Published online Apr 18, 2021. doi: 10.5312/wjo.v12.i4.246
Peer-review started: December 31, 2020
First decision: February 15, 2021
Revised: February 25, 2021
Accepted: March 28, 2021
Article in press: March 28, 2021
Published online: April 18, 2021
Processing time: 101 Days and 21.7 Hours
Infections after anterior cruciate ligament reconstruction (ACLR) are rare. No cases of Salmonella infection have been described to our knowledge.
We describe a rare case of Salmonella infection in a 23-year-old patient following an ACLR. The patient presented with subacute septic arthritis, 26 d after a hamstring autograft ACLR. The pathogen, Salmonella enterica typhimurium was isolated by bacteriological sampling of the first arthroscopic lavage. Two arthroscopic lavages were required, with intravenous antibiotic therapy for two weeks with cefotaxime and ciprofloxacin, followed by oral antibiotics with amoxicillin and ciprofloxacin for a total duration of three months. This approach treated the infection but two years after the septic arthritis, faced with ongoing knee instability due to graft damage, a revision ACLR with a bone-tendon-bone graft was performed. At the last follow-up, full range of knee motion had been achieved and sports activities resumed.
Infection after ACLR is rare and requires an early diagnosis and management in order to treat the infection and prevent arthritis-related joint cartilage destruction and damage to the graft.
Core Tip: Infection after anterior cruciate ligament reconstruction (ACLR) is uncommon, with staphylococci found in more than 90% of cases. We present herein, an exceptional case of Salmonella infection after ACLR. This case highlights the importance of early diagnosis and management: arthroscopic washing for acute infections or arthrotomy for late infections and appropriate antibiotic therapy. The purpose of the surgical treatment is to prevent arthritis-related joint cartilage destruction and to protect the graft.
- Citation: Neri T, Dehon M, Botelho-Nevers E, Cazorla C, Putnis S, Philippot R, Farizon F, Boyer B. Salmonella infection after anterior cruciate ligament reconstruction: A case report. World J Orthop 2021; 12(4): 246-253
- URL: https://www.wjgnet.com/2218-5836/full/v12/i4/246.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i4.246
Septic arthritis after anterior cruciate ligament (ACL) reconstruction (ACLR) is a rare but severe complication, with an incidence between 0.3% and 1.7%[1-3]. The main responsible pathogens are Staphylococci (coagulase-negative and S. aureus) and Streptococci[2,4-6].
Salmonella spp are Gram-negative bacilli, belonging to the enterobacteriaceae family and responsible for digestive infections. Contamination is mainly caused by the consumption of raw or undercooked food (meat, eggs, and dairy products), or food contaminated by the excreta of carrier animals (more rarely by direct animal contact). After a short incubation period, these pathogens are responsible for an inflammatory intestinal syndrome with mucoid bloody diarrhea. Extra-intestinal complications, including osteo-articular complications, are rare (< 1%) and associated with haematogenous spread[7]. Typhoid fever is caused by Salmonella spp and osteo-articular complications occur in less than 1% of cases, arising in one of three possible ways: haematogenous spread, contiguous source, or as a result of vascular insufficiency[8]. The risk factors include sickle cell anaemia, diabetes, systemic lupus erythematosus, lymphoma, liver diseases, previous surgery or trauma, those at extremes of age, and steroid use[9].
We report herein a rare case of Salmonella enterica typhimurium following ACLR. To our knowledge, this is the first case of septic knee arthritis after ACLR due to Salmonella spp.
Twenty-six days after an ACLR, a 23-year-old man showed a deterioration in his general state (asthenia, fever and chills) and local signs of an early knee septic arthritis: pain, heat, redness, and edema.
The patient reported an episode of abdominal pain associated with mucoid bloody diarrhea 10 d before the onset of the arthritis symptoms, which quickly resolved within 48 h. Further questioning on risk factors for Salmonella spp revealed the patient had eaten an egg-based picnic 24 h before these symptoms appeared.
This patient was suffering from chronic instability in his right knee, following a soccer injury. An isolated ACL injury was reported on magnetic resonance imaging (MRI).
Three months after the injury, an ACLR was performed, using a hamstring autograft (semi-tendinosus and gracilis). The procedure was performed under general anesthesia with a tourniquet at the proximal thigh for duration of 40 min. An outside-in drilling technique was used for the femoral tunnel. Femoral and tibial fixation was with interference screws.
The patient followed a standardized post-operative rehabilitation protocol aimed at controlled restoration of range of motion, muscle strength and proprioception. He was discharged the same day full weight bearing assisted with crutches. At 15 d after surgery, a routine consultation was performed to verify the absence of pain, hematoma, wound inflammation and/or serous or purulent discharge.
The patient had a free previous medical history.
A collection in the external femoral approach site was confirmed.
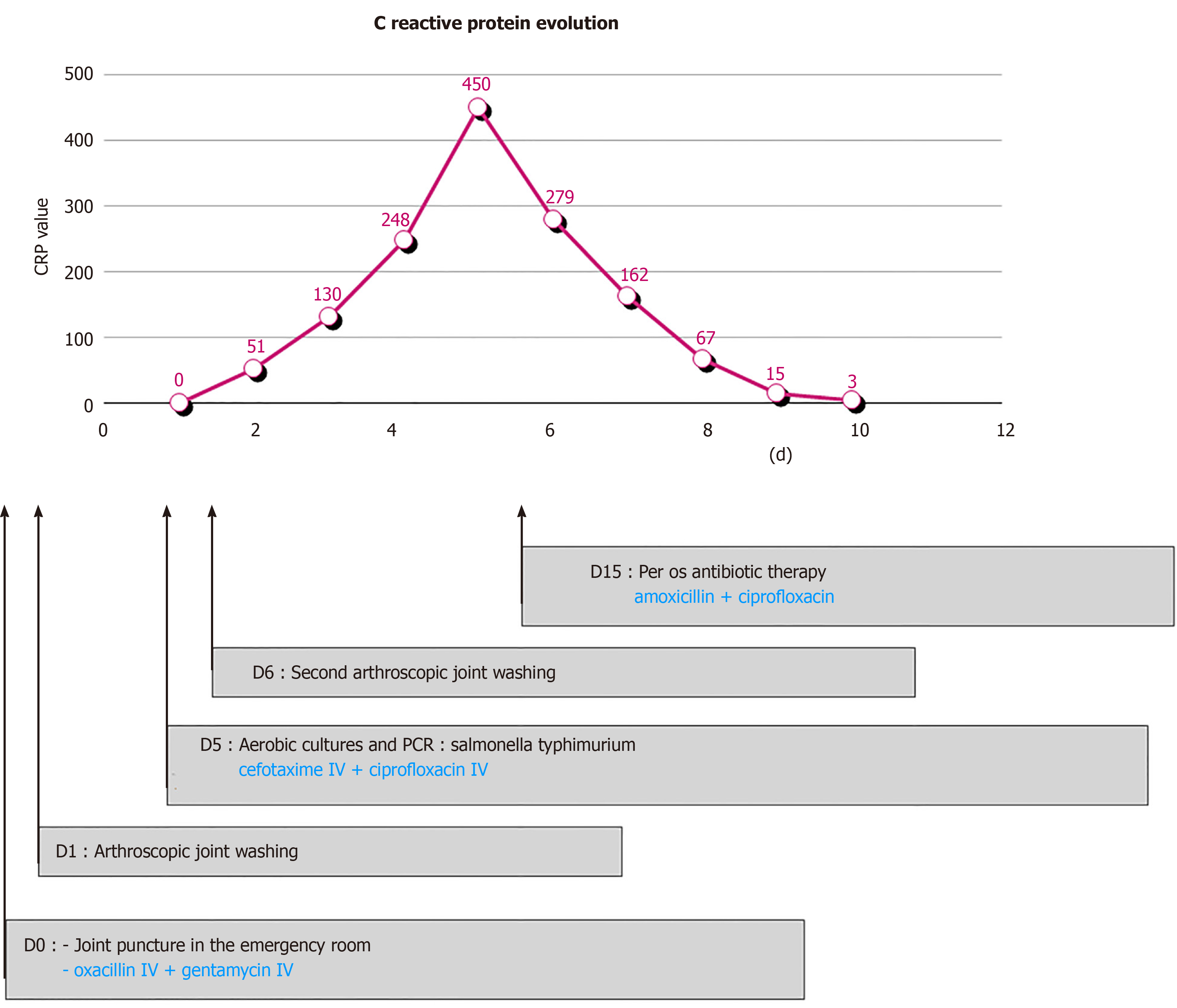
A biological inflammatory syndrome was found with an initially raised C-reactive protein (CRP) of 51 mg/L increasing to 130 mg/L over the first 24 h (Figure 1), alongside a white blood cell count increase from 11000/mm3 to 13300/mm3 during the same period.
The joint fluid aspiration performed in the emergency room showed neutrophils at direct examination and was followed by administration of intravenous antibiotic therapy – oxacillin 2 g every 8 h and gentamycin – 270 mg/d.
A knee ultrasound examination reported an intra-articular effusion. X-rays showed no fracture.
Salmonella enterica typhimurium arthritis following an ACLR.
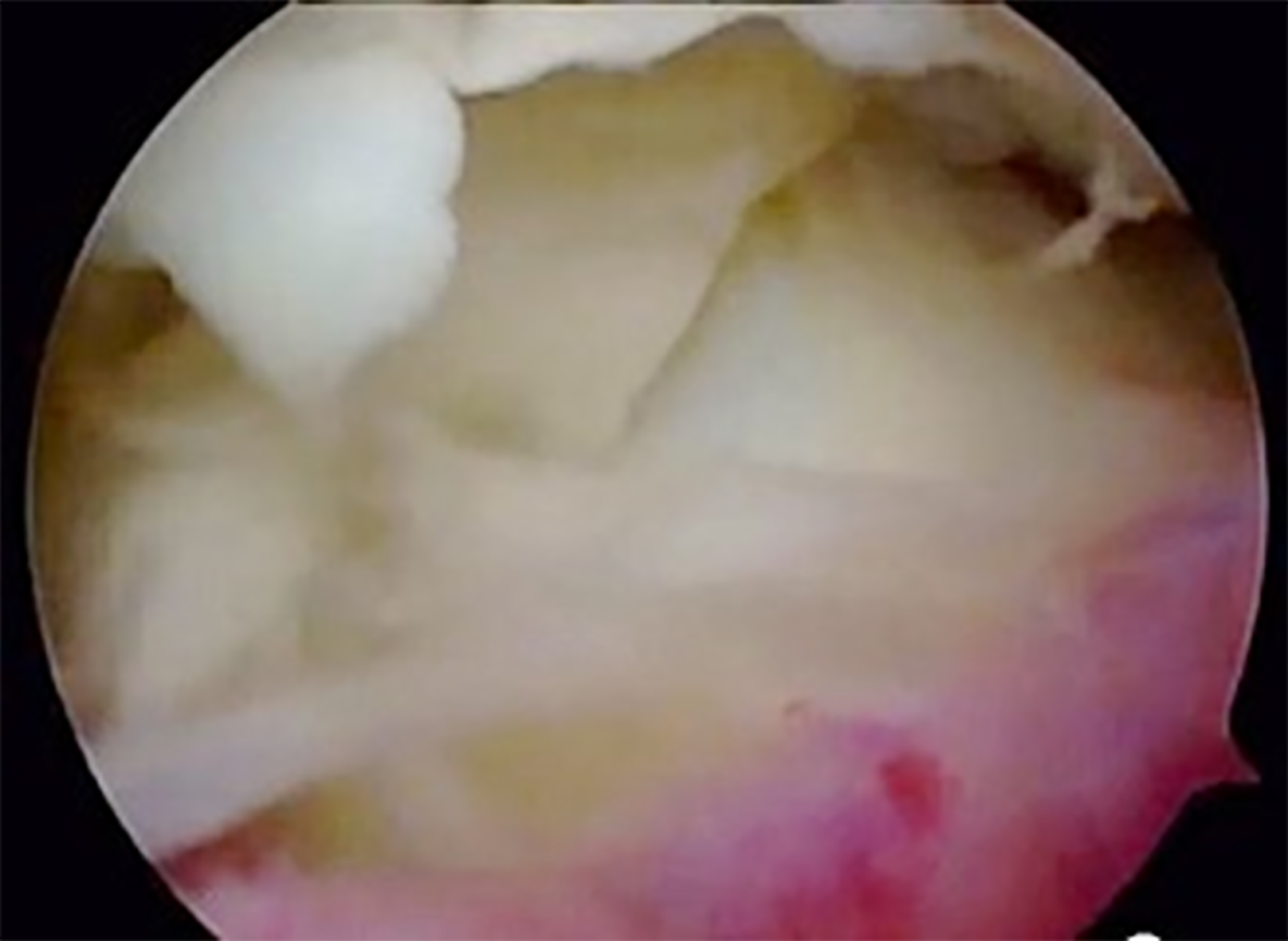
An urgent arthroscopic joint lavage was performed the same day. The joint fluid was serous, not purulent. with few false membranes but without a clear inflammation of the synovial tissue. A surgical approach centered on the scar for the femoral tunnel, was also performed and revealed a separate collection apparently extra-articular. Multiple samples were taken for bacteriological analysis. Intravenous antibiotic therapy with oxacillin at a dose of 2 g every 8 h and gentamycin at a dose of 270 mg per day was continued pending bacteriological results.
The stool and blood cultures returned negative. Samples of joint fluid aspiration and arthroscopic lavage both grew Salmonella typhimurium on aerobic cultures.
This result was also confirmed by polymerase chain reaction (PCR) DNA 16S. Antibiotic therapy was modified to cefotaxime at a dose of 3 g every 6 h and ciprofloxacin at a dose of 400 mg every 8 h.
The clinical and biological evolution was not favorable, on the 6th post-operative day, a knee effusion and a collection in the external femoral approach was seen, as well as a biological inflammatory syndrome, with a CRP increase up to 450 mg/L (Figure 1). Therefore, a second arthroscopic lavage was performed. The external femoral approach was again opened and now revealed a purulent collection, communicating with the joint. Arthroscopy showed purulent joint effusion with false membranes and an inflammatory synovial tissue (Figure 2).
Multiple bacteriological samples were taken, followed by lavage. The samples returned sterile and 16S DNA PCR was negative.
Over the subsequent days, a decrease in local inflammation as well as a decrease in biological inflammatory syndrome was seen, with a normalized CRP (< 5 mg/L) at 1 mo (Figure 1).
A total of 3 wk of hospitalisation was required, with oral antibiotic therapy started after 15 d of intravenous treatment with amoxicillin at a dose of 1 g, 3 times daily and ciprofloxacin at a dose of 500 mg, twice daily for a total of 3 mo of antibiotic therapy.
Two years after the septic episode, the infection was considered cured but a persistent knee instability (with positive Lachmann-Trillat and jerk-tests) persisted. Furthermore, MRI showed a partial rupture of the graft. In order to meet the patient's desire to resume a pivotal sports activity, a revision ACLR was scheduled, using a bone-tendon-bone graft reconstruction combined with a Lemaire procedure. Intra-operatively, a distended and non-functional ACL graft was found. Prophylactic antibiotic therapy with amoxicillin at a dose of 150 mg/kg/d in 4 injections was initiated pending the microbiology results of tissue samples. Antibiotic therapy was stopped on day 5, due to the sterility of the bacteriological samples.
Final follow-up at three years after the surgical revision revealed a full range of stable knee motion, with function similar to the contralateral knee, allowing pivoting sports activities (soccer).
We report an uncommon case of Salmonella enterica typhimurium arthritis in a young patient, following an ACLR.
Infections after ACLR are rare, and no cases of Salmonella infection have been described to our knowledge. The main responsible pathogens are Staphylococci (coagulase-negative and S. aureus) and Streptococci[2,4-6]. This case occurred early after ACLR, however given the history, haematogenous spread from the primary bowel infection is presumed.
Salmonella non typhi have been rarely reported as agents of arthritis. Salmonella is a Gram-negative Bacillus Enterobacteriaceae responsible mainly for digestive infections. The prevalence of joint infections with Salmonella is only 1%[7]. It mainly affects the native hip in children[10]. Some cases of Salmonella arthritis after total hip or knee arthroplasty have been described in the literature (Table 1).
| Ref. | Prosthetic joint | Prosthesis age at time of infection | Symptoms | Microbiology | Surgical treatment | Antibiotic therapy | Failed treatment |
| Chong and Sporer[20], 2005 | THA | 8 mo | Purulent flow | Salmonella choleraesuis | Two-stage revision | IV vancomycin oral ciprofloxacin 6 wk | No |
| Gupta et al[21], 2014 | TKA | 3 yr | Deep joint pain, discomfort for 4 mo | Salmonella enterica serovar Enteritidis | Removal, debridement and arthrodesis after aspiration failed and oral TMP-SMX 12 wk | Oral TMP-SMX 8 wk | No |
| Gupta et al[21], 2014 | THA | 7 yr | Pain | Salmonella enterica serovar Enteritidis | Removal, debridement and arthrodesis after debridement failed and oral amoxicillin 10 mo | IV ampicillin 4 wk | No |
| Gupta et al[21], 2014 | THA | 5 mo | Fever, pain, joint swelling | Salmonella enterica serovar Choleraesuis | Two-stage revision after aspiration failed and oral ampicillin 8 wk | Oral ampicillin4 wk | No |
| Gupta et al[21], 2014 | THA | 4 yr | Fever, pain, swelling | Salmonella enterica serovar Enteritidis | Two-stage revision | Oral ciprofloxacin8 wk | No |
| Gupta et al[21], 2014 | THA | 5 mo | Fever, pain | Salmonella enterica serovar Enteritidis | Two-stage revision | IV ceftriaxone 6 wk | No |
| Gupta et al[21], 2014 | THA | 9 yr | Pain | Salmonella bongori | Two-stage revision after aspiration failed and oral ciprofloxacin 8 wk | IV ceftriaxone 6 wk | No |
| Toth et al[10], 2010 | THA | 2 yr | Pain | Salmonella enteridis | Two-stage revision | Oral ciprofloxacin 6 wk | No |
| Toth et al[10], 2010 | THA | 7 yr | ARDS caused by sepsis and increased uptake aroud the THA (autolog leukocyte scintigraphy) | Salmonella cholerae-suis | Two-stage revision | Oral ciprofloxacin 6 wk | No |
| Sebastian et al[22], 2017 | TKA | 4 yr | Pain, swelling, scare discharge, limitation of range of motion | Salmonella typhimurium | Two-stage revision | Oral ciprofloxacin 6 wk | No |
| Carlile et al[23], 2010 | TKA | 5 yr | Pain, swelling, fluctuant swelling, night sweats and shivering | Salmonella enterica choleraesuis | Two-stage revision | IV cefotaxim 7 d. Oral ciprofloxacin 3 wk | No |
| Jeroense et al[24], 2014 | THA | 5 d | Fever | Group E Salmonella | One-stage revision after lavage failed and ciprofloxacin 4 wk | Oral ciprofloxacine 5 mo | No |
| Jeroense et al[24], 2014 | THA | 13 years | Pain, abscess at the ultrasound | Salmonella enteritidis | One-stage revision | Oral ciprofloxacine 3 mo | No |
Risk factors for bacterial blood-borne knee infections (old age, diabetes, polyarthritis and other immunodeficiency diseases as well as intravenous drug abuse) were not found in this patient, nor were other described risk factors such as concomitant meniscus resection or a history of surgery on the same knee[11].
For ACLR, hamstrings autograft has been reported to be a risk factor for surgical site infection compared to bone-tendon-bone graft reconstruction[5].
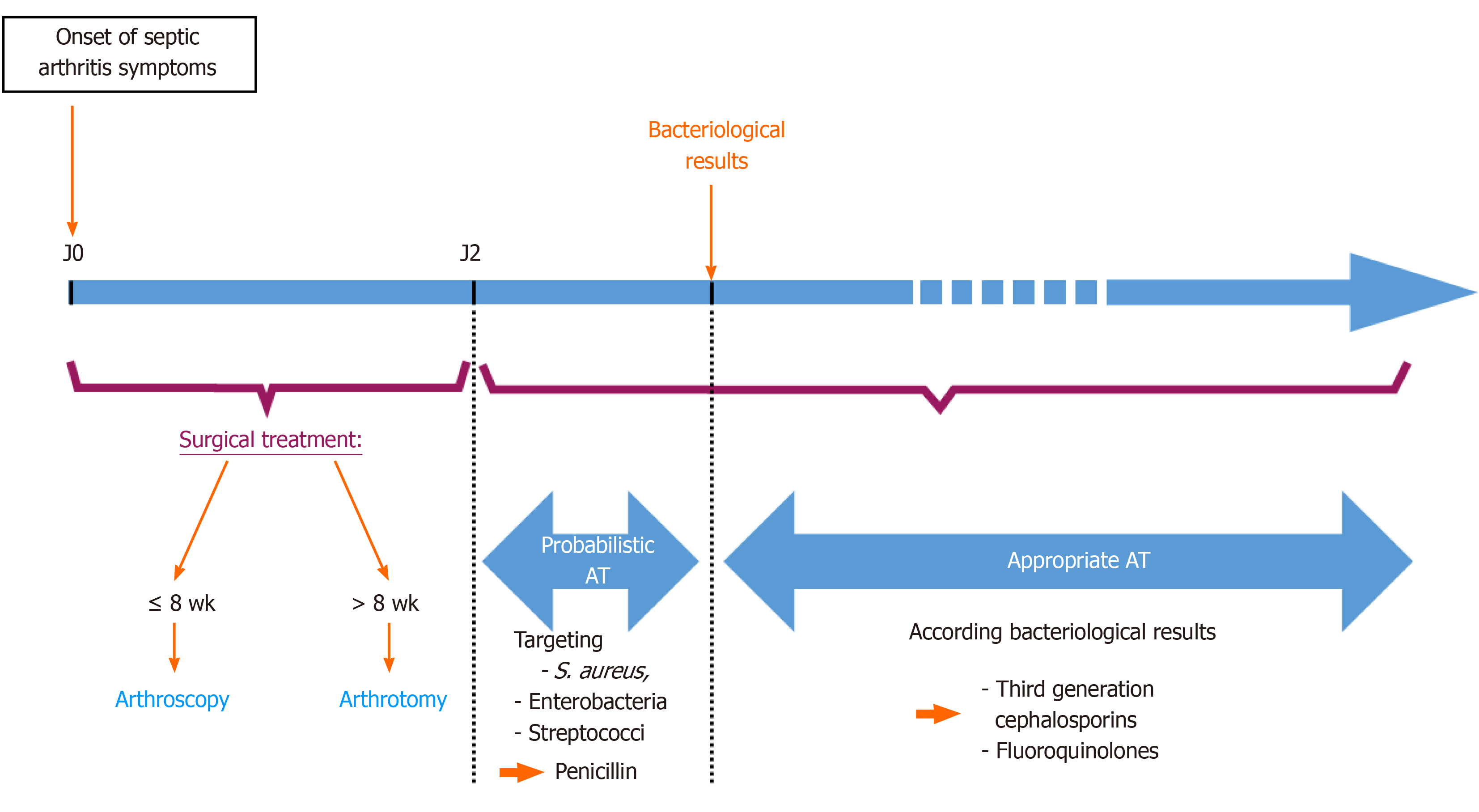
Septic arthritis is an orthopedic emergency. The gold standard of treatment is joint debridement and antibiotic therapy according to the culture results. Smith et al reported that enzymatic destruction begins by the eighth hour after the inoculation. By the 48th hour, 40% of the glycosaminoglycan is lost, and collagen breakdown occurs in a period of few days in septic arthritis[12]. Several studies have shown that the duration between the beginning of symptoms and surgical intervention is the most important prognostic factor for septic arthritis[13,14]. Early diagnosis and management are essential to minimize the risk of graft failure and osteo-articular lesions, which cause stiffness and chronic pain. It is recommended to hospitalise the patient and give the appropriate treatment within 24 h[1,15-17]. The reference treatment is as follows (Figure 3): first surgical treatment by knee debridement and lavage with attempts to protect the graft in most cases and then medical treatment by intravenous antibiotic therapy with penicillin (cloxacillin), initially targets the most frequently encountered pathogens (Staphylococcus aureus, enterobacteria and streptococci).
Blind joint fluid aspiration is not described in the optimal management of infection after ACLR and antibiotic therapy initiated before the first arthroscopic lavage in our patient could have negated the results of bacteriological samples, resulting in a delay in optimal management and therefore decreasing the chances of saving the graft[16]. For acute (less than 2 wk postoperatively) and subacute (between 2 and 8 wk) infections, arthroscopic debridement and lavage can be proposed, while for chronic infections (after 8 wk postoperatively) an open lavage via an arthrotomy has been recommended. Additional lavage may be necessary if the initial treatment fails. The modalities of management, in this case, are ambiguous: some would perform a second lavage arthroscopically and others would prefer an arthrotomy[16,18].
The presence of an abscess in the surgical wound, backed by a CRP increase despite the surgical and antibiotic treatment, justified a second lavage in order to reduce the bacterial inoculum. In this patient case, the subacute infection justified the use of an arthroscopic approach instead of an arthrotomy. An open arthrotomy could also have been performed if arthroscopic treatment had failed. In this case, iterative arthroscopic management has been successful to control the infection. Antibiotic therapy is adapted after analysis of antibiotic sensitivity, and continued until the clinical-biological evolution is satisfactory, an antibiotic treatment of at least 6 wk is recommended[19].
The initial antibiotic therapy used in this patient was appropriate because it is effective for the most frequent pathogens (i.e., staphylococci). It was adapted after antibiotic sensitivities. Salmonella is sensitive to third generation cephalosporins and fluoroquinolones, which were administered to our patient once the bacteriological results were obtained[7,11].
Graft failures are rare in early management of infection[16]. For this patient, the causes may have been as follows: The 24 h delay in treatment due to the aspiration, the absence of graft debridement, the pathogen (since no cases of Salmonella septic arthritis after ACLR are described), but likely primarily due to the two successive surgical procedures that could have compromised the integration of the graft.
Infection after ACLR is uncommon, with staphylococci found in more than 90% of cases. This case highlights the importance of early diagnosis and management: arthroscopic lavage for acute infections or arthrotomy for late infections and appropriate antibiotic therapy. Like any septic joint, early aggressive surgical treatment is required, also aiming to reduce the risk of arthritis-related joint cartilage destruction and damage to the graft.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang HX S-Editor: Zhang H L-Editor: A P-Editor: Xing YX
| 1. | Wee J, Lee KT. Graft infection following arthroscopic anterior cruciate ligament reconstruction: a report of four cases. J Orthop Surg (Hong Kong). 2014;22:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Kim SJ, Postigo R, Koo S, Kim JH. Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics. 2014;37:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Sajovic M, Nič Ar GL, Dernovš Ek MZ. Septic arthritis of the knee following anterior cruciate ligament reconstruction. Orthop Rev (Pavia). 2009;1:e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Kim HJ, Lee HJ, Lee JC, Min SG, Kyung HS. Evaluation of Infection after Anterior Cruciate Ligament Reconstruction during a Short Period. Knee Surg Relat Res. 2017;29:45-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Wang C, Ao Y, Wang J, Hu Y, Cui G, Yu J. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: a retrospective analysis of incidence, presentation, treatment, and cause. Arthroscopy. 2009;25:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Scully WF, Fisher SG, Parada SA, Arrington ED. Septic arthritis following anterior cruciate ligament reconstruction: a comprehensive review of the literature. J Surg Orthop Adv. 2013;22:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Huang DB, DuPont HL. Problem pathogens: extra-intestinal complications of Salmonella enterica serotype Typhi infection. Lancet Infect Dis. 2005;5:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Declercq J, Verhaegen J, Verbist L, Lammens J, Stuyck J, Fabry G. Salmonella typhi osteomyelitis. Arch Orthop Trauma Surg. 1994;113:232-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Arora A, Singh S, Aggarwal A, Aggarwal PK. Salmonella osteomyelitis in an otherwise healthy adult male-successful management with conservative treatment: a case report. J Orthop Surg (Hong Kong). 2003;11:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Tóth K, Janositz G, Kovács G, Sisák K, Rudner E. Successful treatment of late Salmonella infections in total hip replacement - report of two cases. BMC Infect Dis. 2010;10:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Uygur E, Reddy K, Ozkan FÜ, Söylemez S, Aydin O, Senol S. Salmonella enteridis Septic Arthritis: A Report of Two Cases. Case Rep Infect Dis. 2013;2013:642805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Kartus J, Ejerhed L, Sernert N, Brandsson S, Karlsson J. Comparison of traditional and subcutaneous patellar tendon harvest. A prospective study of donor site-related problems after anterior cruciate ligament reconstruction using different graft harvesting techniques. Am J Sports Med. 2000;28:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Wirtz DC, Marth M, Miltner O, Schneider U, Zilkens KW. Septic arthritis of the knee in adults: treatment by arthroscopy or arthrotomy. Int Orthop. 2001;25:239-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Perry CR. Septic arthritis. Am J Orthop (Belle Mead NJ). 1999;28:168-178. [PubMed] |
| 15. | Fong SY, Tan JL. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Ann Acad Med Singap. 2004;33:228-234. [PubMed] |
| 16. | Wang C, Lee YH, Siebold R. Recommendations for the management of septic arthritis after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22:2136-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Abdel-Aziz A, Radwan YA, Rizk A. Multiple arthroscopic debridement and graft retention in septic knee arthritis after ACL reconstruction: a prospective case-control study. Int Orthop. 2014;38:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Williams RJ 3rd, Laurencin CT, Warren RF, Speciale AC, Brause BD, O'Brien S. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Diagnosis and management. Am J Sports Med. 1997;25:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 149] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Indelli PF, Dillingham M, Fanton G, Schurman DJ. Septic arthritis in postoperative anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2002;182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Chong PY, Sporer SM. Case report: Salmonella infection following total hip arthroplasty. Iowa Orthop J. 2005;25:42-43. [PubMed] |
| 21. | Gupta A, Berbari EF, Osmon DR, Virk A. Prosthetic joint infection due to Salmonella species: a case series. BMC Infect Dis. 2014;14:633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Sebastian S, Dhawan B, Malhotra R, Gautam D, Kapil A. Salmonella typhimurium infection in total knee arthroplasty: A case report with review of literature. J Lab Physicians. 2017;9:217-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Carlile GS, Elvy J, Toms AD. Salmonella infection of a total knee replacement. Knee. 2010;17:356-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Jeroense KT, Kuiper JW, Colen S, Schade RP, Saouti R. One-stage revision in two cases of Salmonella prosthetic hip infection. World J Clin Cases. 2014;2:304-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |