping, need for arterial/venous ligation, early thrombosis of vascular repair, ongoing hypotension/continuing resuscitation and delayed treatment with no arterial perfusion for 4-6 h, determine high-risk patients for compartment syndrome development after combined trauma. Early clinical findings related to compartment syndrome are pain on passive movement of the involved compartments’ muscles and patients’ pain considered out of proportion to the injury, while classical 6 Ps (pulselessness, pallor, paresthesia, pain, paralysis, poikilothermia) are frequently not present in trauma patients with possible altered mental status and associated injuries and represent late signs of established compartment syndrome on the injured extremity[110].
Fasciotomies performed concomitantly with limb revascularization are associated with a four-times decrease in eventual amputation. There are also trauma surgeons who routinely perform prophylactic fasciotomies in the presence of high-risk scenarios[112]. This established intraoperative choice is possibly the reason for the low incidence of compartment syndrome after peripheral vascular injury[111]. Moreover, this approach may lead to possible conversion of a closed to open fracture, wound management related complications, altered sensation and chronic limb pain[113]. Still, these potential risks are easily overcome when there is a high chance of developing compartment syndrome and early fasciotomies should be considered in cases of revascularization following vascular trauma, multiple fractures or dislocation and /or multiple arterial injuries[21,112].
Ιn addition to the basic complication of the already-mentioned compartment syndrome, bone and soft-tissue infection following combined musculoskeletal and vascular injuries, represents the major cause for late extremity amputation[114-117]. Gustilo et al[118], classifying open fractures reported an infection rate as high as 42% associated with vascular damage requiring repair (Gustilo IIIC fractures). Moreover, infection seems also being related with vascular thrombosis[119].
Vascular compromise, which often indicates the release of high energy in the fracture area, is a well-established predictive indicator for a nonunion in Gustilo–Anderson grade IIIC injuries[120]. Moreover, the coexistence of even a subclinical compartment syndrome appears to delay or prevent the process of long bones union[121].
Secondary amputations can be expected in 5.5%-28% of Gustilo type IIIC open tibial fractures[122,123]. The main reasons for secondary amputation include delayed revascularization, lack of adequate collateral blood flow, extensive soft tissue necrosis, infection, distal thrombosis, or postoperative arterial disruption[124].
There is increasing evidence that primary or secondary venous insufficiency, particularly in the setting of combined peripheral arterial and venous injuries, may impair the chance of limb salvage. A proposed benefit to venous patency is that maintaining adequate venous drainage of an injured extremity can minimize extremity swelling, hemorrhage, and compartment syndrome. Feliciano argued that these acute benefits are an indication for immediate venous reconstruction, even if the fate of the repair will likely be to thrombose later[102]. Other local vascular complications such as arterial disruption, pseudoaneurysm and emboli may result in a vicious circle of both regional and systemic adverse effects, like myocardial infarction, respiratory distress due to pulmonary embolism, acute renal failure, etc[125].
Even after a successful initial, multidisciplinary-guided treatment of a combined musculoskeletal and vascular injury, long term follow-up is crucial to prevent and detect early possible complications. This follow-up evaluation is performed by both orthopedic and vascular surgeons, with additional consultation from reconstructive surgeons, if necessary[126]. Alternatively, orthopedic surgeons with microsurgical background and competence can follow these patients, as they can solely perform all-around surgery for these combined injuries. Postoperative evaluations can be delayed in cases of segmental fractures, associated bone loss, or infection. In case of team management, the vascular surgeon will evaluate the arterial perfusion and venous flow and determine the shift from prophylactic thromboprophylaxis to antiplatelet agents 3-6 mo postoperatively. The vascular repair should be followed with ABI testing as well as graft duplex scan.
In addition, the continuous cooperation with the radiologists, physiatrists and physiotherapists at the follow-up period ensures rapid recovery without serious complications, or their detection and management in a timely manner. Finally, due to the functional impairment frequently left after these complex injuries, long-term psychological support is often necessary.
Limb salvage in patients with combined musculoskeletal and vascular injuries is distinctly dependent on the severity of soft-tissue injury, the duration of limb ischemia and the early, accurate diagnosis and treatment of vascular damage. In general, the amount of energy transferred on tissues during an injury increases proportionally the possibility of vascular insult[26]. In patients with severe crush injuries associated with extensive bone (segmental shaft fracture or floating joint) and soft-tissue damage, the index of suspicion for associated arterial injury must be high. Closed diaphyseal long bone fractures carry a reported risk of vascular injury of 0.1% but open fractures have a 3.6-fold increase in the chance of vascular injury[27]. It is therefore clear that the basic principle of early detection of a potentially catastrophic vascular lesion in orthopedic trauma must be secured in advance with the high degree of suspicion on the part of the surgeon.
The level of alertness is always determined by (1) The pattern of the injury (open or closed, fracture or dislocation, single or multiple injury, calibration of injury scores); (2) The anatomical area of the injury (open tibial fractures, knee dislocations and supracondylar elbow fractures in children of type III due to Gartland classification have rates of concomitant vascular injuries > 10%)[21,24,26,28,29]; and (3) The type of arterial lesion that the literature correlates with specific trauma patterns. Vascular injuries that are nonocclusive, like arterial spasm, intramural hematomas, or intimal flaps have a satisfactory healing potential with nonoperative treatment, with reported rates of 87%-95%. Therapeutic choices usually consist in monitoring the patient during follow-up, by means of physical examination and ultrasound scan. Single vessel occlusion located peripherally – distal to knee/elbow – does not constitute a risk to the viability of the limb. In these cases, in the absence of mangled extremity or severe soft tissue damage, observation with close clinical and imaging surveillance is a viable option[85,86].
A fundamental dimension of the successful outcome of combined orthopedic and vascular injuries is the speed and accuracy of patient management. Most of the time lost in the treatment approach of the injured patient, after the arrival to the trauma center, concerns the definite diagnosis of the vascular injury and the decision-making procedure. Orthopedic pathology is defined in the majority of cases with a simple X-ray (usually already done in primary or secondary survey). The presence of hard signs has 92%-95% sensitivity for incidence of vascular injuries requiring surgical intervention with a positive predictive value of 95%[33-35]. In these cases, immediate operative intervention – injury site exploration in the presence of any hard sign – is strongly suggested by the literature without further delay for additional advanced vascular imaging (CT or conventional angiography)[36,37]. Rapid decisions are ensured by the use of trauma classifications which assist the surgeons’ judgment in adjunct to clinical evaluation and facilitate in-depth guidance of patients and their families prior to definitive surgical treatment. Predictive factors for complications and poor outcomes in patients with combined musculoskeletal and vascular injuries are evaluated by variable injury scoring systems (MESS, NISSSA, LSI, PSI and HFS)[69,70].
It is also necessary in the future to establish common action protocols between orthopedic, vascular and general surgeons, and for each tertiary trauma center to have a clearly defined action protocol that will be applied in the same way each time and will be included in the training program of young doctors.
As a general rule, in cases with cold ischemia (pulseless limb with no capillary refill) and in patients with prolonged period of warm ischemia (present capillary refill), vascular intervention should be performed first so as to restore perfusion as soon as possible via temporary intraluminal vascular shunt insertion. Fracture fixation can be performed first when there is no evidence of cold or prolonged warm ischemia, especially in concomitant presence of unstable, comminuted fracture pattern. Failure to perform fasciotomies after revascu larization of an acutely ischemic limb is the most common cause of preventable limb loss. Finally, a bed in the intensive care unit ideally should be reserved for early postoperative monitoring. Although open surgical repair has always been the gold standard for treating vascular trauma, the application of endovascular techniques should be a more beneficial future perspective according to specific indications.
Although vascular injuries are rare, they may occur in the context of major combined musculoskeletal trauma. The high index of suspicion, the imaging evaluation, and the timely referral of these patients to organized trauma centers ensure firstly the survival of the patient and the extremity and secondarily the best functional outcome in such challenging cases. Vascular injuries as part of musculoskeletal trauma are usually the result of the release of a high-energy load at the wound site so that the prognosis is strongly determined by the degree of soft-tissue damage, the duration of limb ischemia, the patients’ general medical status and the presence of associated injuries. We therefore consider that distinct guidelines and multidisciplinary team management are sine qua non preconditions in dealing with combined musculoskeletal and vascular injuries of the extremities.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salvi M, Italy S-Editor: Liu JH L-Editor: Kerr C P-Editor: Liu JH
| 1. | Mullis B, Fajardo A, Smith T, Laughlin M. Team Approach: Combined Orthopaedic and Vascular Injury. JBJS Rev. 2017;5:e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Rasmussen TE, Woodson J, Rich NM, Mattox KL. Vascular trauma at a crossroads. J Trauma. 2011;70:1291-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Rich NM, Mattox KL, Hirshberg A. Vascular Trauma. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2004. |
| 4. | Lambert R. surgeon at Newcastle upon Tyne, to Dr. Hunter; giving an account of a new method of treating an aneurysm. Med Observ Inq London. 1762;2:360. |
| 5. | Murphy JB. Resection of arteries and veins injured in continuity-end-to-end of suture-experimental clinical research. Med Rec. 1897;51:73. |
| 6. | Carrel A. The surgery of blood vessels. Bull Johns Hopkins Hosp. 1907;18:18-28. |
| 7. | Guthrie C. Blood Vessel Surgery and Its Applications. London, United Kingdom: Edward Arnold; 1912. |
| 8. | Rich NM, Rhee P. An historical tour of vascular injury management: from its inception to the new millennium. Surg Clin North Am. 2001;81:1199-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Esmarch F. The Surgeons Handbook of the Treatment of the Wounded in War. New York, NY: LW Schmidt; 1878. |
| 10. | Rich NM. Vascular trauma historical notes. Perspect Vasc Surg Endovasc Ther. 2011;23:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Debakey ME, Simeone FA. Battle Injuries of the Arteries in World War II : An Analysis of 2,471 Cases. Ann Surg. 1946;123:534-579. [PubMed] |
| 13. | Hughes CW. The primary repair of wounds of major arteries; an analysis of experience in Korea in 1953. Ann Surg. 1955;141:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | McNamara JH, Brief DK, Beasley W, Wright JK. Vascular injury in Vietnam combat casualties: results of treatment at the 24th Evacuation Hospital 1 July 1967 to 12 August 1969. Ann Surg. 1973;178:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Rich NM, Hughes CW. Vietnam vascular registry: a preliminary report. Surgery. 1969;65:218-226. [PubMed] |
| 16. | Chambers LW, Rhee P, Baker BC, Perciballi J, Cubano M, Compeggie M, Nace M, Bohman HR. Initial experience of US Marine Corps forward resuscitative surgical system during Operation Iraqi Freedom. Arch Surg. 2005;140:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Rotondo MF, Zonies DH. The damage control sequence and underlying logic. Surg Clin North Am. 1997;77:761-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 263] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Kragh JF Jr, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, Holcomb JB. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Ann Surg. 2009;249:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 378] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 19. | Martin MJ, Long WB. Vascular trauma: epidemiology and natural history. In: Cronenwett JL, Johnston KW, eds. Rutherford’s Vascular Surgery. Vol 2. 8th ed. Philadelphia, PA: Saunders; 2014: 2422-2437. |
| 20. | Feliciano DV. For the patient-Evolution in the management of vascular trauma. J Trauma Acute Care Surg. 2017;83:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Mavrogenis AF, Panagopoulos GN, Kokkalis ZT, Koulouvaris P, Megaloikonomos PD, Igoumenou V, Mantas G, Moulakakis KG, Sfyroeras GS, Lazaris A, Soucacos PN. Vascular Injury in Orthopedic Trauma. Orthopedics. 2016;39:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Xu Y, Xu W, Wang A, Meng H, Wang Y, Liu S, Li R, Lu S, Peng J. Diagnosis and treatment of traumatic vascular injury of limbs in military and emergency medicine: A systematic review. Medicine (Baltimore). 2019;98:e15406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 23. | Beranger F, Lesquen H, Aoun O, Roqueplo C, Meyrat L, Natale C, Avaro JP. Management of war-related vascular wounds in French role 3 hospital during the Afghan campaign. Injury. 2017;48:1906-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Feliciano DV, Rasmussen TE. Evaluation and treatment of vascular injuries. In: Browner BD, Jupiter JB, Kretteck C, eds. Skeletal Trauma. Basic Science, Management and Reconstruction. Philadelphia: Elsevier Saunders; 2015: 423e435. |
| 25. | Barmparas G, Inaba K, Talving P, David JS, Lam L, Plurad D, Green D, Demetriades D. Pediatric vs adult vascular trauma: a National Trauma Databank review. J Pediatr Surg. 2010;45:1404-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Halvorson JJ, Anz A, Langfitt M, Deonanan JK, Scott A, Teasdall RD, Carroll EA. Vascular injury associated with extremity trauma: initial diagnosis and management. J Am Acad Orthop Surg. 2011;19:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Monazzam S, Goodell PB, Salcedo ES, Nelson SH, Wolinsky PR. When are CT angiograms indicated for patients with lower extremity fractures? J Trauma Acute Care Surg. 2017;82:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Soni A, Tzafetta K, Knight S, Giannoudis PV. Gustilo IIIC fractures in the lower limb: our 15-year experience. J Bone Joint Surg Br. 2012;94:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Brahmamdam P, Plummer M, Modrall JG, Megison SM, Clagett GP, Valentine RJ. Hand ischemia associated with elbow trauma in children. J Vasc Surg. 2011;54:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | American College of Surgeons. ATLS: Advanced Trauma Life Support for Doctors.8th ed. Chicago, IL: American College of Surgeons, 2008. |
| 31. | Rozycki GS, Tremblay LN, Feliciano DV, McClelland WB. Blunt vascular trauma in the extremity: diagnosis, management, and outcome. J Trauma. 2003;55:814-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Barnes CJ, Pietrobon R, Higgins LD. Does the pulse examination in patients with traumatic knee dislocation predict a surgical arterial injury? J Trauma. 2002;53:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Manthey DE, Nicks BA. Penetrating trauma to the extremity. J Emerg Med. 2008;34:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Gonzalez RP, Falimirski ME. The utility of physical examination in proximity penetrating extremity trauma. Am Surg. 1999;65:784-789. [PubMed] |
| 35. | Inaba K, Branco BC, Reddy S, Park JJ, Green D, Plurad D, Talving P, Lam L, Demetriades D. Prospective evaluation of multidetector computed tomography for extremity vascular trauma. J Trauma. 2011;70:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | US. Army Institute of Surgical Research. Joint Trauma System clinical practice guidelines. Accessed May 11, 2015. Available from: http://www.usaisr.amedd.army.mil/cpgs.html. |
| 37. | Fox CJ, Starnes BW. Vascular surgery on the modern battlefield. Surg Clin North Am. 2007;87:1193-1211, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Miranda FE, Dennis JW, Veldenz HC, Dovgan PS, Frykberg ER. Confirmation of the safety and accuracy of physical examination in the evaluation of knee dislocation for injury of the popliteal artery: a prospective study. J Trauma. 2002;52:247-51; discussion 251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Johansen K, Lynch K, Paun M, Copass M. Non-invasive vascular tests reliably exclude occult arterial trauma in injured extremities. J Trauma. 1991;31:515-9; discussion 519. [PubMed] |
| 40. | Modrall JG, Weaver FA, Yellin AE. Vascular considerations in extremity trauma. Orthop Clin North Am. 1993;24:557-563. [PubMed] |
| 41. | Lynch K, Johansen K. Can Doppler pressure measurement replace "exclusion" arteriography in the diagnosis of occult extremity arterial trauma? Ann Surg. 1991;214:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 97] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Mills WJ, Barei DP, McNair P. The value of the ankle-brachial index for diagnosing arterial injury after knee dislocation: a prospective study. J Trauma. 2004;56:1261-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Hemingway J, Adjei E, Desikan S, Gross J, Tran N, Singh N, Starnes B, Quiroga E. Lowering the Ankle-Brachial Index Threshold in Blunt Lower Extremity Trauma May Prevent Unnecessary Imaging. Ann Vasc Surg. 2020;62:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Gherman D, Dumitrescu CI, Ciocan A, Melincovici CS. Histopathological changes in major amputations due to diabetic foot - a review. Rom J Morphol Embryol. 2018;59:699-702. [PubMed] |
| 45. | Ugwu E, Anyanwu A, Olamoyegun M. Ankle brachial index as a surrogate to vascular imaging in evaluation of peripheral artery disease in patients with type 2 diabetes. BMC Cardiovasc Disord. 2021;21:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Gable DR, Allen JW, Richardson JD. Blunt popliteal artery injury: is physical examination alone enough for evaluation? J Trauma. 1997;43:541-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Miller-Thomas MM, West OC, Cohen AM. Diagnosing traumatic arterial injury in the extremities with CT angiography: pearls and pitfalls. Radiographics. 2005;25 Suppl 1:S133-S142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Itani KM, Burch JM, Spjut-Patrinely V, Richardson R, Martin RR, Mattox KL. Emergency center arteriography. J Trauma. 1992;32:302-6; discussion 306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Kauvar DS, Kraiss LW. Vascular trauma: extremity. In: Cronenwett JL, Johnston KW, eds. Rutherford’s Vascular Surgery. Vol 2. 8th ed. Philadelphia, PA: Saunders; 2014: 2485-2500. |
| 50. | Gagne PJ, Cone JB, McFarland D, Troillett R, Bitzer LG, Vitti MJ, Eidt JF. Proximity penetrating extremity trauma: the role of duplex ultrasound in the detection of occult venous injuries. J Trauma. 1995;39:1157-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Knudson MM, Lewis FR, Atkinson K, Neuhaus A. The role of duplex ultrasound arterial imaging in patients with penetrating extremity trauma. Arch Surg. 1993;128:1033-7; discussion 1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Schwartz M, Weaver F, Yellin A, Ralls P. The utility of color flow Doppler examination in penetrating extremity arterial trauma. Am Surg. 1993;59:375-378. [PubMed] |
| 53. | Cellina M, Gibelli D, Martinenghi C, Oliva G, Floridi C. CT angiography of lower extremities from anatomy to traumatic and nontraumatic lesions: a pictorial review. Emerg Radiol. 2020;27:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Fleiter TR, Mervis S. The role of 3D-CTA in the assessment of peripheral vascular lesions in trauma patients. Eur J Radiol. 2007;64:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Seamon MJ, Smoger D, Torres DM, Pathak AS, Gaughan JP, Santora TA, Cohen G, Goldberg AJ. A prospective validation of a current practice: the detection of extremity vascular injury with CT angiography. J Trauma. 2009;67:238-43; discussion 243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Rieger M, Mallouhi A, Tauscher T, Lutz M, Jaschke WR. Traumatic arterial injuries of the extremities: initial evaluation with MDCT angiography. AJR Am J Roentgenol. 2006;186:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | White PW, Gillespie DL, Feurstein I, Aidinian G, Phinney S, Cox MW, Adams E, Fox CJ. Sixty-four slice multidetector computed tomographic angiography in the evaluation of vascular trauma. J Trauma. 2010;68:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Compton C, Rhee R. Peripheral vascular trauma. Perspect Vasc Surg Endovasc Ther. 2005;17:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Patterson BO, Holt PJ, Cleanthis M, Tai N, Carrell T, Loosemore TM; London Vascular Injuries Working Group. Imaging vascular trauma. Br J Surg. 2012;99:494-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 60. | Polytrauma Guideline Update Group. Level 3 guideline on the treatment of patients with severe/multiple injuries: AWMF Register-Nr. 012/019. Eur J Trauma Emerg Surg. 2018;44:3-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 61. | Llau JV, Acosta FJ, Escolar G, Fernández-Mondéjar E, Guasch E, Marco P, Paniagua P, Páramo JA, Quintana M, Torrabadella P. Multidisciplinary consensus document on the management of massive haemorrhage (HEMOMAS document). Med Intensiva. 2015;39:483-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | Cornero SG, Maegele M, Lefering R, Abbati C, Gupta S, Sammartano F, Cimbanassi S, Chiara O. Predictive Factors for Massive Transfusion in Trauma: A Novel Clinical Score from an Italian Trauma Center and German Trauma Registry. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Welling DR, Burris DG, Hutton JE, Minken SL, Rich NM. A balanced approach to tourniquet use: lessons learned and relearned. J Am Coll Surg. 2006;203:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Feliciano DV. Evaluation and treatment of vascular injuries. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma. Basic Science, Management, and Reconstruction. 4th ed. Philadelphia, PA: Elsevier Saunders; 2009: 323-340. |
| 65. | Feliciano DV, Moore FA, Moore EE, West MA, Davis JW, Cocanour CS, Kozar RA, McIntyre RC Jr. Evaluation and management of peripheral vascular injury. Part 1. Western Trauma Association/critical decisions in trauma. J Trauma. 2011;70:1551-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Korompilias AV, Beris AE, Lykissas MG, Vekris MD, Kontogeorgakos VA, Soucacos PN. The mangled extremity and attempt for limb salvage. J Orthop Surg Res. 2009;4:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Bosse MJ, McCarthy ML, Jones AL, Webb LX, Sims SH, Sanders RW, MacKenzie EJ; Lower Extremity Assessment Project (Leap) Study Group. The insensate foot following severe lower extremity trauma: an indication for amputation? J Bone Joint Surg Am. 2005;87:2601-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | MacKenzie EJ, Bosse MJ, Pollak AN, Webb LX, Swiontkowski MF, Kellam JF, Smith DG, Sanders RW, Jones AL, Starr AJ, McAndrew MP, Patterson BM, Burgess AR, Castillo RC. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87:1801-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Schlickewei W, Kuner EH, Mullaji AB, Götze B. Upper and lower limb fractures with concomitant arterial injury. J Bone Joint Surg Br. 1992;74:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Mullenix PS, Steele SR, Andersen CA, Starnes BW, Salim A, Martin MJ. Limb salvage and outcomes among patients with traumatic popliteal vascular injury: an analysis of the National Trauma Data Bank. J Vasc Surg. 2006;44:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | Johansen K, Daines M, Howey T, Helfet D, Hansen ST Jr. Objective criteria accurately predict amputation following lower extremity trauma. J Trauma. 1990;30:568-72; discussion 572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 374] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 72. | Shanmuganathan R. The utility of scores in the decision to salvage or amputation in severely injured limbs. Indian J Orthop. 2008;42:368-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Trickett RW, Rahman S, Page P, Pallister I. From guidelines to standards of care for open tibial fractures. Ann R Coll Surg Engl. 2015;97:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Jain A, Glass GE, Ahmadi H, Mackey S, Simmons J, Hettiaratchy S, Pearse M, Nanchahal J. Delayed amputation following trauma increases residual lower limb infection. J Plast Reconstr Aesthet Surg. 2013;66:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 75. | MacKenzie EJ, Jones AS, Bosse MJ, Castillo RC, Pollak AN, Webb LX, Swiontkowski MF, Kellam JF, Smith DG, Sanders RW, Jones AL, Starr AJ, McAndrew MP, Patterson BM, Burgess AR. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am. 2007;89:1685-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 76. | Januszkiewicz JS, Mehrotra ON, Brown GE. Calcaneal fillet flap: a new osteocutaneous free tissue transfer for emergency salvage of traumatic below-knee amputation stumps. Plast Reconstr Surg. 1996;98:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | MacKenzie EJ, Bosse MJ, Castillo RC, Smith DG, Webb LX, Kellam JF, Burgess AR, Swiontkowski MF, Sanders RW, Jones AL, McAndrew MP, Patterson BM, Travison TG, McCarthy ML. Functional outcomes following trauma-related lower-extremity amputation. J Bone Joint Surg Am. 2004;86:1636-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 78. | Gonzalez EG, Corcoran PJ, Reyes RL. Energy expenditure in below-knee amputees: correlation with stump length. Arch Phys Med Rehabil. 1974;55:111-119. [PubMed] |
| 79. | Khan MA, Javed AA, Rao DJ, Corner JA, Rosenfield P. Pediatric Traumatic Limb Amputation: The Principles of Management and Optimal Residual Limb Lengths. World J Plast Surg. 2016;5:7-14. [PubMed] |
| 80. | Peng YP, Lahiri A. Spare-part surgery. Semin Plast Surg. 2013;27:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Garcia-Vilariño E, Perez-Garcia A, Salmeron-Gonzalez E, Sanchez-Garcia A, Bas JL, Simon-Sanz E. Avoiding Above-the-Knee Amputation with a Free Tibiofibular-Talocalcaneal Fillet Flap and Free Latissimus Dorsi Flap. Indian J Plast Surg. 2020;53:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Benz D, Balogh ZJ. Damage control surgery: current state and future directions. Curr Opin Crit Care. 2017;23:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 83. | Feliciano DV. Pitfalls in the management of peripheral vascular injuries. Trauma Surg Acute Care Open. 2017;2:e000110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Feliciano DV, Subramanian A. Temporary vascular shunts. Eur J Trauma Emerg Surg. 2013;39:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Frykberg ER, Dennis JW, Bishop K, Laneve L, Alexander RH. The reliability of physical examination in the evaluation of penetrating extremity trauma for vascular injury: results at one year. J Trauma. 1991;31:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 86. | Frykberg ER, Vines FS, Alexander RH. The natural history of clinically occult arterial injuries: a prospective evaluation. J Trauma. 1989;29:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 73] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Simmons JD, Walker WB, Gunter Iii JW, Ahmed N. Role of endovascular grafts in combined vascular and skeletal injuries of the lower extremity: a preliminary report. Arch Trauma Res. 2013;2:40-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Reuben BC, Whitten MG, Sarfati M, Kraiss LW. Increasing use of endovascular therapy in acute arterial injuries: analysis of the National Trauma Data Bank. J Vasc Surg. 2007;46:1222-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 89. | Fowler J, Macintyre N, Rehman S, Gaughan JP, Leslie S. The importance of surgical sequence in the treatment of lower extremity injuries with concomitant vascular injury: A meta-analysis. Injury. 2009;40:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Huynh TT, Pham M, Griffin LW, Villa MA, Przybyla JA, Torres RH, Keyhani K, Safi HJ, Moore FA. Management of distal femoral and popliteal arterial injuries: an update. Am J Surg. 2006;192:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 91. | Tunali O, Saglam Y, Balci HI, Kochai A, Sahbaz NA, Sayin OA, Yazicioglu O. Gustilo type IIIC open tibia fractures with vascular repair: minimum 2-year follow-up. Eur J Trauma Emerg Surg. 2017;43:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Wyrzykowski AD, Feliciano DV. Trauma damage control. In: Feliciano DV, Mattox KL, Moore EE, eds. Trauma. 6th ed. New York, NY: McGraw-Hill; 2008: 851-870. |
| 93. | Glass GE, Pearse MF, Nanchahal J. Improving lower limb salvage following fractures with vascular injury: a systematic review and new management algorithm. J Plast Reconstr Aesthet Surg. 2009;62:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 94. | Allen MJ, Nash JR, Ioannidies TT, Bell PR. Major vascular injuries associated with orthopaedic injuries to the lower limb. Ann R Coll Surg Engl. 1984;66:101-104. [PubMed] |
| 95. | Howard PW, Makin GS. Lower limb fractures with associated vascular injury. J Bone Joint Surg Br. 1990;72:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Lasanianos NG, Kanakaris NK, Dimitriou R, Pape HC, Giannoudis PV. Second hit phenomenon: existing evidence of clinical implications. Injury. 2011;42:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 97. | Grubor P, Milicevic S, Grubor M, Meccariello L. Treatment of Bone Defects in War Wounds: Retrospective Study. Med Arch. 2015;69:260-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41:27-37; table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 548] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 99. | Olesen UK, Eckardt H, Bosemark P, Paulsen AW, Dahl B, Hede A. The Masquelet technique of induced membrane for healing of bone defects. A review of 8 cases. Injury. 2015;46 Suppl 8:S44-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 100. | Feliciano DV, Moore EE, West MA, Moore FA, Davis JW, Cocanour CS, Scalea TM, McIntyre RC Jr. Western Trauma Association critical decisions in trauma: evaluation and management of peripheral vascular injury, part II. J Trauma Acute Care Surg. 2013;75:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 101. | Feliciano DV. Heroic procedures in vascular injury management: the role of extra-anatomic bypasses. Surg Clin North Am. 2002;82:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 102. | Feliciano DV, Mattox KL, Graham JM, Bitondo CG. Five-year experience with PTFE grafts in vascular wounds. J Trauma. 1985;25:71-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 143] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 103. | Subramanian A, Vercruysse G, Dente C, Wyrzykowski A, King E, Feliciano DV. A decade's experience with temporary intravascular shunts at a civilian level I trauma center. J Trauma. 2008;65:316-24; discussion 324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 104. | Inaba K, Aksoy H, Seamon MJ, Marks JA, Duchesne J, Schroll R, Fox CJ, Pieracci FM, Moore EE, Joseph B, Haider AA, Harvin JA, Lawless RA, Cannon J, Holland SR, Demetriades D; Multicenter Shunt Study Group. Multicenter evaluation of temporary intravascular shunt use in vascular trauma. J Trauma Acute Care Surg. 2016;80:359-64; discussion 364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 105. | Parry NG, Feliciano DV, Burke RM, Cava RA, Nicholas JM, Dente CJ, Rozycki GS. Management and short-term patency of lower extremity venous injuries with various repairs. Am J Surg. 2003;186:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Strinden WD, Dibbell DG Sr, Turnipseed WD, Acher CW, Rao VK, Mixter RC. Coverage of acute vascular injuries of the axilla and groin with transposition muscle flaps: case reports. J Trauma. 1989;29:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 107. | Borgquist O, Ingemansson R, Malmsjö M. Wound edge microvascular blood flow during negative-pressure wound therapy: examining the effects of pressures from -10 to -175 mmHg. Plast Reconstr Surg. 2010;125:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 108. | Tahir M, Chaudhry EA, Zimri FK, Ahmed N, Shaikh SA, Khan S, Choudry UK, Aziz A, Jamali AR. [RETRACTED] Negative pressure wound therapy versus conventional dressing for open fractures in lower extremity trauma. Bone Joint J. 2020;102-B:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Costa ML, Achten J, Bruce J, Tutton E, Petrou S, Lamb SE, Parsons NR; UK WOLLF Collaboration. Effect of Negative Pressure Wound Therapy vs Standard Wound Management on 12-Month Disability Among Adults With Severe Open Fracture of the Lower Limb: The WOLLF Randomized Clinical Trial. JAMA. 2018;319:2280-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 110. | British Orthopaedic Association. BOAST 10: diagnosis and management of compartment syndrome of the limbs. Accessed May 20, 2015. Available from: http://www.boa.ac.uk/wp-content/uploads/2015/01/BOAST-10.pdf. |
| 111. | Branco BC, Inaba K, Barmparas G, Schnüriger B, Lustenberger T, Talving P, Lam L, Demetriades D. Incidence and predictors for the need for fasciotomy after extremity trauma: a 10-year review in a mature level I trauma centre. Injury. 2011;42:1157-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 112. | Farber A, Tan TW, Hamburg NM, Kalish JA, Joglar F, Onigman T, Rybin D, Doros G, Eberhardt RT. Early fasciotomy in patients with extremity vascular injury is associated with decreased risk of adverse limb outcomes: a review of the National Trauma Data Bank. Injury. 2012;43:1486-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 113. | Fitzgerald AM, Gaston P, Wilson Y, Quaba A, McQueen MM. Long-term sequelae of fasciotomy wounds. Br J Plast Surg. 2000;53:690-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 114. | Thakore RV, Francois EL, Nwosu SK, Attum B, Whiting PS, Siuta MA, Benvenuti MA, Smith AK, Shen MS, Mousavi I, Obremskey WT, Sethi MK. The Gustilo-Anderson classification system as predictor of nonunion and infection in open tibia fractures. Eur J Trauma Emerg Surg. 2017;43:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 115. | Arnež ZM, Papa G, Ramella V, Novati FC, Ahcan U, Stocco C. Limb and Flap Salvage in Gustilo IIIC Injuries Treated by Vascular Repair and Emergency Free Flap Transfer. J Reconstr Microsurg. 2017;33:S03-S07. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 116. | Warkentien TE, Lewandowski LR, Potter BK, Petfield JL, Stinner DJ, Krauss M, Murray CK, Tribble DR; Trauma Infectious Disease Outcomes Study Group. Osteomyelitis Risk Factors Related to Combat Trauma Open Upper Extremity Fractures: A Case-Control Analysis. J Orthop Trauma. 2019;33:e475-e483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 117. | Wang G, Tang Y, Wu X, Yang H. Masquelet technique combined with microsurgical technique for treatment of Gustilo IIIC open distal tibial fractures: a retrospective single-center cohort study. J Int Med Res. 2020;48:300060520910024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 118. | Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1593] [Cited by in RCA: 1419] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 119. | Brown KV, Ramasamy A, Tai N, MacLeod J, Midwinter M, Clasper JC. Complications of extremity vascular injuries in conflict. J Trauma. 2009;66:S145-S149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 120. | Tian R, Zheng F, Zhao W, Zhang Y, Yuan J, Zhang B, Li L. Prevalence and influencing factors of nonunion in patients with tibial fracture: systematic review and meta-analysis. J Orthop Surg Res. 2020;15:377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 121. | Reverte MM, Dimitriou R, Kanakaris NK, Giannoudis PV. What is the effect of compartment syndrome and fasciotomies on fracture healing in tibial fractures? Injury. 2011;42:1402-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 122. | Seligson D, Ostermann PA, Henry SL, Wolley T. The management of open fractures associated with arterial injury requiring vascular repair. J Trauma. 1994;37:938-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 123. | Alexander JJ, Piotrowski JJ, Graham D, Franceschi D, King T. Outcome of complex vascular and orthopedic injuries of the lower extremity. Am J Surg. 1991;162:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 124. | McNutt R, Seabrook GR, Schmitt DD, Aprahamian C, Bandyk DF, Towne JB. Blunt tibial artery trauma: predicting the irretrievable extremity. J Trauma. 1989;29:1624-1627. [PubMed] |
| 125. | Martin MJ, Perez-Alonso AJ, Asensio JA. Vascular complications and special problems in vascular trauma. Eur J Trauma Emerg Surg. 2013;39:569-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 126. | Boriani F, Ul Haq A, Baldini T, Urso R, Granchi D, Baldini N, Tigani D, Tarar M, Khan U. Orthoplastic surgical collaboration is required to optimise the treatment of severe limb injuries: A multi-centre, prospective cohort study. J Plast Reconstr Aesthet Surg. 2017;70:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |