INTRODUCTION
Acute septic arthritis in children is an orthopaedic emergency. Since the clinical presentation can be similar to other joint pathologies, acute septic arthritis is a diagnostic challenge. This is especially true for infants and neonates, in whom refusal to feed, crying and discomfort with limitations of joint movement can be the presenting symptoms. A delay in diagnosis and inappropriate treatment can result in a devastating damage to the joint with lifelong disability as a consequence[1]. According to laboratory evidence, the loss of glycosaminoglycan in cartilage begins within eight hours after the onset of an infection in a joint[2]. An increase in intracapsular pressure in the hip joint, when not promptly decompressed, may lead to compressive ischemia and avascular necrosis of the femoral head[3]. Therefore, it is important to perform an appropriate diagnostic workup and an optimal treatment of this challenging disease. In 2020, a systematic review was published that stated the test characteristics of history, physical examination and laboratory and image investigations in the evaluation for septic arthritis in children presenting with an acute nontraumatic limp[4]. Recently, we published two systematic reviews with a clear overview of the literature on drainage techniques for septic knee and hip arthritis in children[5,6]. In this evidence-based current concept review we therefore provide an update on the diagnostic workup and treatment of paediatric septic arthritis.
BACKGROUND
Epidemiology
The incidence of septic arthritis is two to seven per 100000 children in Europe and three to four per 100000 in the United States of America[7-9]. The highest incident rates are seen among the group of children aged between zero and four years old[9]. Septic arthritis is typically monoarticulair. The most commonly affected joints are the hip (32%-39%) and knee (26%-47%). Other affected joints are ankle (9%-18%), shoulder (2%-12%), elbow (4%-13%) and wrist (1%-2%)[9-14]. Septic arthritis is 1.4 to 1.7 times more common in males than in females[9,10,12].
Bacteriology
Staphylococcus aureus is the most commonly cultured organism. Other common pathogens are Kingella kingae, Streptococcus pyogenes and Streptococcus pneumoniae[10,15,16]. High prevalence of Salmonella infection is seen in patients with septic arthritis from Africa[17,18]. The causative pathogens overall can vary depending on the child’s age, immunodeficiency, socio-economic factors and vaccination status[9]. Kingella kingae is more frequently isolated among children under 36 mo of age in comparison to older children[15]. Before an effective vaccine, Haemophilus influenzae type B was a very common cause of septic hip arthritis. This pathogen is now rarely reported in well-immunized populations[19-21]. Some causative organisms are less common, but are seen in specific groups. Salmonella typhi can be suspected outside Africa in children with sickle cell disease and has been found in immunoincompetent children[22,23]. Pseudomonas aeruginosa is often found after a wound nearby the joint and Pasteurella canis is found most often after animal bites[23,24]. Neisseria gonorrhoeae should be suspected in sexually active adolescents or in cases of sexual abuse[25].
In 2010, Pääkkönen et al[20], showed in septic hip arthritis in children with culture-positive cases that bacteria grew from the synovial fluid only in 34 percent cases, from blood in only 27 percent cases, and from both joint and blood in 39 percent cases.
DIAGNOSIS
Clinical presentation
The classical presentation of septic arthritis in children is a combination of a painful joint with limited range of movement, the inability to bear weight on the involved limb, fever and malaise[3,26-28]. The symptoms can rapidly progress in hours. At physical examination, effusion, erythema, heat, tenderness to palpation and, in the lower extremities, inability to bear weight can be seen. The affected joint is irritable and is most often held in a position of comfort, one that maximizes intracapsular volume. For example, the hip is flexed, abducted, and externally rotated. A characteristic sign is micromotion tenderness[28]. A recent systematic review showed that the presence of joint tenderness and fever increases the risk of septic arthritis[4]. The presence of fever (≥ 38.5°C) has a positive likelihood ratio (LR) of 2.1 to 18.2. The absence of fever had a negative LR of 0.2 to 0.6. Joint tenderness had a positive LR of 11.4 and a negative LR of 0.3[4].
During infancy, the clinical presentation differs from the presentation in older children. Sepsis is often the first notable presentation of septic arthritis in neonates and infants. The symptoms are comprehensive and include irritability, failure to feed or gain weight and muscular spasm. Also, fever, tachycardia, anaemia and the presence of associated infection are occasionally seen. Involvement of the hip joint must be suspected in any infant with sepsis. The following characteristics at physical examination can be present: pain on palpation or passive movement of the hip, lack of active movement of the leg, asymmetrical buttock creases, unilateral oedema or swelling of an extremity, a buttock or the genitalia[29].
Paediatric septic arthritis can occur several weeks after an upper respiratory infection. In infants and neonates, underlying diseases have been recognized as risk factors for septic arthritis, including respiratory distress syndrome, congenital anomalies and extremely low birth weight[30].
Laboratory studies
The initial laboratory testing for a patient with suspected osteoarticular infection should consist of serum samples with complete blood count, Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and two blood cultures[31,32]. In 1999, Kocher et al[33] identified four predictors that, by combining, had excellent diagnostic performance in differentiating between septic hip arthritis and transient synovitis of the hip in children. These four predictors were a history of fever, non-weight-bearing, an ESR of at least 40 mm/h, and a serum white blood-cell (WBC) count of more than 12000 cells/mm3. Kocher et al[34] concluded that patients with a very high probability of septic arthritis of the hip have three or four positive predictors. They advised that these patients may be good candidates for aspiration in the operating room, given the likelihood that subsequent arthrotomy and drainage will be needed. Patients who have an intermediate probability (two positive predictors) of septic arthritis of the hip may be good candidates for aspiration under ultrasound. Patients who have an extremely low probability (zero or one positive predictors) of septic arthritis of the hip may be appropriate candidates for careful observation without aspiration. After five years, this clinical prediction algorithm was validated in a prospective study[34]. In 2006, Caird et al[35] in a prospective study added an elevated CRP level to the Kocher criteria. They stated that a CRP level of more than 2.0 mg/dL (> 20 mg/L) is a strong independent predictor. A recent systematic review showed that the performances of both clinical risk prediction tools are somewhat lower than originally reported. The predicted probability of septic arthritis for the Kocher criteria ranges from 59.1% to 99.6%; this probability remains similar (60% to 98%) when CRP is added[4].
Imaging
Plain radiographs are the next step in the diagnostic workup of paediatric septic arthritis, mainly to rule out bone changes. Additionally, an increased joint space of the affected septic joint may be visualized on radiographs. In case of suspected hip arthritis, an anteroposterior pelvic radiograph allows assessment of the joint space compared to the contralateral hip.
Both ultrasound and magnetic resonance imaging (MRI) are good non-invasive diagnostic tools without radiation exposure in the evaluation of septic arthritis. Ultrasound is an easily implicated diagnostic tool for detecting the presence of a joint effusion[3]. Joint effusion on ultrasound is seen in 91 percent of patients with septic arthritis[36]. However, it cannot distinguish between sterile, purulent, and hemo-rrhagic fluid accumulations[37]. The data from a negative ultrasound in children with less than 24 h of symptoms should be used with caution and must be interpreted along with a careful history and physical examination[38]. An advantage of ultrasound is that no sedation is required in young children. Furthermore, ultrasound is more sensitive in detecting joint effusion and synovial swelling in children with septic arthritis compared to radiography and MRI[4,36]. One drawback of ultrasound is that it can be user-dependent. In addition, it does not necessarily rule out osteomyelitis or nearby intramuscular abscesses.
Although costly, MRI is the most reliable imaging study for detecting bone and periosteal changes in patients with concomitant osteomyelitis[36,39]. Also, MRI can be used to distinguish septic arthritis of the hip from a psoas abscess and help identify adjacent infection sites. However, in young children sedation in often needed. Although, after the MRI there is a possibility to go straight to the operation room under continuous sedation for a drainage procedure. Recently, an algorithm has been proposed to help identify patients at risk for adjacent infections who would benefit from MRI to identify additional sites of infection. This algorithm contains five variables: older than 3.6 years, CRP > 13.8 mg/L, duration of symptoms > 3 d, platelets < 314 × 10 cells per µL (microliter), and absolute neutrophil count > 8.6 × 10 cells per µL. Patients with three or more risk factors are classified as high risk for having an adjacent infection and would benefit from MRI[40].
Microbiology testing
Synovial fluid analysis by aspiration is an important part of the diagnostic workup when septic arthritis is suspected. Synovial fluid should be sent for white blood cell count, gram stain, culture and antibiotic sensitivity. The diagnosis of acute septic arthritis is highly suggestive when pus is aspirated from the joint, when there is a positive culture of the joint fluid, a positive gram stain of the joint fluid or a WBC count in the joint fluid of > 50000/mm3. Despite appropriate cultures, a notable proportion remains culture negative. Polymerase chain reaction testing of synovial fluid for Kingella kingae (generally seen in children younger than 36 mo of age) and other fastidious pathogens increases detection, particularly in patients who received antibiotics before synovial fluid sampling[41,42].
DIFFERENTIAL DIAGNOSIS
It is important to consider several diseases in the differential diagnosis of septic arthritis[43].
The differentiation between septic hip arthritis and transient synovitis, also known as coxitis fugax, can be difficult because both conditions often present with similarities. Transient synovitis presents as an atraumatic, acutely irritable hip in a child who has progressive symptoms, often sub febrile temperature and refuses to bear weight. Transient synovitis is a self-limiting disorder that is managed nonoperatively and without antibiotics. It typically occurs in children between the ages of three to eight years, with a mean age at presentation of five to six years[44,45]. Most children have symptoms for less than a week at the time of presentation. However, in a retrospective review in 1986, 12 percent of patients had discomfort dating back at least one month[45]. The Kocher criteria can help differentiate between septic arthritis and transient synovitis[33,34]. A transient synovitis is plausible when zero predictors are found.
Juvenile idiopathic arthritis is usually polyarticular and often has gradual onset of symptoms. The first peak is between two to five years of age and the second is between 10 to 14 years of age. Joints are warm and markedly swollen, but not especially painful. The symptoms tend to be worst upon rising in the morning. Joint involvement is generally symmetric and most frequently affected locations are the knees, wrists and ankles. The hip is rarely the initial joint. Children with systematic onset of juvenile idiopathic arthritis and intermittent fever, often have a skin rash[46].
Lyme arthritis needs to be considered in lyme disease endemic areas. About 90 percent of children with Lyme disease present with erythema migrans, which is an early stage of the disease[47]. In six percent an arthritis can present, but arthritis is the most common manifestation of late Lyme disease. Monoarthritis of the knee is most common, but Lyme arthritis may also cause an asymmetric oligoarthritis. The affected joint is usually swollen and may be tender, but the pain is less intense and the range of motion greater as compared to bacterial arthritis. Besides, fever is uncommon[48,49].
In addition to clinical presentation and laboratory studies, plain radiographs should eliminate fracture and other structural diagnoses. For example, in children with pain in the hip or knee joint, plain radiographs are used to exclude slipped capital femoral epiphysis and Legg-Calvé-Perthes disease. Legg-Calvé-Perthes is a syndrome of idiopathic osteonecrosis (avascular necrosis) of the hip. It typically presents as hip pain and/or limp of acute or insidious onset in children between the ages of 3 to 12 years of age, with a peak incidence between five to seven years of age[50]. Stress fractures are rarely seen in children, but they can occur in athletes engaged in endurance sports. Sometimes the radiographs of Legg-Calvé-Perthes and stress fractures are negative and MRI is needed to confirm the diagnosis.
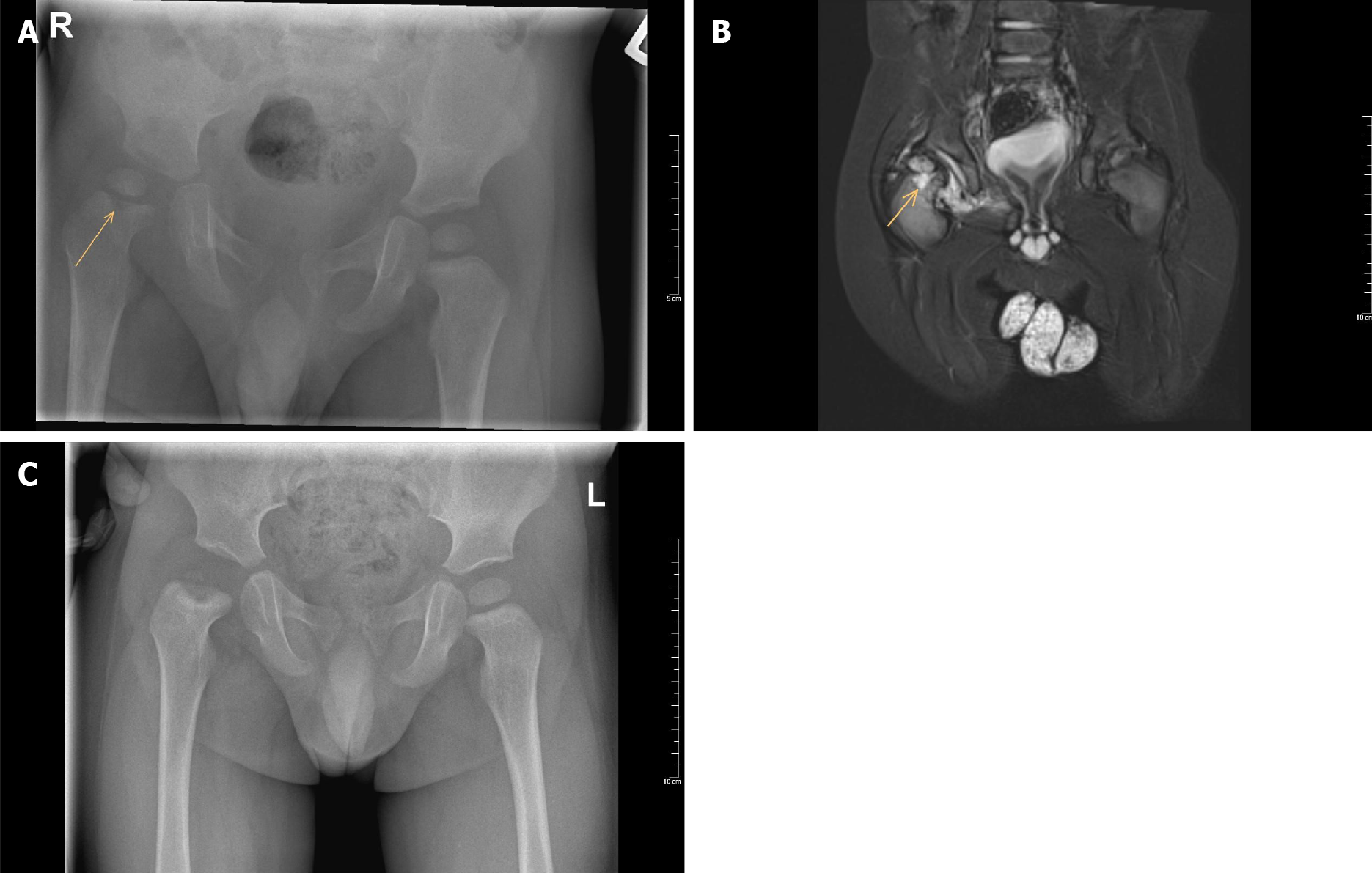
An MRI can also be used when osteomyelitis, pyomyositis, subperiosteal abscess, cellulitis, intramuscular abscess, or tumour are still in the differential diagnosis. MRI is the gold standard imaging technique for osteomyelitis[51]. The tibia and femur are the most commonly affected bones in children with osteomyelitis. A systematic review showed that the clinical features of osteomyelitis include fever (60%), localized pain (70%), reduced range of movement (50%) and reduced weight-bearing (50%)[51]. In contrast to isolated septic arthritis, the child with osteomyelitis usually allows some joint movement and pain-free range of motion with gentle examination. Osteomyelitis can occur next to septic arthritis (Figure 1).
Figure 1 Radiograph images.
A: Plain anteroposterior pelvic radiograph of a one-year-old boy with septic hip arthritis showing concomitant osteomyelitis of the proximal femur at the right side (arrow); B: T2 magnetic resonance imaging coronal view confirms joint effusion, suggestive of hip arthritis, and increased signal of the metaphysis, suggestive of osteomyelitis (arrow); C: Plain anteroposterior radiograph of the same boy after six months follow-up, which shows avascular necrosis of the femoral head.
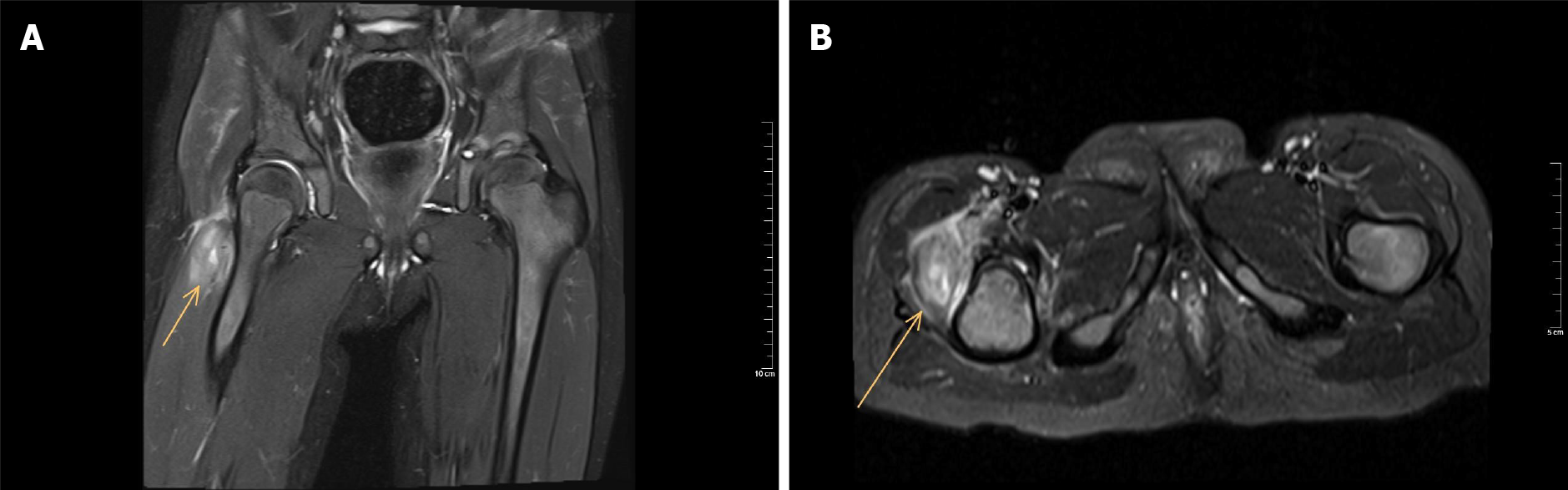
Pyomyositis is a purulent infection of skeletal muscle that arises from haematogenous spread[52]. It commonly manifests as a local abscess but may also present as a diffuse inflammatory or a rapidly progressing myonecrotic process. The quadriceps, gluteal, and iliopsoas muscles are the most commonly affected anatomic sites[53]. It is classically an infection of the tropics (Africa and the South Pacific), although it has been recognized in temperate climates. Trauma has been postulated as a predisposing factor for pyomyositis. Pyomyositis presents with fever and pain with cramping localized to a single muscle group. On physical examination, exquisite muscle tenderness, oedema, and/or fluctuance of the involved muscle group may be present. MRI is the optimal imaging technique, because it is highly sensitive for muscle inflammation (Figure 2)[53].
Figure 2 T2 magnetic resonance imaging of a three-year-old girl with pyomyositis of the vastus lateralis at the right side (arrow).
There is no excessive fluid in the hip joint space. A: Coronal view; B: Sagittal view.
TREATMENT AND FOLLOW-UP
Drainage procedures
Paediatric septic arthritis can be treated by arthrocentesis (articular needle aspiration) with or without irrigation, arthroscopy or arthrotomy. All procedures are followed by antibiotics. Each of the drainage techniques have advantages and disadvantages within the different joints. Arthrocentesis, usually ultrasound-guided, has the advantage of a minimally invasive and short procedure. Generally, this can be used as a first procedure in different joints. However, in the very young, arthrocentesis requires an anaesthetic. Arthrocentesis without anaesthesia or sedation can be an anxiety-producing and painful experience. Advantages of arthroscopy include direct visualization of the joint, the ability to perform a complete debridement of the necrotic synovium and a thorough irrigation of the joint with minimal operative morbidity[54,55]. An arthrotomy gives a good overview of the joint and allows a thorough irrigation, but a disadvantage is a larger incision with more scar tissue. The anterior approach is the most mentioned approach for arthrotomy in paediatric septic hip arthritis[6].
Recent systematic reviews showed a clear overview of the literature on drainage techniques for septic knee and hip arthritis in children[5,6]. It was concluded that knee arthroscopy might have a lower risk of additional drainage procedures as compared to arthrocentesis and arthrotomy in paediatric septic knee arthritis[5]. In septic hip arthritis, arthrocentesis and arthroscopic procedures may have a higher risk of additional drainage procedures in comparison with arthrotomy. Nonetheless, arthrotomy in septic hip arthritis might be associated with inferior outcomes on the long term[6]. However, the studies about the optimal drainage procedure of the several joints were diverse and the scientific quality was generally low[5,6].
Antibiotics
Antibiotic coverage should start in suspected cases as soon as cultures and synovial fluid samples are collected and the joint has been drained, unless the patient is septic[26,27]. Most surgeons agree that preoperative antibiotics should be avoided in the management of paediatric septic arthritis, because MacLean et al[56] showed that it leads to additional washouts and complications.
In consultation with the infectious disease team, the patient is transitioned to oral antibiotics after clinical and laboratory improvement, see Figure 3. It has been reported that the treatment with large doses of well-absorbed antimicrobials for 10 d (started intravenously for a few days only) is as effective as a 30 d treatment in children with septic arthritis, provided that the clinical response is good and the CRP level normalizes quickly[10]. However, the ideal duration of treatment has not yet been determined.
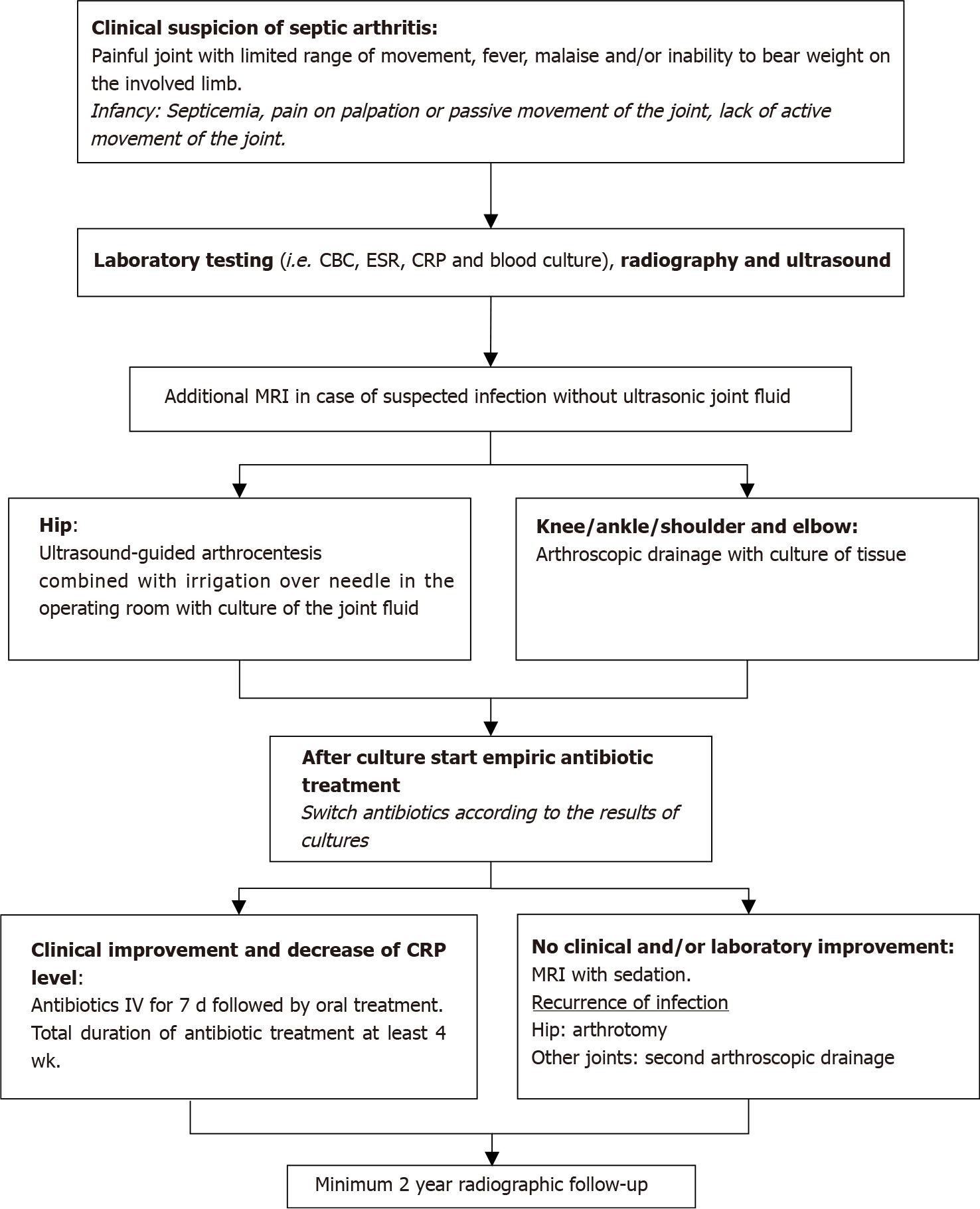
Figure 3 Diagnostic and treatment algorithm for paediatric septic arthritis.
CBC: Complete blood count; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; WBC: White blood cell; MRI: Magnetic resonance imaging; IV: Intravenous.
Follow-up
After the drainage procedure it is important to monitor the clinical and laboratory outcomes. Peltola et al[10] showed in a prospective trial that the CRP level and ESR can increase the first few days after starting the therapy. The highest scores were found on day two and three. A second or third drainage procedure is not exceptional[5,6].
The duration of symptoms between onset and the procedure is negatively associated with the prognosis, especially in infants and neonates with septic hip arthritis[30]. Septic hip arthritis can lead to serious musculoskeletal sequelae, which include: leg length discrepancy, pathologic hip dislocation, a hip joint surface irregularity, coxa magna or avascular necrosis (Figure 1C)[30]. Close follow-up with radiographic observation of at least two years is recommended.
RECOMMENDATIONS FOR FUTURE RESEARCH
There is a need for clinical risk prediction tools of paediatric septic arthritis to be prospectively validated[4]. Furthermore, the current literature about drainage techniques of paediatric septic arthritis is diverse and the quality is generally low[5,6]. Future prospective studies should ideally endeavour larger numbers of patients, define an established diagnosis of acute septic arthritis, report the delay between the first symptoms and the diagnosis, randomize treatment, and provide adequate follow-up time.