Published online Aug 18, 2021. doi: 10.5312/wjo.v12.i8.565
Peer-review started: April 24, 2021
First decision: June 7, 2021
Revised: June 14, 2021
Accepted: July 9, 2021
Article in press: July 9, 2021
Published online: August 18, 2021
Processing time: 108 Days and 22.4 Hours
The quantitative alpha-defensin enzyme-linked immunosorbent assay (ELISA) demands a prior synovial fluid centrifugation, whereas this processing is not routinely required prior to the alpha-defensin lateral flow test.
To evaluate whether a prior synovial fluid centrifugation could lead the lateral flow performance to achieve comparable results to ELISA during periprosthetic joint infection (PJI) diagnosis.
Fifty-three cases were included in this study: 22 classified as PJI and 31 classified as aseptic cases, according to Musculoskeletal Infection Society 2013 criteria. Synovial fluid samples were submitted to centrifugation, and the supernatant was evaluated by ELISA and lateral flow tests. The sensitivity (SE), specificity (SP) and accuracy of each method were calculated as well as the agreement between those two methods.
In all of the 31 samples from aseptic patients, alpha-defensin ELISA and lateral flow tests showed negative results for infection. Regarding the 22 infected patients, the lateral flow test was positive in 19 cases (86.4%) and the ELISA was positive in 21 (95.5%). Sensibility, SP and accuracy were, respectively, 86.4% (95%CI: 65.1%-97.1%), 100% (95%CI: 88.8%-100%) and 93.2% (95%CI: 82.8%-98.3%) for the lateral flow test and 95.5% (95%CI: 77.2%-99.9%), 100% (95%CI: 88.8%-100%) and 98.1% (95%CI: 89.9%-100%) for ELISA. An agreement of 96.2% between those methods were observed. No statistical difference was found between them (P = 0.48).
Alpha-defensin lateral flow test showed high SE, SP and accuracy after a prior synovial fluid centrifugation, achieving comparable results to ELISA. Considering the lower complexity of the lateral flow and its equivalent performance obtained in this condition, a prior centrifugation might be added as a valuable step to enhance the PJI diagnosis.
Core Tip: This was a prospective study seeking to evaluate whether the synovial fluid centrifugation prior to the alpha-defensin lateral flow test leads to comparable results in relation to the alpha-defensin enzyme-linked immunosorbent assay (ELISA) during periprosthetic joint infection of the knee. Prior centrifugation of the synovial fluid showed to achieve high sensitivity, specificity and accuracy for the lateral flow test during periprosthetic joint infection diagnosis, leading to similar results in comparison to alpha-defensin ELISA.
- Citation: Abdo RCT, Gobbi RG, Leite CBG, Pasoto SG, Leon EP, Lima ALLM, Bonfa E, Pécora JR, Demange MK. Performance of alpha-defensin lateral flow test after synovial fluid centrifugation for diagnosis of periprosthetic knee infection. World J Orthop 2021; 12(8): 565-574
- URL: https://www.wjgnet.com/2218-5836/full/v12/i8/565.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i8.565
Total knee arthroplasty (TKA) is one of the most successful orthopedic procedures, providing excellent improvement in knee pain, function and quality of life[1]. With the population ageing and the growth incidence of symptomatic osteoarthritis, an increased number of TKA has been observed over the years[2,3]. Despite the most satisfactory results, several complications can occur after TKA, such as long-term pain, periprosthetic fractures, and joint infection[4]. Periprosthetic joint infection (PJI) after TKA is a catastrophic postoperative complication, that ranges from 0.5% to 3% of cases[5-7]. PJI can lead to serious consequences, including death[8], and accounts for a quarter of TKA revision surgeries[6], leading to a substantial economic impact on the healthcare system[9].
Although timing and precision of PJI diagnosis is critical for the patient's evolution, there is no one-hundred percent exam to provide its confirmation. For that reason, the Musculoskeletal Infection Society (MSIS) has developed a score for unifying PJI definition[10,11]. Considering the most updated criteria, alpha-defensin has been included as a new biomarker during the investigation of PJI[10].
Alpha-defensin is a neutrophil-released antimicrobial peptide[12] that increases in response to pathogens[13]. Nowadays, both the synovial alpha-defensin tests available [the quantitative enzyme-linked immunosorbent assay (ELISA) and the qualitative lateral flow test] provide important information during the investigation of PJI[14]. However, given the higher performance of ELISA, this test has a slight advantage[15,16]. The lateral flow test, despite the inferior performance, offers benefits regarding the ease of use, time-efficiency and cost[14]. One potential reason that could reduce the measurement of the lateral flow test is regarding the differences between fluid processing. While the synovial fluid sample has to be centrifuged preceding ELISA measurement, the same processing is not routinely performed before the lateral flow, according to the manufacturer's instructions. Thus, the maintenance of cellular debris and other particles within the synovial fluid could interfere in the results.
Here, we aimed to evaluate the performance of the alpha-defensin lateral flow test post synovial fluid centrifugation, and compare these results with the synovial alpha-defensin ELISA. Our hypothesis was that a prior centrifugation of the synovial fluid would achieve high sensitivity and specificity to predict knee PJI, leading to equivalent performance as alpha-defensin ELISA.
The study was approved by the local Institutional Review Board (2179456). Written informed consent was obtained from each patient prior to participation.
We conducted a prospective, cross-sectional diagnostic study to assess the performance of the alpha defensin lateral flow measured after synovial fluid centrifugation in patients under investigation of chronic knee PJI. Inclusion and exclusion criteria are displayed in Table 1.
| Inclusion criteria | Exclusion criteria |
| Any of the following suspicious signs or symptoms of chronic knee PJI (more than 90 d), as following: | Acute signs or symptoms of knee infection (less than 90 d) |
| Persistent knee pain (more than 3 mo), without other apparent cause | |
| Persistent joint effusion (more than 3 mo) | |
| Persistent local heat (more than 3 mo) | |
| Presence of draining sinus | |
| Early failure of the prosthesis (less than 5 yr) | |
| Radiographic findings suggesting infection[33] | |
| Have not used antibiotics for at least 4 wk before the evaluation | Insufficient synovial fluid volume during knee aspiration |
| Insufficient data for fulfilling the periprosthesis infection criteria[11] |
The primary outcome was to evaluate the sensitivity, specificity and accuracy of the lateral flow test post fluid centrifugation. Secondarily, we assessed the performance of the alpha-defensin ELISA in the same population of study, and compared the results between both modalities.
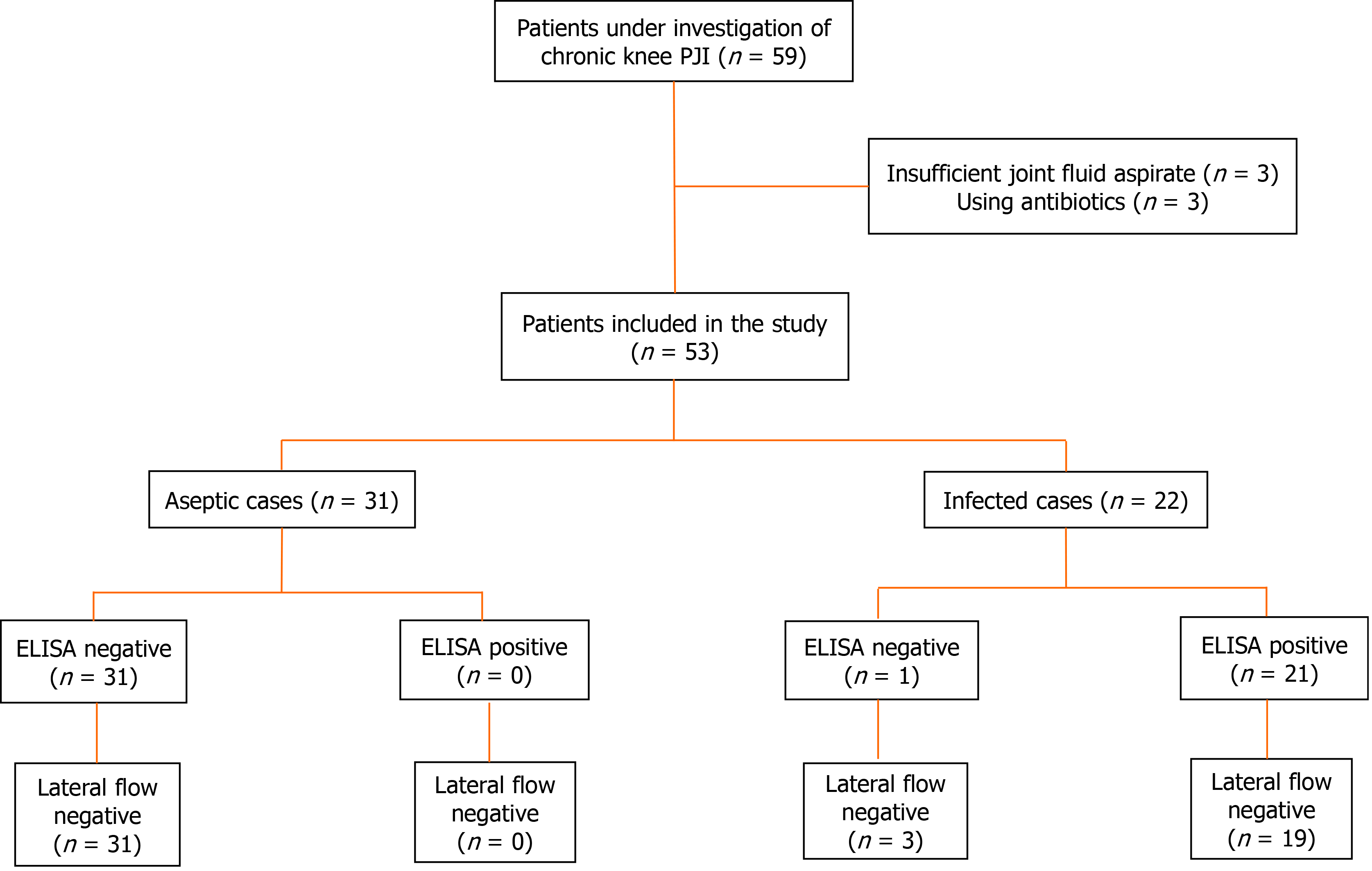
Initially, 59 patients were selected. Of these, three patients had insufficient joint fluid aspirate for analysis, and three patients were using antibiotics, being excluded from the study. A total of 53 patients were included. Figure 1 represents the flowchart of enrolled patients. The recruitment was performed between August 2016 and July 2019.
Among those 53 patients, 22 were diagnosed as infected, and 31 as aseptic. The revised MSIS 2013 criteria were used for the diagnosis of knee PJI[11].
Demographic data was recorded. Clinical examination and laboratory evaluations, including serum C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were collected on the same day as joint aspiration. Knee aspiration was conducted using the superolateral approach, with a 21-gauge needle and a 20-mL syringe. The possible maximum volume of synovial liquid was collected. In this study, at least 3 mL of joint fluid was required for proper analysis. All aspiration procedures were performed by the same author (RCTA).
After that, the synovial fluid samples were referred to the laboratory within 2 h. Part of the fluid from each sample was sent to analysis for cell count, percentage of polymorphonuclear leukocytes and cultures from aerobic, anaerobic and fungi. The remaining fluid was centrifuged for 10 minutes at 2700 rpm to separate all cell debris and particles. The supernatant was collected and divided into two aliquots, as following: approximately 1 mL of synovial fluid was referred to the qualitative alpha-defensin lateral flow test; the rest of the fluid was stored at -80° C until further immunoassay analyses. To quantify synovial alpha-defensin using ELISA, approximately 1.5 to 2 mL of synovial fluid was needed.
For the qualitative measurement, a lateral flow test (Synovasure® Zimmer-Biomet, Warsaw, IN, United States) was used according to the manufacturer's label. The centrifuged synovial fluid sample was diluted in the dilution buffer supplied by the kit, and deposited on the Synovasure® device. The qualitative result was read after 10 min. The result was considered positive for PJI if two lines appeared in the reading panel, regardless of its intensity.
For the quantitative alpha-defensin test, the commercial alpha-defensin (HNP1-3) ELISA kit (Hycult Biotech®, Uden, Netherlands) was employed. This ELISA kit is used to determine human HNP1-3. All assays were optimized and performed in duplicate by an experienced laboratory technician. The dilution optimization of the synovial fluid at 1:5000 was performed to decrease the effects of the fluid viscosity on the assay. Results were generated in optical density units (OD) using a spectrophotometer. The results in OD were plotted on the vertical axis with the corresponding concentration values on the horizontal axis (logarithmic scale). The concentration and the dilution factor were multiplied to reach alpha-defensin values in mg/L. The assay was optimized to operate at a cutoff value of 5.2 mg/L, based on previous studies[17,18].
Shapiro-Wilk test was applied to assess normality. Continuous variables were expressed as descriptive analysis, and categorical variables were expressed as proportions. To compare continuous variables, unpaired t-test or Mann-Whitney test were used, as appropriate. Fisher's exact test was applied to compare categorical variables. The cutoff value was obtained using the Receiver Operator Curve (ROC) through the SPSS software® (version 25.0 for Mac; SPSS, Chicago, IL), giving the results as a semiquantitative signal-to-cutoff ratio (S/CO) of 1.0. Sensitivity, specificity and accuracy (and 95%CI) of each method were calculated using the MSIS 2013 criteria as standard. The agreement between ELISA and lateral flow test was evaluated based on the percentage of concordant results, and McNemar's test was performed to calculate the statistical difference between those two alpha-defensin tests. Statistical significance was set at P < 0.05.
To further investigate whether draining sinus has influenced the tests results, we also calculated sensitivity, specificity and accuracy of each method excluding patients with fistulization.
Of the 53 patients included in the study, 31 were considered without infection (aseptic cases) and 22 were classified as infected. Table 2 shows the patient demographics.
| Total | Aseptic cases | Infected cases | P value | |
| n | 53 | 31 | 22 | |
| Sex | 0.221 | |||
| Male | 14 (26.4) | 6 (19.4) | 8 (36.4) | |
| Female | 39 (73.6) | 25 (80.6) | 14 (63.6) | |
| Age (range) | 68 (47-85) | 67 (47-85) | 70 (52-85) | 0.302 |
| Laterality | > 0.991 | |||
| Right knee | 28 (52.8) | 16 (51.6) | 12 (54.5) | |
| Left knee | 25 (47.2) | 15 (48.4) | 10 (45.5) | |
| Inflammatory disease | 12 (22.6) | 7 (22.6) | 5 (22.7) | > 0.991 |
| RA | 10 (18.9) | 7 (22.6) | 3 (13.6) | |
| Gout | 2 (3.8) | 0 | 2 (9.1) | |
| Sinus tract | 4 (7.5) | 0 | 4 (18.2) | |
| Alpha-defensin S/CO | 2.21 ± 2.73 | 0.28 ± 0.13 | 4.93 ± 2.28 | < 0.013 |
In relation to the aseptic cases, all lateral flow tests showed negative results for infection. Likewise, alpha-defensin ELISA showed a mean S/CO of 0.28 ± 0.13, which was considered negative for all cases.
Regarding the infected patients, lateral flow showed positive results in 19 cases (86.4%). The 3 false negatives occurred in patients with sinus tract. The ELISA presented 21 positive (95.5%) (mean S/CO-4.93 ± 2.28) and one negative result (S/CO-0.24). Similarly, this false negative case referred to a patient with draining sinus.
Lateral flow test showed a sensitivity of 86.4% (95%CI: 65.1%-97.1%), a specificity of 100% (95%CI: 88.8%-100%) and an accuracy of 93.2% (95%CI: 82.8%-98.3%). Alpha-defensin ELISA presented a sensitivity of 95.5% (95%CI: 77.2%-99.9%), a specificity of 100% (95%CI: 88.8%-100%) and an accuracy of 98.1% (95%CI: 89.9%-100%). Table 3 summarizes those findings.
| Aseptic | Infected | Sensitivity (95%CI) | Specificity (95%CI) | Accuracy (95%CI) | |
| ELISA | 95.5% (77.2%-99.9%) | 100% (88.8%-100%) | 98.1% (89.9%-100%) | ||
| Negative | 31 | 1 | |||
| Positive | 0 | 21 | |||
| Lateral flow | 86.4% (65.1%-97.1%) | 100% (88.8%-100%) | 93.2% (82.8%-98.3%) | ||
| Negative | 31 | 3 | |||
| Positive | 0 | 19 |
In terms of ROC analysis, area under curve was 93.2% (95%CI: 84.6%-100%) for the lateral flow test and 97.9% (95%CI: 93.6%-100%) for ELISA. The agreement between lateral flow and ELISA was observed in 51 cases (96.2%; 95%CI: 87.0%-99.5%). The two disagreement cases were false negatives for the lateral flow. No statistical difference between those two tests were found (P = 0.48).
Given that all false positive results occurred in patients with sinus tract, we performed an exploratory analysis to evaluate whether the lateral flow and the ELISA would change after excluding those selected patients (4 patients with sinus tract). In this situation, a sensitivity of 100% (95%CI: 81.5%-100%), specificity of 100% (95%CI: 88.8%-100%), accuracy of 100% (95%CI: 92.8%-100%) and agreement of 100% (95%CI: 92.9%-100%) were found for both tests (Table 4).
| Aseptic | Infected | Sensitivity (95%CI) | Specificity (95%CI) | Accuracy (95%CI) | |
| ELISA | 100% (81.5%-100%) | 100% (88.8%-100%) | 100% (92.8%-100%) | ||
| Negative | 31 | 0 | |||
| Positive | 0 | 18 | |||
| Lateral flow | 100% (81.5%-100%) | 100% (88.8%-100%) | 100% (92.8%-100%) | ||
| Negative | 31 | 0 | |||
| Positive | 0 | 18 |
The findings of this study reinforce our hypothesis that a prior synovial fluid centrifugation before the lateral flow measurement provides high sensitivity, specificity and accuracy, leading to comparable performance of the alpha-defensin ELISA, so far the best method to measure synovial alpha-defensin[15]. This preliminary finding may bring a novel concept to the major topic of PJI.
Diagnosis of PJI is frequently defiant, particularly in chronic infections in which the clinical symptoms might be subtle and inflammatory markers might be normal[19]. In this regard, a great need for new diagnostic tests is observed[20]. Alpha-defensin is a small antimicrobial peptide that acts as part of the host's innate immune response against pathogens[12]. After the pathogen insult, the release of alpha-defensin increases, and a rapid interaction of this peptide with the pathogen's membrane occurs. As a consequence, the membrane depolarizes, and the pathogen is killed[21]. Under a knee infection, the concentration of alpha-defensin elevates into the joint. Indeed, this synovial fluid biomarker has been studied for PJI diagnosis, providing exciting findings in terms of sensitivity and specificity[14,22]. It has been demonstrated that, even in the presence of inflammatory disease or antibiotic use, the results are similar[17]. Here, we opted to exclude patients using antibiotics to avoid potential bias. However, we did include patients with inflammatory diseases, which in fact did not influence those tests' performance. Due to its relevance and applicability, alpha-defensin has been included as a diagnostic criterion in the updated consensus of PJI[10].
Currently, there are two commercially available methods for the determination of synovial alpha-defensin. The quantitative laboratory-based ELISA, that requires a centrifuged synovial fluid to assess the concentration of alpha-defensin[17], and the qualitative lateral flow test. As mentioned, both tests have shown to be successful for the investigation of PJI, with ELISA presenting the best performance[17,18,23,24]. However, alpha-defensin ELISA is much more complex, requiring a laboratory structure and an experienced professional to be performed. Conversely, the lateral flow test can be done by the physician at any location, and the result is rapidly expressed within 10 minutes.
As suggested by our team, one potential reason for the inferior results regarding the lateral flow test is that, during its execution, fluid centrifugation is not performed (in accordance with the manufacturer's instruction). Consequently, some particles and cellular debris could lead to false results. Although some evidence shows that blood contamination does not influence the lateral flow reading[19], the sample processing is not equivalent between ELISA and lateral flow test, which may interfere in the device reading[15,16]. In this study, we indirectly suggest this plausible issue, since a favorable performance of the lateral flow test was reached after centrifugation. Here, we obtained a sensitivity of 86.4%, a specificity of 100% and an accuracy of 93.2%, superior values than the ones observed in some previous non-centrifuged studies. Indeed, sensitivity of approximately 67%-69%, specificity of 93%-94%, and accuracy of 85% were previously reported for the lateral flow test[25,26]. It is noteworthy to mention that, although some recent systematic reviews and meta-analyses present higher pooled values for the lateral flow, the moderate-to-high heterogeneity among the included studies compels careful interpretation. Even so, the 83% sensitivity and 94% specificity found in these studies are still slightly lower than the achieved here[14,27]. In our series, the centrifuged lateral flow performed similarly to ELISA, which also demonstrated excellent results in concordance to the literature[16,27].
Some authors have described false positive results using the lateral flow test in cases of metallosis[23,25] and crystal deposition disease[28,29]. This current study did not find any false positive case, despite the presence of four patients with gout. Once again, the centrifugation might improve the measurement by removing these particles. On the other hand, one false negative (by ELISA) and three false negatives (by the lateral flow test) were observed. All of those occurred in patients with sinus tract, as previously shown[30,31]. Although it was not directly investigated here, we speculate that the fistulization tends to drain the synovial fluid, avoiding the accumulation of pathogen and alpha-defensin within the knee. Considering the presence of draining sinus as a confirmation of PJI diagnosis, additional investigation would not be required. In this regard, excluding these specific patients, a sensitivity, specificity, accuracy and agreement of 100% were obtained for both tests.
The study has several limitations. First, we did not perform a direct comparison between centrifuged and non-centrifuged samples for the lateral flow test. Due to the high cost of lateral flow test in our region when this preliminary study was designed, we decided to compare these initial findings with the literature. As we know, there are several studies presenting remarkable data[14,25,26,32]. Further comparative trials are necessary and might add stronger conclusions. In addition, understanding the reason for false positive cases in patients with crystal arthropathy or metallosis, and the beneficial effects of synovial fluid centrifugation in these contexts may be valuable for its proper management. Moreover, despite the prospective design, the study was not randomized. Given the rarity of the cases that fit in our study, a randomization is impracticable. Therefore, we provide interesting data showing that a prior centrifugation may improve the lateral flow test performance. Considering the ease of execution and interpretation of the lateral flow, the addition of this prior step deserves further investigation and, potentially, a place in the PJI diagnosis.
In conclusion, we have identified that an extra step of synovial fluid centrifugation prior to the alpha-defensin lateral flow test achieved high sensitivity, specificity and accuracy. The results obtained using this methodology were comparable to those obtained with the alpha-defensin ELISA. Furthermore, centrifuged lateral flow demonstrated performance values slightly higher than the previously reported in the literature. Therefore, the use of the alpha-defensin lateral flow post synovial fluid centrifugation may represent a novel and interesting strategy during the PJI investigation given its lower complexity and equivalent performance in comparison to ELISA.
Periprosthetic joint infection (PJI) is a serious postoperative complication that leads to severe morbidity as well as substantial financial burden to the healthcare system. Currently, two synovial alpha-defensin tests [the quantitative enzyme-linked immunosorbent assay (ELISA) and the qualitative lateral flow test] are available and provide important information during PJI investigation, with the ELISA presenting slightly superior performance. However, the lateral flow test offers benefits in terms of the ease of use, time-efficiency and cost.
While the synovial fluid sample has to be centrifuged preceding ELISA, prior centrifugation is not routinely performed to the lateral flow test. The maintenance of synovial fluid debris could potentially interfere in the lateral flow results.
This study aimed to evaluate the performance of the alpha-defensin lateral flow test with prior synovial fluid centrifugation and compare the results with the synovial alpha-defensin ELISA.
In this prospective study, 53 cases of total knee arthroplasty were evaluated: 22 classified as PJI and 31 classified as aseptic knees. Synovial fluid samples were collected and submitted to centrifugation, and the supernatant was evaluated by lateral flow test and ELISA. Sensitivity, specificity, and accuracy of each method as well as the agreement between those two methods were calculated.
Alpha-defensin ELISA and lateral flow tests showed negative results for infection in all 31 aseptic patient samples. In regard to the 22 infected cases, the lateral flow test showed positive results in 19 cases (86.4%) whereas the ELISA was positive in 21 cases (95.5%). Sensibility, specificity, and accuracy were 86.4% (95%CI: 65.1%-97.1%), 100% (95%CI: 88.8%-100%) and 93.2% (95%CI: 82.8%-98.3%), respectively, for the lateral flow test and 95.5% (95%CI: 77.2%-99.9%), 100% (95%CI: 88.8%-100%) and 98.1% (95%CI: 89.9%-100%) for ELISA. Agreement of 96.2% between these two methods were found, without statistical difference between them (P = 0.48).
Alpha-defensin lateral flow test with prior synovial fluid centrifugation showed high sensitivity, specificity, and accuracy, achieving comparable results to ELISA. Given the lower complexity of the lateral flow test, a prior centrifugation might be a valuable strategy to enhance its performance.
Prior synovial fluid centrifugation may be a novel and interesting strategy to improve the lateral flow performance during the PJI diagnosis. Further investigation is required to clarify its actual benefit.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: M'Koma A S-Editor: Ma YJ L-Editor: A P-Editor: Xing YX
| 1. | Steinhaus ME, Christ AB, Cross MB. Total Knee Arthroplasty for Knee Osteoarthritis: Support for a Foregone Conclusion? HSS J. 2017;13:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Sloan M, Premkumar A, Sheth NP. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 1449] [Article Influence: 207.0] [Reference Citation Analysis (1)] |
| 3. | Price AJ, Alvand A, Troelsen A, Katz JN, Hooper G, Gray A, Carr A, Beard D. Knee replacement. Lancet. 2018;392:1672-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 518] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 4. | Lu J, Han J, Zhang C, Yang Y, Yao Z. Infection after total knee arthroplasty and its gold standard surgical treatment: Spacers used in two-stage revision arthroplasty. Intractable Rare Dis Res. 2017;6:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Martínez-Pastor JC, Maculé-Beneyto F, Suso-Vergara S. Acute infection in total knee arthroplasty: diagnosis and treatment. Open Orthop J. 2013;7:197-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Mallon CM, Gooberman-Hill R, Moore AJ. Infection after knee replacement: a qualitative study of impact of periprosthetic knee infection. BMC Musculoskelet Disord. 2018;19:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Parvizi J, Ghanem E, Sharkey P, Aggarwal A, Burnett RS, Barrack RL. Diagnosis of infected total knee: findings of a multicenter database. Clin Orthop Relat Res. 2008;466:2628-2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Gottfriedsen TB, Schrøder HM, Odgaard A. Transfemoral Amputation After Failure of Knee Arthroplasty: A Nationwide Register-Based Study. J Bone Joint Surg Am. 2016;98:1962-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 2012; 27: 61-5. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1225] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 10. | Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty 2018; 33: 1309-1314. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 885] [Cited by in RCA: 1410] [Article Influence: 201.4] [Reference Citation Analysis (0)] |
| 11. | Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29:1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 691] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 12. | Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1030] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 13. | Brook M, Tomlinson GH, Miles K, Smith RW, Rossi AG, Hiemstra PS, van 't Wout EF, Dean JL, Gray NK, Lu W, Gray M. Neutrophil-derived alpha defensins control inflammation by inhibiting macrophage mRNA translation. Proc Natl Acad Sci U S A. 2016;113:4350-4355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Balato G, de Matteo V, Ascione T, Di Donato SL, De Franco C, Smeraglia F, Baldini A, Mariconda M. Laboratory-based vs qualitative assessment of α-defensin in periprosthetic hip and knee infections: a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2020;140:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Suen K, Keeka M, Ailabouni R, Tran P. Synovasure 'quick test' is not as accurate as the laboratory-based α-defensin immunoassay: a systematic review and meta-analysis. Bone Joint J. 2018;100-B:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Ahmad SS, Hirschmann MT, Becker R, Shaker A, Ateschrang A, Keel MJB, Albers CE, Buetikofer L, Maqungo S, Stöckle U, Kohl S. A meta-analysis of synovial biomarkers in periprosthetic joint infection: Synovasure™ is less effective than the ELISA-based alpha-defensin test. Knee Surg Sports Traumatol Arthrosc. 2018;26:3039-3047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-Defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96:1439-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 18. | Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Booth RE Jr, Parvizi J. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473:198-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Renz N, Yermak K, Perka C, Trampuz A. Alpha Defensin Lateral Flow Test for Diagnosis of Periprosthetic Joint Infection: Not a Screening but a Confirmatory Test. J Bone Joint Surg Am. 2018;100:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 20. | Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int J Artif Organs. 2012;35:923-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin Accuracy to Diagnose Periprosthetic Joint Infection-Best Available Test? J Arthroplasty. 2016;31:456-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472:3254-3262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 23. | Bonanzinga T, Zahar A, Dütsch M, Lausmann C, Kendoff D, Gehrke T. How Reliable Is the Alpha-defensin Immunoassay Test for Diagnosing Periprosthetic Joint Infection? Clin Orthop Relat Res. 2017;475:408-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 24. | Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472:4006-4009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 25. | Kasparek MF, Kasparek M, Boettner F, Faschingbauer M, Hahne J, Dominkus M. Intraoperative Diagnosis of Periprosthetic Joint Infection Using a Novel Alpha-Defensin Lateral Flow Assay. J Arthroplasty. 2016;31:2871-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Sigmund IK, Holinka J, Gamper J, Staats K, Böhler C, Kubista B, Windhager R. Qualitative α-defensin test (Synovasure) for the diagnosis of periprosthetic infection in revision total joint arthroplasty. Bone Joint J. 2017;99-B:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | Kuiper JWP, Verberne SJ, Vos SJ, van Egmond PW. Does the Alpha Defensin ELISA Test Perform Better Than the Alpha Defensin Lateral Flow Test for PJI Diagnosis? Clin Orthop Relat Res. 2020;478:1333-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Plate A, Stadler L, Sutter R, Anagnostopoulos A, Frustaci D, Zbinden R, Fucentese SF, Zinkernagel AS, Zingg PO, Achermann Y. Inflammatory disorders mimicking periprosthetic joint infections may result in false-positive α-defensin. Clin Microbiol Infect 2018; 24: 1212.e1-1212. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Partridge DG, Gordon A, Townsend R. False-positive synovial fluid alpha-defensin test in a patient with acute gout affecting a prosthetic knee. Eur J Orthop Surg Traumatol. 2017;27:549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Adams JR, Schwartz AJ. False-negative synovial alpha-defensin. Arthroplast Today. 2017;3:239-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Gehrke T, Lausmann C, Citak M, Bonanzinga T, Frommelt L, Zahar A. The Accuracy of the Alpha Defensin Lateral Flow Device for Diagnosis of Periprosthetic Joint Infection: Comparison with a Gold Standard. J Bone Joint Surg Am. 2018;100:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 32. | Balato G, Franceschini V, Ascione T, Lamberti A, D'Amato M, Ensini A, Baldini A. High performance of α-defensin lateral flow assay (Synovasure) in the diagnosis of chronic knee prosthetic infections. Knee Surg Sports Traumatol Arthrosc. 2018;26:1717-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Math KR, Scheider R. Imaging of the Painful TKR. In: Scuderi GR, Tria AJ, editors. Surgical Techniques in Total Knee Arthroplasty. New York, NY: Springer New York, 2002: 351-367. |