Published online May 18, 2021. doi: 10.5312/wjo.v12.i5.292
Peer-review started: January 16, 2021
First decision: January 24, 2021
Revised: February 18, 2021
Accepted: April 9, 2021
Article in press: April 9, 2021
Published online: May 18, 2021
Processing time: 115 Days and 19 Hours
Maximum surgical blood order schedules were designed to eliminate unnecessary preoperative crossmatching prior to surgery in order to conserve blood bank resources. Most protocols recommend type and cross of 2 red blood cell (RBC) units for patients undergoing surgery for treatment of hip fracture. Preoperative hemoglobin has been identified as the strongest predictor of inpatient transfusion, but current maximum surgical blood order schedules do not consider preoperative hemoglobin values to determine the number of RBC units to prepare prior to surgery.
To determine the preoperative hemoglobin level resulting in the optimal 2:1 crossmatch-to-transfusion (C:T) ratio in hip fracture surgery patients.
In 2015 a patient blood management (PBM) program was implemented at our institution mandating a single unit-per-occurrence transfusion policy and a restrictive transfusion threshold of < 7 g/dL hemoglobin in asymptomatic patients and < 8 g/dL in those with refractory symptomatic anemia or history of coronary artery disease. We identified all hip fracture patients between 2013 and 2017 and compared the preoperative hemoglobin which would predict a 2:1 C:T ratio in the pre PBM and post PBM cohorts. Prediction profiling and sensitivity analysis were performed with statistical significance set at P < 0.05.
Four hundred and ninety-eight patients who underwent hip fracture surgery between 2013 and 2017 were identified, 291 in the post PBM cohort. Transfusion requirements in the post PBM cohort were lower (51% vs 33%, P < 0.0001) than in the pre PBM cohort. The mean RBC units transfused per patient was 1.15 in the pre PBM cohort, compared to 0.66 in the post PBM cohort (P < 0.001). The 2:1 C:T ratio (inpatient transfusion probability of 50%) was predicted by a preoperative hemoglobin of 12.3 g/dL [area under the curve (AUC) 0.78 (95% confidence interval (CI), 0.72-0.83), Sensitivity 0.66] in the pre PBM cohort and 10.7 g/dL [AUC 0.78 (95%CI, 0.73-0.83), Sensitivity 0.88] in the post PBM cohort. A 50% probability of requiring > 1 RBC unit was predicted by 11.2g/dL [AUC 0.80 (95%CI, 0.74-0.85), Sensitivity 0.87] in the pre PBM cohort and 8.7g/dL [AUC 0.78 (95%CI, 0.73-0.83), Sensitivity 0.84] in the post-PBM cohort.
The hip fracture maximum surgical blood order schedule should consider preoperative hemoglobin in determining the number of units to type and cross prior to surgery.
Core Tip: Implementation of patient blood management programs has led to a decrease in transfusion needs in hip fracture surgery patients. Preoperative hemoglobin plays a significant role in determining transfusion needs in these patients. Maximum surgical blood order schedules should be adjusted based on preoperative hemoglobin values to reduce unnecessary blood product waste and conserve resources.
- Citation: Amin RM, Puvanesarajah V, Chaudhry YP, Best MJ, Rao SS, Frank SM, Hasenboehler EA. Reducing unnecessary crossmatching for hip fracture patients by accounting for preoperative hemoglobin concentration. World J Orthop 2021; 12(5): 292-300
- URL: https://www.wjgnet.com/2218-5836/full/v12/i5/292.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i5.292
Patients with hip fractures constitute a particularly frail patient population due to their elevated comorbidity burden[1]. Given the nature of the injury and poor health status of this population, many of these patients require perioperative allogeneic red blood cell (RBC) transfusions[2,3]. Transfusions in this population are not without risk as they are independently associated with surgical site infection and 1-year mortality and also represent a limited resource[4-7]. As such, substantial focus has been placed upon reducing the use of allogeneic RBCs in this population[8]. Several measures including quicker time to surgery, conservative transfusion practices, and use of anti-fibrinolytic agents have been employed to successfully to reduce the transfusion incidence from 50% of patients to 30%-35% more recently[8-13].
As blood transfusion rates have decreased, adjusting the number of units pre-operatively cross-matched is an important consideration. Over-crossmatching increases both the number of units taken out of the available blood bank inventory and the likelihood of discarding those units as they become closer to their storage duration limit[14]. To successfully reduce unneeded type-and-cross (T&C) of blood prior to major surgical procedures, maximum surgical blood order schedules (MSBOS) have been widely adopted[15]. Several algorithms for calculation have been employed with a consensus placed on optimum crossmatch to transfusion (C:T) ratio of 2:1 for any surgical procedures[14,16,17]. These base crossmatching recommendations on surgical procedure to be performed without incorporation of patient specific variables [19]. Patient specific factors have been previously shown to better predict RBC needs but are not employed in MSBOS[19-21].
Nationally, most institutions practice crossmatching 2 units RBC ahead of hip fracture surgery[17]. These guidelines may be based on RBC utilization with a transfusion threshold of < 8 g/dL, as recommended by the American Association of Blood Banks and American Academy of Orthopaedic Surgeons[23,24], however, an increasing body of recent literature has demonstrated that transfusion thresholds of Hgb < 7 g/dL are safe in this population[10]. As such, using this crossmatch generalization for all hip fracture patients inadvertently results in over-crossmatching for many patients, while potentially under cross-matching for a small subset of very frail patients. It stands to reason that more accurate cross-matching practices could be determined by using broadly understood patient specific factors, including preoperative hemoglobin, which, although not typically considered in the MSBOS, has been identified as one of the strongest predictors of transfusion requirements[2,25-27].
In an era of increased emphasis on value-based care, incorporation of simple, predictive patient factors is necessary to prevent unneeded crossmatching. With our implementation of a widely utilized restrictive transfusion strategy (hemoglobin transfusion threshold of < 7 g/dL in asymptomatic patients, < 8 g/dL in history of coronary artery disease, and single RBC unit per occurrence) we sought to redefine clinically significant anemia (the Hgb level which predicts a 50% transfusion rate) to reduce the number of units crossmatched for this population.
Institutional review board approval was obtained for this study. Documentation of consent was waived by our institutional review board as this was a minimal risk retrospective review of electronic medical record data.
We reviewed the medical records of 498 consecutive patients admitted to our level 2 trauma center from January 2013 to May 2017. Patients with Arbeitsgemeinschaft für Osteosynthesefragen classification fractures of 31A1, 31A2, 31A3, 31B1, 31B2, 31B3, 31C1, 31C2, 31C3 were eligible for inclusion. Patients were excluded if they were both admitted and discharged by the acute care general surgery trauma service or if the fracture was oncologic in nature. The prior exclusion criteria were based on the high likelihood of additional, nonorthopaedic, surgical procedures in these population per our institutional admitting guidelines.
Beginning in January 2015 a patient blood management (PBM) program was implemented at our institution. This program included lowering postoperative RBC transfusion threshold to Hgb < 7 g/dL in hemodynamically stable patients and < 8 g/dL in patients with a history of coronary artery disease or symptomatic anemia (hypotension and/or tachycardia refractory to fluid management, lightheadedness, and lethargy), and single-unit pRBC transfusions followed by reassessment before transfusion of additional units. Antifibrinolytic agents such as tranexamic acid were not used in our patient population. Based on the implementation date of January 1, 2015, patients were stratified into a pre-PBM group (January 2013 through December 2014, n = 201) and a post-PBM group (January 2015 through May 2017, n = 297) for analysis.
Pre-, peri- and post-operative outcomes were analyzed. The primary outcome was the admission hemoglobin which predicted the 2:1 C:T ratio (50% probability of a transfusion event during the entire hospital stay) in the pre- and post-PBM cohorts. The secondary outcome was the admission hemoglobin which predicted a 50% probability of multiple unit transfusion in the pre- and post- PBM cohorts. The mean RBC units transfused per patient was calculated for the pre- and post-PBM cohorts and compared using the Kruskal-Wallis test. Predictive modeling was utilized to generate a receiver operating curve and determine the pre- and post-PBM hemoglobin threshold which predicted a 50% transfusion event rate during the entire admission. This is consistent with previously described methods[16,28]. 95% confidence intervals (CI) were reported for each area under the curve (AUC) value. Two-sided t-tests were used for univariate analysis of continuous variables, and chi-square tests were used for categorical variables. A value of P < 0.05 (two-sided) was considered statistically significant. Analyses were performed using JMP, version 13.0.0, software (SAS Institute, Cary, NC).
Preoperative characteristics of the two cohorts are demonstrated in Table 1. The post-PBM cohort had a slightly higher rate of congestive heart failure. There were no differences in preoperative use of anticoagulation, dual antiplatelet therapy, cardiopulmonary risk factors, need for transfusion at Hgb < 8 g/dL instead of < 7 g/dL, fracture location, or treatment type. The preponderance of patients in both cohorts had intertrochanteric fractures treated by cephalomedullary nailing.
| Variable | Pre-PBM (n = 201) (January 2013 – December 2014) | Post-PBM (n = 297) (January 2015 – May 2017) | P value | ||
| mean ± SD | mean ± SD | ||||
| Age (yr) | 77 ± 15 | 76 ± 16 | 0.68 | ||
| Case mix index1 | 1.9 ± 0.6 | 1.9 ± 0.9 | 0.36 | ||
| Preoperative Hb, g/dL | 12.1 ± 1.9 | 12.1 ± 2.0 | 0.96 | ||
| Male sex | 58 (29) | 110 (37) | 0.26 | ||
| Cardiac disease | 42 (20.3) | 67 (23.0) | |||
| Congestive heart failure | 11 (5.3) | 19 (6.5) | 0.04 | ||
| Pacemaker | 18 (8.7) | 37 (12.7) | 0.57 | ||
| Chronic kidney disease | 18 (8.7) | 37 (12.7) | 0.15 | ||
| COPD | 30 (14.5) | 47 (16.2) | |||
| Requiring home oxygen | 9 (4.3) | 14 (4.8) | |||
| Anti-platelet agent use | 80 (38.7) | 103 (35.4) | 0.57 | ||
| Anticoagulation use | 27 (12.0) | 48 (16.5) | 0.29 | ||
| Extracapsular Fracture | 127 (61) | 169 (58) | 0.46 | ||
| Independent in ADL | 152 (73.4) | 215 (73.9) | 0.91 | ||
| Cephalomedullary nail | 122 (59) | 161 (55) | 0.78 | ||
The mean RBC units transfused per patient in the post-PBM cohort was 0.66, significantly lower than the 1.15 RBC units per patient in the pre-PBM cohort (P < 0.001). In the post-PBM cohort, both the percentage of patients requiring one or more units of RBCs declined significantly (51% vs 33%, P < 0.0001) (Table 1).
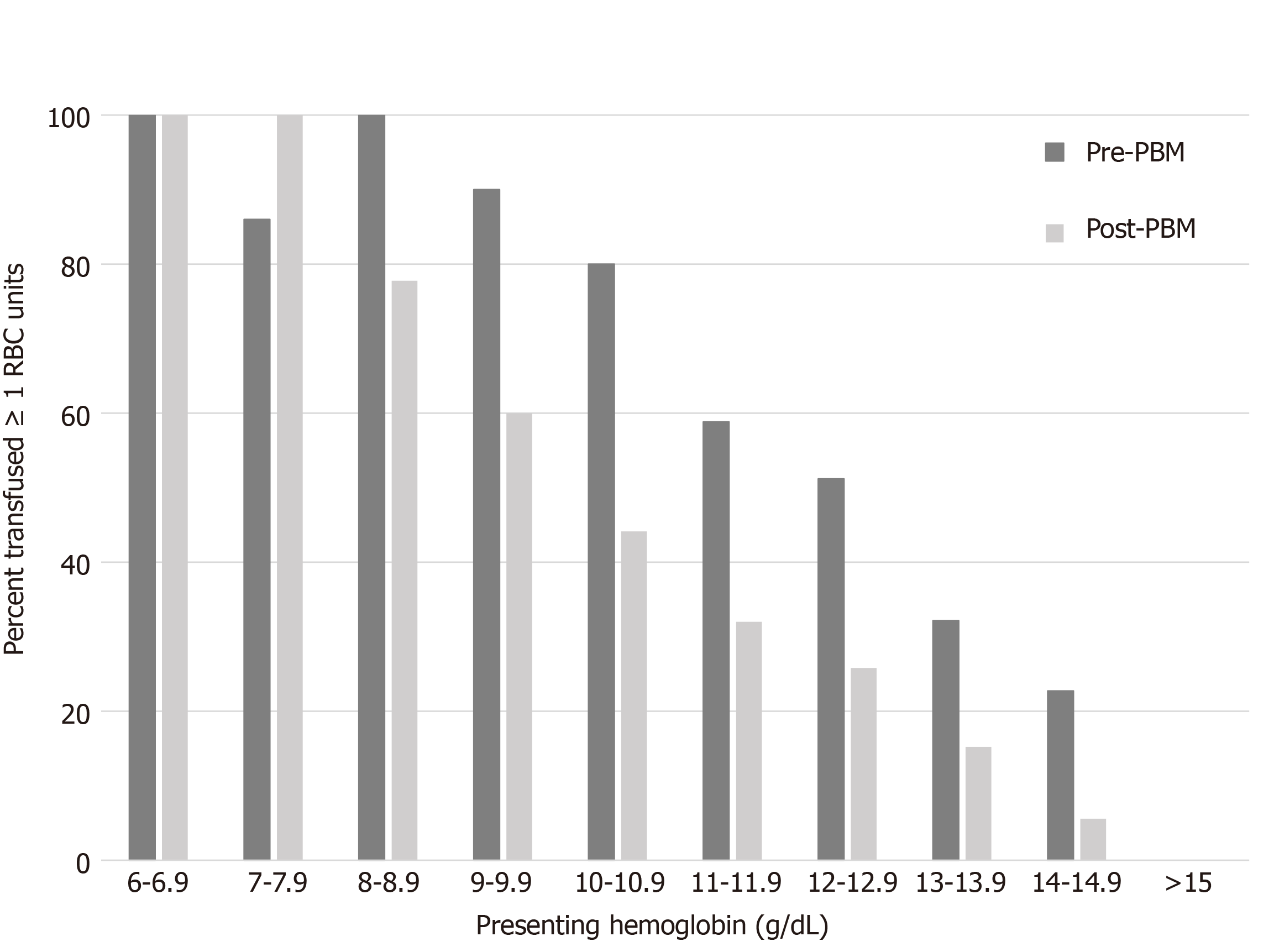
With implementation of the PBM program, a 50% probability of a transfusion event in the post PBM cohort was predicted by a preoperative hemoglobin of Hgb 10.7 [AUC 0.78 (95%CI, 0.73-0.83), Sensitivity 0.88]. For those patients in our study with a preoperative hemoglobin of > 10.7 g/dL, implementation of a single unit crossmatch policy would have resulted in 6.3% of our population initially undercrossmatched by 1 pRBC and 5.2% by > 1 unit. For the pre-PBM cohort a 50% probability of transfusion event was predicted by a preoperative Hgb of 12.3 [AUC 0.78 (95%CI, 0.72-0.83), Sensitivity 0.66] (Figure 1).
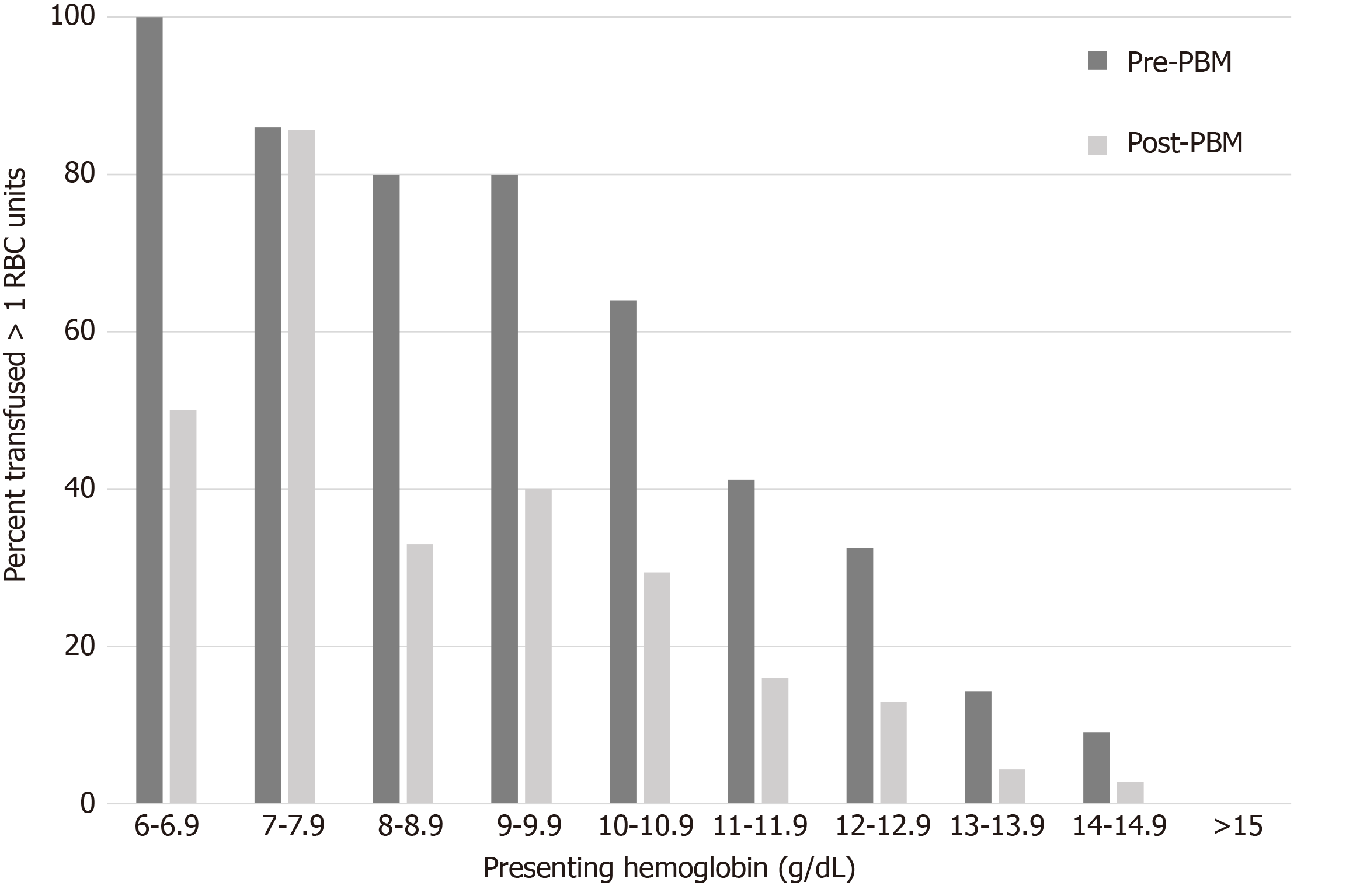
Multiple unit transfusion status was substantially different between the two cohorts. The pre-PBM cohort had a 50% probability of multiple unit transfusion at a preoperative hemoglobin of 11.2 [AUC 0.80 (95%CI, 0.74-0.85), Sensitivity 0.87] (Figure 2) whereas this value was hemoglobin 8.7 [AUC 0.78 (95%CI, 0.73-0.83), Sensitivity 0.84] (Figure 2) in the post-PBM cohort. For those patients in our study with a preoperative hemoglobin of > 8.7 g/dL, use of a 2:1 unit crossmatch policy would have resulted in undercrossmatching 4.3% of our population by 1 unit and 2.9% by > 1 unit for the entire hospitalization.
With PBM programs and improvements in peri-operative care of hip fracture patients, the RBC transfusion requirements of this population have declined. Thus, the routine crossmatch of 2 units preoperatively may not be necessary. Our data suggest that with a restrictive transfusion threshold of Hgb < 7 g/dL and single unit per transfusion occurrence, a substantial number of patients require only a type-and-screen (T&S), or T&C of one RBC unit during their inpatient hospitalization. Specifically, the ideal preoperative blood orders may be a T&C of 2 units with a preoperative Hgb < 9 g/dL, a T&C of 1 unit with a preoperative Hgb < 11 g/dL, and a T&S when the preoperative Hgb is > 11 g/dL.
Though the hip fracture patient population is well-studied in the literature, data regarding hemoglobin thresholds for optimal preoperative blood ordering are relatively scarce. Multiple studies have found that preoperative hemoglobin values of 7-12 g/dL predict perioperative transfusion, though few have used this information to determine a cross-matching algorithm[27,29,30]. To our knowledge, only one study has risk stratified the number of units to crossmatch preoperatively based on starting preoperative hemoglobin for patients with hip fracture. The 2002 study advocated for crossmatching of 2 units for Hgb > 12.5 g/dL and 4 units for Hgb 10-12.5 g/dL. Given the vast aforementioned improvements in the care of patients with hip fracture of the past decade and the transfusion threshold of 10 g/dL utilized in this 2002 study, these recommendations are likely not suitable for current practice[31].
While there are many individual preoperative patient characteristics that can be utilized to predict transfusion requirements, the use of preoperative hemoglobin is likely the simplest marker which is well understood by all ordering providers. Though time to surgery[32], fracture site (intra-capsular vs extra capsular)[3,12], and anesthesia type[33] predict blood utilization, the associations have not been documented as thoroughly as that of preoperative hemoglobin[32]. Moreover, the decision to preoperatively allocate blood products is considered by multiple independent practitioners (emergency medicine, anesthesiology, internal medicine, and surgeons). Incorporating the aforementioned factors, which are not as easily obtainable as preoperative hemoglobin, may not be reasonable and may result in reversion to generalized cross matching practices for all patients.
The benefits to both the patient and health system with reduction of unneeded preoperative crossmatching are substantial. Unneeded crossmatching removes RBC units from the available inventory for the general population placing increasing strain on blood banks by requiring a larger volume of stored RBCs, and pushes blood products closer to their expiration date[34]. Although longer RBC storage duration does not appear to influence clinical outcomes[35,36], wastage of product reaching the expiration date could be problematic.
This study is not without limitations. First, our study is also retrospective in nature and requires prospective validation. We do not advocate for proceeding to the operating room without obtaining a T&S, which at our institution costs 5-fold more than a crossmatch. Second practitioners may fear underordering products in the event there is a need for emergency release (uncrossmatched type-O) blood. However, implementation of a restrictive MSBOS has been shown to not increase the rate of emergency release transfusions[37]. Moreover, data suggest less than 5% of hip fracture patients require intraoperative transfusion, and that these 5% of patients are highly predictable with multiple independent risk factors[30]. Additionally, with electronic remote blood issue and electronic crossmatching the time from the need for urgent blood products in the OR to delivery has been reduced substantially[38-39]. Should emergent release blood be needed, type-O blood has been shown in multiple studies to be exceedingly safe in all patients[37,40,41]. Type-O blood is associated with a 0.2% incidence of mild delayed hemolytic reaction which is lower than the risk of major ABO transfusion reaction due to clerical errors and wrong unit transfusion[37], the decision to obtain only a type and screen ahead of the OR as opposed to crossmatching 1 unit may cause concern for delays in availability, however the electronic crossmatch has mitigated this problem for patients with a negative antibody screen[39].
Our findings suggest that a hemoglobin < 11 g/dL and < 9 g/dL warrants a T&C of only 1 and 2 units, respectively, and a hemoglobin > 11g/dL warrants consideration of only a T&S. The MSBOS may over allocate RBC units in the era of restrictive transfusion thresholds and we propose that preoperative hemoglobin levels should be considered in determining the number of units to type and cross prior to surgery.
Blood product utilization is becoming increasingly scrutinized in orthopaedic surgery as restrictive transfusion triggers and conservative blood management strategies have become more common.
As transfusion frequency decreases through implementation of restrictive blood management practices, a rethinking of preoperative blood product allocation is required. Rather than using standardized maximum surgical blood order schedules (MSBOS), we wanted to investigate the ideal type and cross ratios for hip fracture patients while accounting for preoperative hemoglobin values.
The aims of this study were to characterize and compare the ideal 2:1 crossmatch to transfusion ratio in hip fracture patients before and after the implementation of a restrictive blood management policy at our institution.
A retrospective review was conducted of all operatively treated hip fractures at our institution from January 2013 through May 2017. Cases were split up based on whether they occurred before or after implementation of a patient blood management (PBM) program (January 2015). Receiver operating curve analyses were used to determine the preoperative hemoglobin levels predicting 50% transfusion events in the pre- and post-PBM cohorts.
Implementation of the PBM resulted in a significant decrease in transfusion requirements from the pre- to post-PBM cohorts (51% vs 33%, P < 0.0001). Additionally, the post-PBM cohort was much less likely to receive multiple transfusions. Compared to the pre-PBM cohort, the post-PBM cohort had a much lower preoperative hemoglobin value that predicted a 50% transfusion probability.
In order to more appropriately allocate blood product resources, hip fracture MSBOS should be updated to reflect current restrictive transfusion strategies and should consider preoperative patient hemoglobin values.
Further study at other institutions is warranted to validate the generalizability of our findings. To help conserve resources, additional MSBOS studies are warranted in other orthopaedic trauma surgery procedures as well.
This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhu CT S-Editor: Liu M L-Editor: A P-Editor: Xing YX
| 1. | Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 434] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 2. | Desai SJ, Wood KS, Marsh J, Bryant D, Abdo H, Lawendy AR, Sanders DW. Factors affecting transfusion requirement after hip fracture: can we reduce the need for blood? Can J Surg. 2014;57:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Puckeridge G, Terblanche M, Wallis M, Fung YL. Blood management in hip fractures; are we leaving it too late? BMC Geriatr. 2019;19:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Ercin E, Bilgili MG, Sari C, Basaran SH, Tanriverdi B, Edipoglu E, Celen KM, Cetingok H, Kural C. Risk factors for mortality in geriatric hip fractures: a compressional study of different surgical procedures in 785 consecutive patients. Eur J Orthop Surg Traumatol. 2017;27:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Carow J, Carow JB, Coburn M, Kim BS, Bücking B, Bliemel C, Bollheimer LC, Werner CJ, Bach JP, Knobe M. Mortality and cardiorespiratory complications in trochanteric femoral fractures: a ten year retrospective analysis. Int Orthop. 2017;41:2371-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Dodd AC, Bulka C, Jahangir A, Mir HR, Obremskey WT, Sethi MK. Predictors of 30-day mortality following hip/pelvis fractures. Orthop Traumatol Surg Res. 2016;102:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Heyes GJ, Tucker A, Marley D, Foster A. Predictors for Readmission up to 1 Year Following Hip Fracture. Arch Trauma Res. 2015;4:e27123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Qiu M, Zhang X, Cai H, Xu Z, Lin H. The impact of hemocoagulase for improvement of coagulation and reduction of bleeding in fracture-related hip hemiarthroplasty geriatric patients: A prospective, single-blinded, randomized, controlled study. Injury. 2017;48:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Alvi HM, Thompson RM, Krishnan V, Kwasny MJ, Beal MD, Manning DW. Time-to-Surgery for Definitive Fixation of Hip Fractures: A Look at Outcomes Based Upon Delay. Am J Orthop (Belle Mead NJ). 2018;47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Amin RM, DeMario VM, Best MJ, Shafiq B, Hasenboehler EA, Sterling RS, Frank SM, Khanuja HS. A Restrictive Hemoglobin Transfusion Threshold of Less Than 7 g/dL Decreases Blood Utilization Without Compromising Outcomes in Patients With Hip Fractures. J Am Acad Orthop Surg. 2019;27:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Farrow LS, Smith TO, Ashcroft GP, Myint PK. A systematic review of tranexamic acid in hip fracture surgery. Br J Clin Pharmacol. 2016;82:1458-1470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Morris R, Rethnam U, Russ B, Topliss C. Assessing the impact of fracture pattern on transfusion requirements in hip fractures. Eur J Trauma Emerg Surg. 2017;43:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Fazal MA, Bagley C, Garg P. Predictors for perioperative blood transfusion in elderly patients with extra capsular hip fractures treated with cephalo-medullary nailing. Chin J Traumatol. 2018;21:16-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Woodrum CL, Wisniewski M, Triulzi DJ, Waters JH, Alarcon LH, Yazer MH. The effects of a data driven maximum surgical blood ordering schedule on preoperative blood ordering practices. Hematology. 2017;22:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Friedman BA, Oberman HA, Chadwick AR, Kingdon KI. The maximum surgical blood order schedule and surgical blood use in the United States. Transfusion. 1976;16:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 158] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Hall TC, Pattenden C, Hollobone C, Pollard C, Dennison AR. Blood Transfusion Policies in Elective General Surgery: How to Optimise Cross-Match-to-Transfusion Ratios. Transfus Med Hemother. 2013;40:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Hayse S. Maximum Surgical Blood Ordering Schedule. [cited 10 January 2021]. Available from: https://www.gov.nl.ca/hcs/files/bloodservices-pdf-max-surgical-blood-order.pdf. |
| 18. | Barreto SG, Singh A, Perwaiz A, Singh T, Singh MK, Chaudhary A. Maximum surgical blood order schedule for pancreatoduodenectomy: a long way from uniform applicability! Future Oncol. 2017;13:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Nuttall GA, Santrach PJ, Oliver WC, Jr. , Ereth MH, Horlocker TT, Cabanela ME, Trousdale RT, Schroeder DR. Possible guidelines for autologous red blood cell donations before total hip arthroplasty based on the surgical blood order equation. Mayo Clin Proc. 2000;75:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Nuttall GA, Horlocker TT, Santrach PJ, Oliver WC, Jr. , Dekutoski MB, Bryant S. Predictors of blood transfusions in spinal instrumentation and fusion surgery. Spine (Phila Pa 1976). 2000;25:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 184] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Nuttall GA, Santrach PJ, Oliver WC, Jr. , Ereth MH, Horlocker TT, Cabanela ME, Trousdale RT, Bryant S, Currie TW. A prospective randomized trial of the surgical blood order equation for ordering red cells for total hip arthroplasty patients. Transfusion. 1998;38:828-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, Gernsheimer T, Holcomb JB, Kaplan LJ, Katz LM, Peterson N, Ramsey G, Rao SV, Roback JD, Shander A, Tobian AA. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 758] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 23. | Roberts KC, Brox WT, Jevsevar DS, Sevarino K. Management of hip fractures in the elderly. J Am Acad Orthop Surg. 2015;23:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 24. | Gupta PB, DeMario VM, Amin RM, Gehrie EA, Goel R, Lee KHK, Yang WW, Khanuja HS, Sterling RS, Ness PM, Frank SM. Patient Blood Management Program Improves Blood Use and Clinical Outcomes in Orthopedic Surgery. Anesthesiology. 2018;129:1082-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Nuttall GA, Santrach PJ, Oliver WC, Jr. , Horlocker TT, Shaughnessy WJ, Cabanela ME, Bryant S. The predictors of red cell transfusions in total hip arthroplasties. Transfusion. 1996;36:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 106] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Madsen CM, Jorgensen HL, Norgaard A, Riss T, Jantzen C, Pedersen OB, Duss BR, Lauritzen JB. Preoperative factors associated with red blood cell transfusion in hip fracture patients. Arch Orthop Trauma Surg. 2014;134:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Adunsky A, Lichtenstein A, Mizrahi E, Arad M, Heim M. Blood transfusion requirements in elderly hip fracture patients. Arch Gerontol Geriatr. 2003;36:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Viberg B, Gundtoft PH, Schønnemann J, Pedersen L, Andersen LR, Titlestad K, Madsen CF, Lauritsen J, Overgaard S. Introduction of national guidelines for restrictive blood transfusion threshold for hip fracture patients--a consecutive cohort study based on complete follow-up in national databases. J Orthop Surg Res. 2018;13:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Kumar D, Mbako AN, Riddick A, Patil S, Williams P. On admission haemoglobin in patients with hip fracture. Injury. 2011;42:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Dillon MF, Collins D, Rice J, Murphy PG, Nicholson P, Elwaine JM. Preoperative characteristics identify patients with hip fractures at risk of transfusion. Clin Orthop Relat Res. 2005;439:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Morritt DG, Morritt AN, Kelley SP, Stone MH. Blood ordering protocol based on proposed surgical implant in fractured neck of femur patients. Ann R Coll Surg Engl. 2005;87:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Garcia-Erce JA, Cuenca J, Solano VM. [Predictive factors for transfusion requirements in patients over 65 years old with subcapital hip fracture]. Med Clin (Barc). 2003;120:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Norouzi A, Behrouzibakhsh F, Kamali A, Yazdi B, Ghaffari B. Short-term complications of anesthetic technique used in hip fracture surgery in elderly people. Eur J Transl Myol. 2018;28:7355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Bardyn M, Rappaz B, Jaferzadeh K, Crettaz D, Tissot JD, Moon I, Turcatti G, Lion N, Prudent M. Red blood cells ageing markers: a multi-parametric analysis. Blood Transfus. 2017;15:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 35. | Heddle NM, Cook RJ, Arnold DM, Liu Y, Barty R, Crowther MA, Devereaux PJ, Hirsh J, Warkentin TE, Webert KE, Roxby D, Sobieraj-Teague M, Kurz A, Sessler DI, Figueroa P, Ellis M, Eikelboom JW. Effect of Short-Term vs. Long-Term Blood Storage on Mortality after Transfusion. N Engl J Med. 2016;375:1937-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 36. | Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, Granger S, Bennett-Guerrero E, Blajchman MA, Scavo V, Carson JL, Levy JH, Whitman G, D’Andrea P, Pulkrabek S, Ortel TL, Bornikova L, Raife T, Puca KE, Kaufman RM, Nuttall GA, Young PP, Youssef S, Engelman R, Greilech PE, Miles R, Josephson CD, Bracey A, Cooke R, McCullough J, Hunsaker R, Uhl L, McFarland JG, Park Y, Cushing MM, Klodell CT, Karanam R, Roberts PR, Dyke C, Hod EA, Stowell CP. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 368] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 37. | Frank SM, Oleyar MJ, Ness PM, Tobian AAR. Reducing unnecessary preoperative blood orders and costs by implementing an updated institution-specific maximum surgical blood order schedule and a remote electronic blood release system. Anesthesiology. 2014;121:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Staves J, Davies A, Kay J, Pearson O, Johnson T, Murphy MF. Electronic remote blood issue: a combination of remote blood issue with a system for end-to-end electronic control of transfusion to provide a "total solution" for a safe and timely hospital blood transfusion service. Transfusion. 2008;48:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | White MJ, Hazard SW, 3rd, Frank SM, Boyd JS, Wick EC, Ness PM, Tobian AAR. The evolution of perioperative transfusion testing and blood ordering. Anesth Analg. 2015;120:1196-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Dutton RP, Shih D, Edelman BB, Hess J, Scalea TM. Safety of uncrossmatched type-O red cells for resuscitation from hemorrhagic shock. J Trauma. 2005;59:1445-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Flommersfeld S, Mand C, Kühne CA, Bein G, Ruchholtz S, Sachs UJ. Unmatched Type O RhD+ Red Blood Cells in Multiple Injured Patients. Transfus Med Hemother. 2018;45:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |