Published online Nov 18, 2022. doi: 10.5312/wjo.v13.i11.1015
Peer-review started: August 26, 2021
First decision: November 17, 2021
Revised: December 1, 2021
Accepted: October 27, 2022
Article in press: October 27, 2022
Published online: November 18, 2022
Processing time: 450 Days and 0.7 Hours
Although the impact of microbial infections on orthopedic clinical outcomes is well recognized, the influence of viral infections on the musculoskeletal system might have been underestimated.
To systematically review the available evidence on risk factors and musculoskeletal manifestations following viral infections and to propose a pertinent classification scheme.
We searched MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), the Re
Six human and four animal studies were eligible for inclusion in the qualitative synthesis. Hepatitis C virus was implicated in several peri- and post-operative complications in patients without cirrhosis after major orthopedic surgery. Herpes virus may affect the integrity of lumbar discs, whereas Ross River and Chikungunya viruses provoke viral arthritis and bone loss.
Evidence of moderate strength suggested that viruses can cause moderate to severe arthritis and osteitis. Risk factors such as pre-existing rheumatologic disease contributed to higher disease severity and duration of symptoms. Therefore, based on our literature search, the proposed clinical and pathogenetic classification scheme is as follows: (1) Viral infections of bone or joint; (2) Active bone and joint inflammatory diseases secondary to viral infections in other organs or tissues; and (3) Viral infection as a risk factor for post-surgical bacterial infection.
Core Tip: Viral infections can include multiple orthopedic manifestations, thus resulting in significant distress. In addition, the outcome of orthopedic surgeries may be influenced by certain chronic viral infections such as hepatitis C virus. There is evidence of autoimmune-mediated mechanisms, immunosuppression, and perhaps direct viral infection provoking this, although the precise mechanisms have yet to be fully understood. In this review, a classification scheme was proposed. However, further research is needed to unveil the relative contributions of the identified mechanisms and develop novel preventative and treatment strategies.
- Citation: Sidiropoulos K, Christofilos SI, Tsikopoulos K, Kitridis D, Drago L, Meroni G, Romanò CL, Kavarthapu V. Viral infections in orthopedics: A systematic review and classification proposal. World J Orthop 2022; 13(11): 1015-1028
- URL: https://www.wjgnet.com/2218-5836/full/v13/i11/1015.htm
- DOI: https://dx.doi.org/10.5312/wjo.v13.i11.1015
Fracture-related and periprosthetic joint infections (PJIs) represent dreadful complications of orthopedic surgery[1]. Although the impact of microbial infections has been well documented, the influence of viral infections on orthopedics might have been underestimated[2]. The first investigation into the probable cause of bone reactions brought on by viral infections was conducted in 1962 by Marcowa[3], who examined the tick-borne encephalitis virus's ability to cause mice to develop tibial osteitis histologically and radiographically. The potential cause of bone reaction due to viral infections was firstly studied in 1962 by Marcowa[3], who histologically and radiographically evaluated tick-borne encephalitis virus, which induced tibial osteitis in mice. Since then, evidence has suggested that viral agents, such as parvovirus B19, hepatitis B, C virus, Human immunodeficiency virus (HIV), and alphavirus, cause viral arthritis with an estimated incidence of 1% of all acute arthritides[4].
Furthermore, an elevated risk of total hip arthroplasty (THA) revision has been documented in HIV patients 90 days post-procedure[5]; whereas sepsis, pneumonia, microbial joint infection, and revision surgery are more ubiquitous in hepatitis C virus (HCV)/hepatitis B virus (HBV) patients after total joint arthroplasty[5]. In addition, orthopedic manifestations of Alphaviridae have been observed[6], with Chikungunya virus (CHIKV), Ross River virus (RRV), and Sindbis virus being implicated and with viral ribonucleic acid (RNA) being present in joints for months post-infection[6]. Flaviviridae are also relevant as arthralgia occurs in 23%-80% of Zika virus infections[7]. In addition, a decrease in Alkaline Phosphatase (ALP) production by osteoblasts post-infection tends to delay their maturation[8].
Arthritis is a significant pain generator in HIV patients and is mediated by the premature de
Additionally, it would be reasonable to broadly categorize the orthopedic signs and symptoms of viral infections into three somewhat similar groups. More precisely, manifestations can be provoked by the inflammatory response or direct infection during the acute phase of the illness. This could be the case in Flaviviridae members, such as the Zika virus and Alphaviridae member RRV[7,8]. In addition, the Chikungunya virus (CHIKV) causes orthopedic manifestations mainly via autoimmune mechanisms such as cross-reactivity[6]. Lastly, certain viral infections could predispose to microbial infections due to immunosuppression. Examples would be HIV and HCV/HBV[5]. Of note, in the case of HIV, this was more commonly documented in the pre-highly active antiretroviral therapy (HAART) era[5].
In this present systematic review, we sought to systematically evaluate the risk factors for developing persistent arthritis after a viral infection and the impact of viral infections on musculoskeletal clinical outcomes. Lastly, we sought to categorize the musculoskeletal manifestations of viral infections acc
This review included human and animal model studies exploring bone and joint manifestations secondary to human viral infections. Observational studies, papers that did not report clinical outcomes, rheumatological articles, and papers that assessed patients under 18 years of age were excluded. Moreover, case series with less than ten subjects were discarded to increase the validity and credibility of our reporting. We searched MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), the Reference Citation Analysis, and Scopus for completed studies published before January 30, 2021. We also considered the trial registries of ClinicalTrials.gov, the EU clinical trial register, and the Australian New Zealand Clinical Trials Registry to search for completed yet unpublished studies. The search terms for MEDLINE were 'clinical trials', 'case series,' 'viral infection,' and 'bone’/joint'. KT and KS conducted the literature search independently without any language restrictions. Articles were deduplicated and examined for eligibility using title and article screening. Subsequently, a full-text evaluation of the remainder of the articles was performed. Any discrepancies between authors in the study selection procedure were resolved through discussion. KT and KS independently extracted relevant information from the included full-text articles, including any risk factors for persistent musculoskeletal manifestations.
Two reviewers (KT and DK) assessed the quality of the included studies using SYRCLE's risk of bias tool[12] for animal studies, Newcastle-Ottawa Scale for case-control studies[13], Wylde score for Registry Studies[14], and Moga score for case series[15]. For the included animal studies, the following domains were considered: sequence generation, baseline characteristics (i.e., sex, age, weight), allocation concealment, random housing, identical housing conditions, blinding of caregivers, random outcome assessment, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, other bias (i.e., contamination, pooling drugs, the influence of funders, units of analysis errors, design risk, new animals added for dropouts). Regarding case-control studies, we assessed the adequacy of case definition, representativeness, control selection and definition, comparability of cases and controls based on design analysis, ascertainment of exposure on the same method for both cases and controls, and non-response rates. For registry studies, we evaluated the following domains: consecutive patients, representativeness, percentage of follow-up, and minimization of potential confounding. In addition, we checked the quality of the included case studies against the 18-criteria checklist included in the Moga score[15].
The primary outcome measure of the present systematic review was bone and joint manifestations after direct viral infections other than those associated with abnormal autoimmune responses. The secondary outcomes included the general impact of viruses on clinical features and the study of any risk factors for developing persistent musculoskeletal manifestations following viral infections.
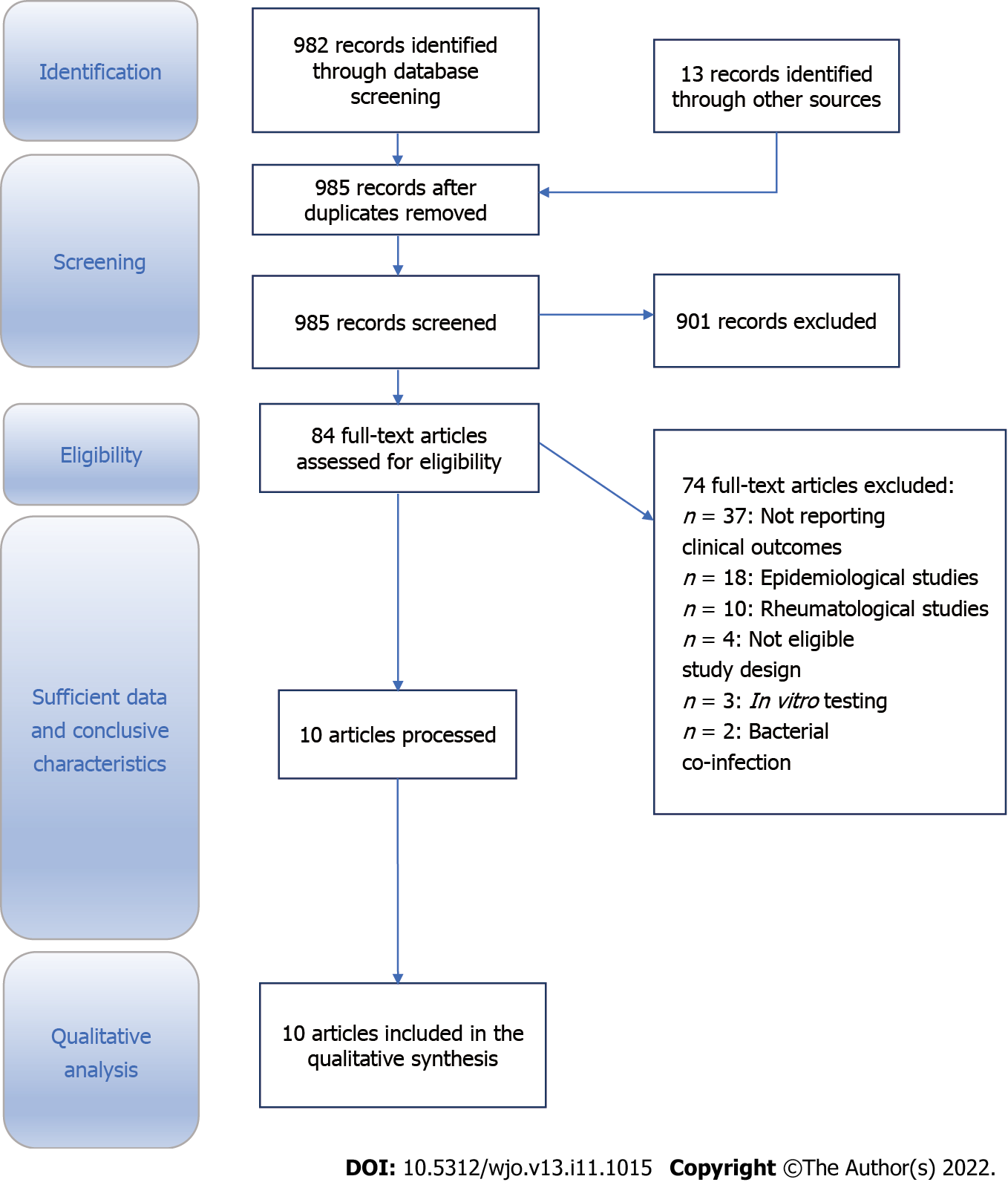
The literature search yielded 995 potentially relevant records. After removing duplicates, the remaining 985 articles were screened for eligibility. Following title and abstract evaluation, 84 articles were eligible for inclusion. The full texts were assessed, and ten articles were included for systematic review (Figure 1). Of these papers, two addressed treatment strategies, and three dealt with arthroplasties in patients with HCV. In addition, six papers involved humans looking at Chikungunya, HCV, and RRV[16-25].
For the included animal studies, the domains of follow-up, minimization of potential confounding, representativeness, baseline characteristics (i.e., sex and age), other bias (i.e., contamination, pooling drugs, influence of funders, unit of analysis errors, design risk, new animals added for dropouts) and adequate definitions of cases, selection and definition of controls, ascertainment of the same exposure to control and cases, and comparability of cases and controls were considered to be at low risk of bias (Supplementary Table 1). Moreover, the following domains were judged to be at unclear risk of bias: sequence generation, allocation concealment, random housing, blinding of caregivers, random outcome assessment, blinding of outcome assessors, incomplete outcome data, and selective outcome reporting (Supplementary Table 1). Furthermore, the domains of identical housing conditions were found to be at an unclear risk of bias (Supplementary Table 1). Regarding the case-control studies, the Newcastle-Ottawa score was used, and the only domain found to have an unclear risk of bias was the non-response rate for both included papers (Supplementary Table 2). On the contrary, the adequacy and representativeness of cases and the selection and definition of controls had a low risk of bias (Supplementary Table 2). Lastly, the comparability of cases and controls based on design, and the ascertainment of exposure, were deemed to be at low risk of bias (Supplementary Table 2). Furthermore, following an appraisal of the two included registry studies, representativeness was deemed adequate because the articles were multicenter with sufficient follow-up (Supplementary Table 3). Lastly, the case series of Alpantaki et al[25] and Soden et al[22] were evaluated utilizing the Moga score (Supplementary Table 4), with the former study reaching a sum of 13 and the latter achieving a sum of 10 (Supplementary Table 4).
| Author (year) | Study design | Inoculated groups and number of subjects | Risk factors/outcome measures | Outcomes | Follow-up |
| Best (2014) | Retrospective cohort study of non-cirrhotic HCV positive individuals and HCV negative patients who underwent TKA/THA in the USA from 1990-2007 | Group 1: 26444 HCV patients, 14452 subjected to THA (51.2% males) and 11992, to TKA (36.5% males); Group 2: 8336822 HCV negative patients, 2968679 subjected to THA (42.6% males), and 5370202 subjected to TKA (36.2% males) | Gender, Age, LOHS, Comorbidities, post-operative bleeding, thrombocytopenia, transfusion reaction, cardiac complications, peripheral vascular complications, urinary complications, acute renal failure, myocardial infarction, pulmonary embolism, pneumonia, deep venous thrombosis, blood transfusion, osteomyelitis, and infection | LOHS, age, rates of total complications, and post-operative bleeding | Not specified |
| Chowdhury (2017) | Retrospective registry study with a propensity-matched cohort including HCV patients and uninfected participants who have undergone TKA, THA, and spine procedures in the USA between 2006-2014 | Group 1: 1131 (52% males) with HCV; Group 2: 95161 (56% males) non-HCV individuals, and after propensity score matching, 1131 non-HCV patients were matched to the HCV group, and the cohort consisted of 2262 patients | Mortality within 30- or 90-d, readmission, and complications within 30 and 90 d | Mortality assessment, complication, and readmission rate evaluation | Up to 30 and 90 d post-operatively or upon complication |
| Pour (2011) | Retrospective case-control study with a control group matched at a 2:1 ratio with asymptomatic HCV patients subjected to THA and TKA from 1995-2006 in the USA | Group 1: n = 39 (29 males) HCV patients who have undergone THA; Group 2: n = 80 (60 males) patients who have undergone THA (control group); Group 3: n = 32 (15 males) HCV patients subjected to TKAGroup 4: 64 (30 males) patients subjected to TKA (control group) | Αge, gender, BMI, need for transfusion, preoperative PLTS, LOHS, and the complication rate | Complication assessment (wound, mechanical, fracture, reoperation, revision); Length of hospital stay | 101 mo (range 66-140) for the HCV patients subjected to THA; 94 mo (range 45-131 mo) for the control group subjected to THA, 117 months (range 67-150 mo) for the HCV patients subjected to TKA; 98 mo (range 49-133 mo) for the control group subjected to TKA |
| Author (year) | Study design | Inoculated groups and number of subjects | Outcomes | Follow-up |
| Chen (2014) | In vitro and in vivo animal interventional study | For the in vitro experiment: 21-day-old male and female C57BL/6 WT mice were inoculated in the thorax with 104 PFU of RRV. Those mice received 500 μg of anti-IL-6 antibody injections on days 0 and 2, 4, 6, 8 post-infection. For the in vivo investigation: Serum samples from 14 Ross River virus patients (7M and 7F) were obtained, and serum from 13 healthy individuals (7M). Synovial fluid samples from 12 RRV-induced polyarthritis patients (6M) were retrieved and from 6 healthy individuals (3M). | The animal part of the study investigated whether RRV replicates in the bone (murine model) and viral titers were measured. μCT imaging was used to assess the impact of the infection on bone architecture and loss. The role of IL-6 on bone loss was evaluated. The human part of the study looked at OPG, RANKL, and TRAP5b levels in RRV-positive patients. | Not specified |
| Soden (2000) | Prospective observational study involving humans | Biopsy tissue from inflamed knees from 12 patients was retrieved. | Histological examination of the synovial membrane. RT-PCRto look for the presence of viral RNA | The follow-up was performed at 3-mo intervals until 6 m following symptom resolution |
| Author (year) | Virus information | Study design | Inoculated groups and number of subjects | Outcome measures | Follow-up |
| Chang (2017) | Colombian patients infected by CHIKV | Case-control study of 38 participants with CHIVK and chronic arthritis and 10 location-matched controls without CHIKV or arthritis | Group 1: Out of 907 patients who were clinically (424) and laboratory (483) confirmed for CHIKV, 38 individuals with chronic arthritis post-infection were selected; Group 2: 10 matched controls without CHIKV/arthritis were considered | Synovial fluid samples were analyzed by PCR, and mass spectrometry for viral proteins. No virus could be detected. | Not specified |
| Chen (2015) | Chikungunya virus from infected patients' serum. CHIKV-mCherry strain was also used | In vitro study utilizing serum from 14 CHIK patients and 7 healthy individuals; In vivo animal study utilizing 25 d-old C57BL/6 mice infected with CHIKV/mCherry | Group 1: Serum from 14 CHIKV patients (8F 6M) was collected between the 3rd and 22nd week post-infection; Group 2: Serum from 7 (4F and 3M) healthy individuals was also used; Group 1: 25 d-old mice were infected with 20 μL 105 PFU CHIKV-mCherry in the ventral side of the foot. Group 2: Consisted of the control group of mice. | For the in vitro study: Serum RANKL/OPG ratio was measured; For the in vivo study: Day 3 post-infection peak swelling was measured until day 10. Levels of RANKL and OPG were measured inside the joint during days 1, 3, 7 and 15 post-infection. | Days 1, 3, 7, and 15 post-infection |
| Goupil (2016) | Chikungunya virus SVO 476-96 | In vivo animal study featuring IRF 3/7 C57BL/6 mice (M and F > 8 wk old) and C57BL/6J mice (> 8 wk old only F) | Group 1: 11 IRF mice were intradermally injected with 2 × 104 PFU; Group 2: 9 control C57BL/6j mice were injected with 2 × 104 PFU | Intact hindlimbs were collected and scanned μCT to evaluate the difference between the morphology of two types of mice (joint, trabecular bone). Histopathological evaluation was also performed. On day 5, post-infection 4 mice were euthanized due to being lethargic, and 6 mice died due to rapid progression of the illness | For the IRF mice 1, 2, 3, 5, 6, 7th day post-infection;For the C57BL/6J mice 7, 14, 21st day post-infection |
| Hawman (2013) | Chikungunya SL15649 from serum sample | In vivo animal study featuring 3-week-old C57BL/6J mice and congenial rag_/- mice | Group 1: 55 mice were inoculated in the left rear footpad with 103 PFU (10 μL). MAbs (200 μg each of CHK-152 and CHK-166) were administered intraperitoneally on days -1 and 3 for prophylaxis studies. For therapeutic studies, MAbs were administered on days 21 and 25 post-infection; Group 2: Control mice were subject to mock-infection | Duration of CHIKV infection in tissues was assessed, and associated histopathological changes were evaluated | Day 3 and weeks 1, 2, 4, 6, 12, 16 |
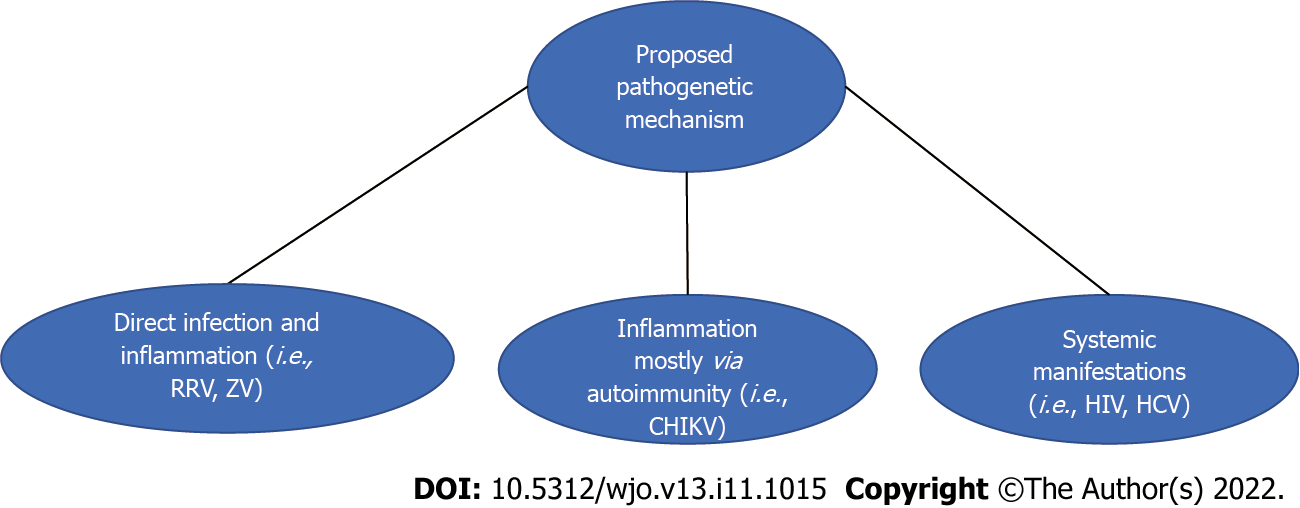
| Proposed pathogenetic mechanism | Viruses involved | Evidence supporting the proposed mechanism |
| Direct infection and subsequent inflammation | Ross Rover and Zika viruses | RRV causes arthritis with its RNA coinciding with the appearance of symptoms; Evidence of human osteoblasts being primarily infected |
| Inflammation primarily through autoimmune mechanisms such as cross-reactivity | CHIKV | CHIKV causes arthritis in the absence of evident infection. Other supporting information includes rheumatoid factor negative RA and exclusive presence in the synoviocytes. Animal studies implicate a potential role of primary infection |
| Systemic manifestations resulting in immunosuppression | HCV and HIV | Greater rates of microbial infections in HCV patients post-surgery. Greater rates of infections post-surgery pre-HAART. High rates of mechanical and medical comorbidities |
Three studies related to HCV infection were identified[18-20]. In particular, Best et al[18] published a retrospective cohort study in 2015 looking at non-cirrhotic HCV patients subjected to total hip and knee arthroplasty. Half of the included cases were subjected to total hip arthroplasty (THA) (approximately 50% males), and the rest to total knee arthroplasty (TKA) (40% males) (Table 1). Likewise, Chowdhury et al[19] published a retrospective study in 2017 to assess the effect of HCV infection 90 days after TKA, THA, and spine surgery. This study included 2262 patients, half HCV positive (Table 1). Moreover, Pour et al[20] investigated HCV-positive patients relative to matched controls with a 1:2 ratio in 2011 and included individuals who underwent THA and TKA from 1995-2006 in the US (Table 1). Risk factors in the article published by Best et al[18] included length of hospital stay (LOHS), age, gender, comorbidities, post-operative bleeding, thrombocytopenia, transfusion reaction, cardiac complications, respiratory and renal complications, as well as osteomyelitis, and infection. In the study of Chowdhury et al[19], age, race, readmission, and death within 30 or 90 days post-operatively were identified as outcome measures/risk factors (Table 1). Lastly, those identified by Pour et al[20] were age, gender, BMI, preoperative platelets, complication rate, and LOHS (Table 1). Best et al[18] noted that patients from the HCV-positive group presented fewer comorbidities such as diabetes mellitus, hypertension, cardiovascular disease, and osteoporosis as well as a shorter LOHS (5.3 ± 3.4 compared with 5.4 ± 5.1 days in the non-HCV group, P < 0.001)[18]. In the above study, the overall complication rate was higher in the HCV group with prosthetic joint infection (OR was 9.5 [95%CI 8.3 to 10.8], P < 0.001)[18]. More specifically, acute renal failure and peripheral vascular complications showed an OR of 8 (95%CI 7.4 to 8.6, P < 0.001) and 4.8 (95%CI 4.3 to 5.4, P < 0.001), respectively[18]. It is underlined that stratification of the 'patients' cohort into THA and TKA revealed a significant difference in the comorbidities of these patients[18]. Other complications noted in the HCV group were deep venous thrombosis and pulmonary embolism, pneumonia, post-operative bleeding, and a higher blood transfusion rate[18]. Similar results were presented by Pour et al[20], who noted a statistically significant difference when the complications of revision hip or knee arthroplasty were compared between the two study groups (P < 0.05)[20]. When comparing the results of Best and Pour's studies, the only difference was the LOHS [18,20]. Furthermore, Chowdhury et al[19]; reported higher readmission and mortality rates in the HCV group after THA, TKA, lumbar interbody fusion, decompression, and discectomy.
The role of a Herpes virus infection in intervertebral disc degeneration was studied by Alpantaki et al[25]. More precisely, 16 consecutive patients (8 males) with a mean age of 40 years undergoing discectomy within six months of lumbar disc herniation were included. Those individuals constituted the study group, while two patients with thoracolumbar burst fractures formed the control group[25]. Moreover, material from the herniated or fractured disc and peripheral blood samples were sampled intraoperatively. Polymerase chain reaction (PCR) detected Herpesviridae DNA in 13 study group subjects[25].
Regarding blood samples, seropositivity of patients was assessed with IgM and IgG assays for HSV-1 and Cytomegalovirus (CMV)[25]. Moreover, the surrounding tissues of the herniated disc were tested by qRT-PCR for mRNA levels of TNF-α and IL-6[25]. Herpes simplex virus type-1 (HSV-1) DNA was detected in 9/16 subjects, and CMV DNA was found in 6 subjects, while 2/16 subjects had a co-infection with both species[25]. On the contrary, DNA from HSV-2, Varicella-Zoster Virus, Epstein-Barr Virus, Human Herpes Virus 6, 7, and 8 were not found in any participants, and the control group tested negative for Herpesviridae DNA[25]. In addition, the IgG serological tests were positive in 13/16 subjects with PCR positivity for viral DNA, whereas all subjects were negative for IgM antibodies, indicating the absence of an acute reaction at the time of surgical excision[25]. Furthermore, IL-6, TNF-a, and viral mRNA in the study group were two to three times higher than in the control group[25]. This is the only indication that the Herpesviridae evoke disc herniation in individuals, regardless of age or sex[25].
We note that almost all Alphaviruses can cause joint manifestations. For instance, the RRV is detectable in the serum within 7-10 days after the initial symptoms, with synovial fluid infiltration by mononuclear cells being a common phenomenon throughout the disease. Soden et al[22] assessed synovial membrane biopsies of inflamed knees weeks after the initial symptoms of RRV infection (Table 2), whereas Chen et al[16] studied the effects of the RRV on human osteoblasts, bone loss in an established murine model, and viral arthralgia. Soden et al[22] also used RT-PCR to detect viral RNA from synovial membrane biopsy samples that were histologically evaluated with standard H&E staining, immunohistochemistry, and TRIzol treatment[22]. Chen et al[16] included 21-d-old male and female C57BL/6 WT mice inoculated with 104 PFU RRV T48 strain (Table 2). This study replicated bone infection and implemented μCT to assess bone loss in WT mice (Table 2). In addition, it compared the RANKL/osteoprotegerin (OPG) levels in the serum of healthy and RRV-infected individuals (Table 2). Soden et al[22] detected RRV RNA in the synovial membrane in 2 subjects 5 weeks after the onset of symptoms, with almost all subjects presenting with detectable histological abnormalities, including minor lining layer hyperplasia, vascular proliferation, and mononuclear cell infiltration[22]. This study proved that RRV affects the joints by directly triggering an inflammatory reaction and is also detectable weeks after the initial symptoms[22]. Chen et al[16] detected high viral titers in the femur, tibia, patella, and foot, mainly in osteoblastic bone cells. Notably, high viral levels were detected until day 21 post-infection[16]. By day 15, post-infection μCT imaging showed an evident bone loss in the tibial epiphysis, metatarsal joints, and vertebrae, accompanied by a decrease in trabecular thickness and a reduction in the growth plate[16]. By contrast, these findings were not noticed in the control group[16]. Lower OPG and higher RANKL levels were observed in the study group, while serum TRAP5b levels were also higher[16]. These findings indicate increased osteoclastogenesis in humans, similar to that observed in a murine model[16]. It is worth noting that the RRV also has a tropism for osteocytes[16].
Four studies looking at CHIKV alphavirus joint manifestations fulfilled our eligibility criteria (Table 3). Chang et al[23] recruited 907 clinically and laboratory-confirmed CHIKV-infected patients. Of these, 38 presented with chronic knee arthritis and were deemed eligible for selection. Furthermore, a control group with ten location-matched individuals was considered (Table 3). Chen et al[21] studied bone loss after CHIKV infection by recruiting 14 CHIKV patients (6 males) and a control group consisting of 7 healthy individuals (3 males) (Table 3). The second part of the experiment included 25 day-old C57BL/6 mice infected with CHIKV-mCherry (Table 3). Goupil et al[24] studied bone and cartilage loss during CHIKV infection by employing two groups of mice featuring IRF 3/7 with deficient type 1 interferon response and adult wild-type C57BL/6. The study group consisted of 11 IRF mice and the control group of 9 C57BL/6 mice[24]. Hawman et al[17] studied the persistence of the viral RNA and its role in 'joint pathology. Two groups of 3-wk-old C57BL/6J WT mice and Rag1-/- with a lack of T and B cells were formed, and a control group was also included. Chang et al[23] collected synovial fluid samples for viral culture and performed qRT-PCR and mass spectrometry for the detection/quantification of viral genome and proteins, respectively (Table 3). Moreover, serum samples were analyzed for CRP, IgM, IgM-RF, anti-cyclic citrullinated peptide (anti-CCP), and selected cytokine and chemokine levels[23]. Chen et al[21] collected serum from the 3rd to the 22nd post-infection week and compared the RANKL/OPG of the 14 CHIKV patients and the seven healthy participants. In the second part of the study, 25 d old C57BL/6 mice were infected with CHIKV-mCherry (20 μL of 105 PFU at the ventral side of the foot), and a control group was injected with saline (Table 3)[21]. They were followed up on the 1st, 3rd, 7th, and 15th days post-infection[21]. Goupil et al[24] injected IRF 3/7 mice with IFN-1 deficiency, and C56BL/6 WT mice, with 2 × 104 PFU CHIKV SVO 476-96 at the caudoventral aspect of the hindfoot[24]. In addition, intact hindlimbs were collected from both groups and scanned via μCT to evaluate differences in the morphology of joints and the trabecular bones post-infection[24]. Furthermore, histopathological analysis was performed using hematoxylin, eosin, and Mason's Trichrome staining[24]. Hawman et al[17] utilized CHIKV patients' serum to inoculate Rag1-/- mice which lacked T and B cells, and WT mice with CHIKV SL 15649 in the left rear footpad[17]. Viral titers were measured, and histopathological analysis was performed[17]. Chang et al[23] found no evidence of viral infection, and therefore it was concluded that either CHIKV is exclusively found in synovial tissue cells or it provokes arthritis through autoimmune mechanisms. Chen et al[21] found higher RANKL levels in the CHIKV patients and almost the same OPG levels (Table 3) in the CHIKV and control groups. This finding indicated an osteoclastic condition during the infection. From a clinical point of view, edema was greatest on the third day of CHIKV-mCherry mice follow-up, which was eventually resolved by day 10[21]. Moreover, the pro-osteoclastic microenvironment was created early after the acute infection as RANKL/OPG was elevated from day one and remained high thereafter[21]. In addition, CHIKV was replicated in a murine bone and induced bone loss of 25% relative to uninfected mice[21]. The immune response resulted in arthritis on the 3rd post-infection day, featuring elevated MCP-2/CCL8 and increased cellularity[21]. Goupil et al[24] found that C57BL/6J mice on the 7th post-infection day suffered from moderate dermatitis/dermal edema, extensive degeneration/necrosis of skeletal muscles, minimal periostitis, mononuclear/neutrophilic synovitis, and equivocal cartilage necrosis. On day 14 post-inoculation, mild to moderate dermatitis was observed, as well as extensive skeletal muscle degeneration/necrosis with early evidence of regeneration, extensive periostitis, and persistent synovitis with distal joint involvement[24]. On day 21 post-infection, the following findings were documented: minimal/mild dermatitis, resolving necrosis/inflammation of muscles (immature fibrosis), extensive periostitis with periosteal bone proliferation, subacute lymphoplasmacytic synovitis, synovial hypertrophy/fibrosis, and cartilage necrosis[24]. The tendons showed variable mild peritendonitis from day seven and minimal myocyte necrosis in the contralateral feet[24]. When the IRF type mice were assessed by Goupil et al[24], the following findings were observed by the fourth day post-infection: multifocal mild to moderate epidermic necrosis, mild neutrophilic dermatitis/edema, rare vascular necrosis, mild myofiber degeneration, periosteal necrosis, and minimal inflammation of tendons and cartilage. At the same time, the synovium presented with multifocal degeneration/necrosis affecting a few joints. Subsequently, extensive synovitis in multiple joints was documented[24]. By day 7, these findings worsened with extensive epidermal necrosis, extensive vascular necrosis, moderate myofiber degeneration/necrosis, bone marrow and periosteum necrosis, articular cartilage necrosis, and fibrinosuppurative synovitis in the majority of joints[24]. The tendons only presented mild inflammation[24]. It was concluded that the bone and joint manifestations resulted from acute viral infection rather than autoimmune-mediated mechanisms[24]. Hawman et al[17] documented that viral inoculation of Rag1-/- mice that lacked T and B cells resulted in higher virus titers. In addition, the histopathological analysis presented more intense synovitis, arthritis, and tendonitis[17]. These findings support the notion that joint manifestations of CHIKV infection directly result from the infection rather than the host's immune system[17]. It should be noted that the authors of the included studies proposed possible treatment strategies for CHIKV infection. In particular, Chen et al[21] used an inhibitor of monocyte chemoattractant protein (MCP) (i.e., Bindarit) twice daily intraperitoneally (100 mg/kg) in mice which reduced joint swelling and bone loss but not viral titers[21]. Hawman et al[17], on the other hand, proved that tissue-specific administration of monoclonal antibodies reduced the viral RNA in these tissues.
It is undeniable that viral infections pose a substantial yet unclear impact on the musculoskeletal system. Most aspects have not yet been studied sufficiently, mainly due to the lack of technological advancements until the 21st century. In light of the above, a systematic review was designed to delineate the risk factors and orthopedic clinical outcomes secondary to viral infections. More specifically, the effects of chronic HCV infection on TKA and THA, as well as the role of Herpesviridae on lumbar disc degeneration, were addressed. Moreover, the musculoskeletal effects of the Chikungunya virus and RRV-mediated chronic arthritis were examined.
HCV is a significant cause of orthopedic complications such as an oligoarthritis of large and middle joints or rarely a rheumatoid arthritis-like illness[26]. A higher level of post-operative complications and a higher mortality rate were noted in the HCV group despite the patients being younger and having fewer medical comorbidities[18]. Nevertheless, the hospitalization of HCV patients was shorter, perhaps due to them being transferred to different units to have their complications addressed[18]. A possible explanation would be the circulating autoantibodies leading to decreased lymphoproliferation. As a result of lymphoproliferation, predisposition to infection, leukocytoclastic vasculitis[27], and glomerulonephritis[27,28] develop. In addition to the above features of HCV patients, a hypercoagulability profile was observed[18].
It should be noted that HCV has been shown to induce thrombocytopenia and impaired platelet function[29,30,31], predisposing to higher rates of bleeding[18]. Pour et al[20] also documented increased reoperation rates, higher mechanical complications, and hospital stays in the HCV group. To be more precise, complications included periprosthetic femoral fractures, femoral implant loosening, and hip dislocation secondary to migration of acetabular implant that required a revision of the THA[20]. On the other hand, chronic HCV disease was associated with multiple extrahepatic manifestations such as diabetes mellitus and thyroiditis, thrombocytopenia, glomerulonephritis, inflammatory myositis, arthralgia, mixed connective tissue disease, leukocytoclastic vasculitis, and lymphadenopathy. These can be attributed to circulating autoantibodies that could alter the physiological process of healing[20]. In addition, low-key inflammation can alter the function of platelets, further compounding the pathophysiologic mechanism[20]. Another aspect worth mentioning is the potential difference in the socio-economic level of HCV-infected individuals and that of healthy participants[20]. Furthermore, ancillary liver effects were investigated by Chowdhury et al[19], and it was thought that they were implicated in immunosuppression and impaired wound healing. These findings confirm the significance of HCV infection in post-operative outcomes and highlight the importance of including HCV testing in the preoperative workup.
Regarding the potential of Herpesviridae being a possible cause of disc degeneration, Alpantaki et al[25] studied 16 patients undergoing discectomy six months after lumbar disc herniation subjected to Herpesviridae DNA testing[25]. Positivity for at least one species (most commonly HSV) was found in 13[25]. Possible mechanisms implicated in disc degeneration could be the vascular channels formed during fetal development that remain patent until the 4th-6th year of life, as well as migrating macrophages and retrograde axoplasmic transport[25]. However, it remains unclear whether degeneration is solely secondary to the upregulation of inflammatory cytokines or whether viral-induced cell death could also contribute[32]. In addition, it has been postulated that Herpesvirus 6 could cause Langerhans' Histiocytosis[33]. This rare disease affecting children of 1 to 4 years of age has predominantly bone involvement, and, more often than not, the first presentation of the disease is a pathological fracture[33].
Furthermore, CHIKV has a cyclical pattern of epidemics from 7 to 20 years and affects countries neighboring the Indian Ocean, Central Africa, China, Italy, and France. After transmission via a mosquito bite, the virus multiplies locally and is then transferred to the whole host body via lymphoid organs and the bloodstream. Mononuclear cell infiltration and viral replication in muscles and joints cause severe pain and arthritis. Although CHIKV infection is self-limiting, arthritis/arthralgia occurs for a particular amount of time due to the immune response or an active viral reservoir in joints[34]. In 2018, a systematic review and meta-analysis of 2415 individuals suffering from CHIKV infection in the US revealed that 52% of the patients appeared to have persistent arthritis 10 to 72 weeks after the primary infection[35]. It has been thought that arthritis may develop due to epigenetic modifications of macrophages which present a more aggressive cell behavior[36]. Another possible cause could be the concomitant presence of seronegative rheumatoid arthritis, although this has been doubted by Chen et al[21]. Moreover, Chen et al[21] noted that Alfaviridae, such as RRV, could infect primary human osteoblasts and cause the production of inflammatory cytokines, thus promoting osteoclastogenesis[37-39]. In addition, when analyzed with μCT, it was clear that Alfaviridae could also lead to bone loss. Interestingly, after treatment with IL-6 inhibitors, bone loss was blocked, thus highlighting the central role of inflammation in pathogenesis[16]. Finally, it is essential to stress the impact of CHIVK-mediated arthritis as 82% of chronically infected patients present with arthritis that substantially impacts their quality of life[23].
In the current review, we have proposed a classification system for the pathogenesis of viral infection (Table 4 and Figure 2). Regarding the first proposed category addressing the viral infections of bones and joints, we noted that the Alphavirdae member RRV replicated in murine bones[16]. To address this proposed mechanism further, we note that human osteoblasts could be infected with RRV and produce inflammatory cytokines such as IL-6. In addition, in the inflamed knees of affected patients, RRV RNA was present five weeks after the onset of symptoms[22]. However, some patients presented symptoms in the absence of detectable viruses[22], and this finding is partially congruent with the initial hypothesis that RRV provokes orthopedic manifestations through primary infection and inflammation[22].
Regarding the second proposed category, active bone and joint inflammatory diseases occur secondary to viral infections in other organs or tissues. CHIKV indeed causes arthralgia/arthritis without being directly detectable[23]. No evidence of it was found via RT-PCR, mass spectrometry, and culture of synovial fluid, thus agreeing with the initial hypothesis of it causing arthritis principally via cross-reactivity and suggesting immunomodulatory agents in its treatment[23]. Although, productive infection of musculoskeletal cells was reported by Chen et al[21]. In addition, Hawman et al[17] presented animal evidence revealed persistent viral infection, but safe extrapolations to human biology cannot be made based on this finding.
Lastly, the third proposed category included viral infection as a risk factor for post-surgical bacterial infection. To elaborate, we reported that HCV predisposes to immunosuppression[18] with associated increased post-surgical complications in HCV patients[19], in addition to compromised liver function and wound healing[19]. Pour et al[20] confirmed this finding as wound complications requiring antibiotics/wound debridement were noticeably more common in HCV patients post-surgery.
The persistence of the viral genome or proteins in host cells could represent the major risk factor for chronic manifestations after the initial infection. However, no data prove any association between Epstein-Barr and rheumatoid arthritis[40]. In the setting of CHIKV infection, joint manifestations resemble inflammatory arthropathies[41], and their severity depends on the levels of cytokines. We highlight that further studies should be performed to clarify that[42].
We recognize that the lack of a consistent definition of virus-induced rheumatoid arthritis and the wide variety of musculoskeletal manifestations secondary to viral infections complicates the picture for clinicians and health policymakers. In addition, the limited number of studies addressing the above issues and the uncertainty introduced by the moderate-to-low quality of evidence of the included articles further contribute to this vagueness. It has been shown that some tropism exists for cells such as osteocytes, synovial cells, and chondrocytes. However, the pathophysiology of the infection or inflammation, the underlying processes, and the reasons for lingering symptoms are still unknown. Towards this direction, we advocate that future research should aim for the development of novel treatment options based on the underlying mechanisms.
It is highlighted that all articles relating to COVID-19 were excluded as the vast majority were based on expert opinions and observational studies with limited follow-up and sample size. However, some aspects of this pandemic should be commented on. First, it is unknown whether osteoporosis and osteonecrosis are two common findings after COVID-19 infection, but the potential role of corticosteroid administration as a part of the therapeutic regime cannot be overlooked[43]. Another clinical finding worth mentioning is the higher incidence of late (i.e., a few weeks following COVID-19 diagnosis) spinal epidural abscesses. This could be explained by 'patients' immunosuppression or the occurrence of nosocomial superinfection[44]. Finally, the binding of COVID-19 spike protein to functional receptors of ACE2, also expressed in human bone marrow, could be a possible explanation for the decreased bone matrix and early muscle disorders[45]. Although the above considerations represent some early indications of the potential connection of COVID-19 with orthopedic clinical outcomes, we underline that a safe conclusion cannot be drawn, given the limited available literature and considerable risk of bias.
Viral infections pose a major concern for microbiologists and orthopedic surgeons, given the high incidence of chronic arthritis and its detrimental effect on patients' quality of life[23]. While the literature on this topic was sparse and heterogenous, the negative influence of viruses on orthopedic surgical outcomes is evident[18-20]. We highlight that arthralgia, myalgia, and transient arthritis could result from viral infection or secondary immune processes, although each mechanism's contribution is still relatively unclear. Therefore, we advocate that the present systematic review raises awareness of the implications of viral infections in orthopedics and acts as a guide for orthopedic surgeons to classify them in a clinical and pathogenetic fashion.
While the influence of microbial infections on orthopedic clinical outcomes is well documented, the impact of viral infections on the musculoskeletal system has been inadequately investigated.
Although microbial infections have been studied extensively in orthopedics, the impact of viral infections on orthopedics has not been sufficiently investigated. In addition, we are unaware of any classifications relating to viral infections in the orthopedic literature.
In this article, we looked at the risk factors for persistent arthritis development after a viral infection and the impact of viral infections on orthopedic clinical outcomes. In addition, we categorized orthopedic manifestations of viral infections relative to their causative mechanism.
An extensive literature search was performed to identify completed studies published before January 30, 2021. MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), the Reference Citation Analysis, and Scopus were searched for articles evaluating risk factors and bone/joint manifestations of viral infection in animals and humans. In addition, we assessed the quality of the included articles utilizing SYRCLE's risk of bias tool for animal studies, the Moga score for case series, the Wylde score for registry studies, and the Newcastle-Ottawa Scale for Case-control studies.
Ten articles were included in the systematic review. Of these, two dealt with treatment strategies and another three with arthroplasties in patients with hepatitis C virus (HCV). In addition, six articles addressed human beings investigating Chikungunya, HCV, and RRVs. After major orthopedic surgery, HCV was implicated in several peri- and post-operative complications. Herpes virus may affect the integrity of lumbar discs, whereas Ross River and Chikungunya viruses negatively influence bones and/or joints, resulting in viral arthritis and bone loss.
Viral infections pose a significant burden in orthopedics due to the significant impact on patient quality of life. We have demonstrated a connection between viral infections and orthopedic surgical outcomes. We highlight that arthralgia, myalgia, and transient arthritis could result from viral infection or secondary immune processes, although each mechanism's contribution is still relatively unclear. We advocate that the present systematic review raises awareness of the implications of viral infections in orthopedics and acts as a guide for orthopedic surgeons to classify them in a clinical and pathogenetic fashion.
We recommend that a consistent definition of virus-induced rheumatoid arthritis be developed. Furthermore, we suggest that further high-quality articles investigating novel treatment options based on the underlying mechanisms be conducted.
Provenance and peer review: Invited article; Externally peer-reviewed.
Peer-review model: Single-blind
Specialty type: Orthopedics
Country/Territory of origin: Greece
Peer-review ' 'report's scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ge X, China; Luo ZW, China S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Wu YXJ
| 1. | Metsemakers WJ, Morgenstern M, Senneville E, Borens O, Govaert GAM, Onsea J, Depypere M, Richards RG, Trampuz A, Verhofstad MHJ, Kates SL, Raschke M, McNally MA, Obremskey WT; Fracture-Related Infection (FRI) group. General treatment principles for fracture-related infection: recommendations from an international expert group. Arch Orthop Trauma Surg. 2020;140:1013-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 2. | Drago L, Romanò CL, Morelli I, Benzakour T. Viral Bone Infection: A Neglected Disease? Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Markowa J. Osteitis produced experimentally in mice by the virus of tick-borne encephalitis. J Bone Joint Surg Am. 1962;44B:722-734. [DOI] [Full Text] |
| 4. | Marks M, Marks JL. Viral arthritis. Clin Med (Lond). 2016;16:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 5. | Kildow BJ, Politzer CS, DiLallo M, Bolognesi MP, Seyler TM. Short and Long-Term Post-operative Complications Following Total Joint Arthroplasty in Patients With Human Immunodeficiency Virus, Hepatitis B, or Hepatitis C. J Arthroplasty. 2018;33:S86-S92.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | McCarthy MK, Morrison TE. Persistent RNA virus infections: do PAMPS drive chronic disease? Curr Opin Virol. 2017;23:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Wimalasiri-Yapa BMCR, Yapa HE, Huang X, Hafner LM, Kenna TJ, Frentiu FD. Zika Virus and Arthritis/Arthralgia: A Systematic Review and Meta-Analysis. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Mumtaz N, Koedam M, van den Doel PB, van Leeuwen JPTM, Koopmans MPG, van der Eerden BCJ, Rockx B. Zika virus infection perturbs osteoblast function. Sci Rep. 2018;8:16975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Mody GM, Parke FA, Reveille JD. Articular manifestations of human immunodeficiency virus infection. Best Pract Res Clin Rheumatol. 2003;17:265-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Raynaud-Messina B, Bracq L, Dupont M, Souriant S, Usmani SM, Proag A, Pingris K, Soldan V, Thibault C, Capilla F, Al Saati T, Gennero I, Jurdic P, Jolicoeur P, Davignon JL, Mempel TR, Benichou S, Maridonneau-Parini I, Vérollet C. Bone degradation machinery of osteoclasts: An HIV-1 target that contributes to bone loss. Proc Natl Acad Sci USA. 2018;115:E2556-E2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Pattison JR. B19 virus--a pathogenic human parvovirus. Blood Rev. 1987;1:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2511] [Cited by in RCA: 2425] [Article Influence: 220.5] [Reference Citation Analysis (2)] |
| 13. | Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 551] [Article Influence: 68.9] [Reference Citation Analysis (82)] |
| 14. | Wylde V, Beswick AD, Dennis J, Gooberman-Hill R. Post-operative patient-related risk factors for chronic pain after total knee replacement: a systematic review. BMJ Open. 2017;7:e018105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Moga C, Guo B, Schopflocher D, Harstall C. Development of a Quality Appraisal Tool for Case Series Studies Using a Modified Delphi Technique. Edmonton AB: Institute of Health Economics. 2012. |
| 16. | Chen W, Foo SS, Rulli NE, Taylor A, Sheng KC, Herrero LJ, Herring BL, Lidbury BA, Li RW, Walsh NC, Sims NA, Smith PN, Mahalingam S. Arthritogenic alphaviral infection perturbs osteoblast function and triggers pathologic bone loss. Proc Natl Acad Sci USA. 2014;111:6040-6045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Hawman DW, Stoermer KA, Montgomery SA, Pal P, Oko L, Diamond MS, Morrison TE. Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response. J Virol. 2013;87:13878-13888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 18. | Best MJ, Buller LT, Klika AK, Barsoum WK. Increase in perioperative complications following primary total hip and knee arthroplasty in patients with hepatitis C without cirrhosis. J Arthroplasty. 2015;30:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Chowdhury R, Chaudhary MA, Sturgeon DJ, Jiang W, Yau AL, Koehlmoos TP, Haider AH, Schoenfeld AJ. The impact of hepatitis C virus infection on 90-day outcomes following major orthopaedic surgery: a propensity-matched analysis. Arch Orthop Trauma Surg. 2017;137:1181-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Pour AE, Matar WY, Jafari SM, Purtill JJ, Austin MS, Parvizi J. Total joint arthroplasty in patients with hepatitis C. J Bone Joint Surg Am. 2011;93:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Chen W, Foo SS, Taylor A, Lulla A, Merits A, Hueston L, Forwood MR, Walsh NC, Sims NA, Herrero LJ, Mahalingam S. Bindarit, an inhibitor of monocyte chemotactic protein synthesis, protects against bone loss induced by chikungunya virus infection. J Virol. 2015;89:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Soden M, Vasudevan H, Roberts B, Coelen R, Hamlin G, Vasudevan S, La Brooy J. Detection of viral ribonucleic acid and histologic analysis of inflamed synovium in Ross River virus infection. Arthritis Rheum. 2000;43:365-369. [PubMed] [DOI] [Full Text] |
| 23. | Chang AY, Martins KAO, Encinales L, Reid SP, Acuña M, Encinales C, Matranga CB, Pacheco N, Cure C, Shukla B, Ruiz Arteta T, Amdur R, Cazares LH, Gregory M, Ward MD, Porras A, Rico Mendoza A, Dong L, Kenny T, Brueggemann E, Downey LG, Kamalapathy P, Lichtenberger P, Falls O, Simon GL, Bethony JM, Firestein GS. Chikungunya Arthritis Mechanisms in the Americas: A Cross-Sectional Analysis of Chikungunya Arthritis Patients Twenty-Two Months After Infection Demonstrating No Detectable Viral Persistence in Synovial Fluid. Arthritis Rheumatol. 2018;70:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Goupil BA, McNulty MA, Martin MJ, McCracken MK, Christofferson RC, Mores CN. Novel Lesions of Bones and Joints Associated with Chikungunya Virus Infection in Two Mouse Models of Disease: New Insights into Disease Pathogenesis. PLoS One. 2016;11:e0155243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Alpantaki K, Katonis P, Hadjipavlou AG, Spandidos DA, Sourvinos G. Herpes virus infection can cause intervertebral disc degeneration: a causal relationship? J Bone Joint Surg Br. 2011;93:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Galossi A, Guarisco R, Bellis L, Puoti C. Extrahepatic manifestations of chronic HCV infection. J Gastrointestin Liver Dis. 2007;16:65-73. [PubMed] |
| 27. | Panzer S, Seel E, Brunner M, Körmöczi GF, Schmid M, Ferenci P, Peck-Radosavljevic M. Platelet autoantibodies are common in hepatitis C infection, irrespective of the presence of thrombocytopenia. Eur J Haematol. 2006;77:513-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Buezo GF, García-Buey M, Rios-Buceta L, Borque MJ, Aragües M, Daudén E. Cryoglobulinemia and cutaneous leukocytoclastic vasculitis with hepatitis C virus infection. Int J Dermatol. 1996;35:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Mayo MJ. Extrahepatic manifestations of hepatitis C infection. Am J Med Sci. 2003;325:135-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Olariu M, Olariu C, Olteanu D. Thrombocytopenia in chronic hepatitis C. J Gastrointestin Liver Dis. 2010;19:381-385. [PubMed] |
| 31. | Cines DB, Liebman H, Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. 2009;46:S2-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 32. | Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976). 2000;25:3005-3013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 357] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 33. | Manfredi M, Corradi D, Vescovi P. Langerhans-cell histiocytosis: a clinical case without bone involvement. J Periodontol. 2005;76:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Caglioti C, Lalle E, Castilletti C, Carletti F, Capobianchi MR, Bordi L. Chikungunya virus infection: an overview. New Microbiol. 2013;36:211-227. [PubMed] |
| 35. | Edington F, Varjão D, Melo P. Incidence of articular pain and arthritis after chikungunya fever in the Americas: A systematic review of the literature and meta-analysis. Joint Bone Spine. 2018;85:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 701] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 37. | Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005;146:1991-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 38. | Souza PP, Lerner UH. The role of cytokines in inflammatory bone loss. Immunol Invest. 2013;42:555-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 39. | Binder NB, Niederreiter B, Hoffmann O, Stange R, Pap T, Stulnig TM, Mack M, Erben RG, Smolen JS, Redlich K. Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat Med. 2009;15:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Almohmeed YH, Avenell A, Aucott L, Vickers MA. Systematic review and meta-analysis of the sero-epidemiological association between Epstein-Barr virus and multiple sclerosis. PLoS One. 2013;8:e61110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Krutikov M, Manson J. Chikungunya Virus Infection: An Update on Joint Manifestations and Management. Rambam Maimonides Med J. 2016;7. [PubMed] [DOI] [Full Text] |
| 42. | Chirathaworn C, Chansaenroj J, Poovorawan Y. Cytokines and Chemokines in Chikungunya Virus Infection: Protection or Induction of Pathology. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Disser NP, De Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, Toresdahl BG, Rodeo SA, Casey EK, Mendias CL. Musculoskeletal Consequences of COVID-19. J Bone Joint Surg Am. 2020;102:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 253] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 44. | Talamonti G, Colistra D, Crisà F, Cenzato M, Giorgi P, D'Aliberti G. Spinal epidural abscess in COVID-19 patients. J Neurol. 2021;268:2320-2326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Tao H, Bai J, Zhang W, Zheng K, Guan P, Ge G, Li M, Geng D. Bone biology and COVID-19 infection: Is ACE2 a potential influence factor? Med Hypotheses. 2020;144:110178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |