INTRODUCTION
Prosthetic joint infection (PJI) is one of the most dreadful complications of arthroplasty. Effective prophylactic strategies are essential to reduce the incidence of these difficult to treat and burdensome infections. The mode of prophylactic use of antibiotic-loaded bone cement (ALBC) is a frequent surgical practice in cemented hip and knee replacement. The idea behind delivering antibiotics directly into the vulnerable joint compartment is that local concentrations well above the minimum inhibitory concentration of the pathogen can be achieved without exposing the patient to major risks of side effects. With this mechanism ALBC may form an additional antimicrobial frontline in situ and complement the routine systemic antibiotic prophylaxis. The combined systemic and local antibiotic administration may be even more important in situations where the efficacy of the systemic prophylaxis is experiencing increasing limitations due to the spread of resistant bacteria to the commonly used perioperative antibiotics cefazolin or cefuroxime[1,2]. The Scandinavian registries and, most recently, the National Registry of United Kingdom have demonstrated that the additional use of ALBC reduces the revision risk in cemented hip and knee replacement[3-6]. It can be further speculated that this effect is more significant if specific cement brands are analyzed due to different antibiotic elution capacities of the cement polymers in commercial ALBC brands[7,8].
In view of the demographic changes, arthroplasty surgeons today face the challenge to operate on an increasing number of older patients suffering from several major comorbidities. Numerous clinical studies have provided evidence that important patient-related disorders predispose patients to a higher operational risk of infections than on average[9-11]. This is also true for the more complex surgical procedures of revision arthroplasty which is frequently associated with longer operation times and a higher invasiveness leading to a PJI incidence of 5% and more[12]. Significantly increased infection rates of 4%-6% are also reported in the frail cohort of femoral neck fracture (FNF) patients on an emergency trauma track which does not leave time for preoperative health optimization strategies or for decolonization protocols of multi-drug resistant bacteria[13,14]. In order to counteract the higher infection risks in such patient cohorts, one may hypothesize that a more optimized and risk-adjusted antibiotic prophylaxis strategy may have a positive impact on the PJI incidence. This may include (1) modification of the routine perioperative antibiotic prophylaxis regimen by either extending the duration[15] or by adding a second antibiotic to the standard drug (e.g., vancomycin or teicoplanin to a cephalosporin)[16] or (2) use of high dose local antibiotic combinations. Given the controversial outcomes regarding the first option and the substantial risks of side effects associated with prolonged systemic antibiotic exposure[17], a risk-adjusted strategy with dual ALBC might be a more attractive and “easy-to-apply” option in the theatre. The more potent antimicrobial growth inhibition found in vitro and the significantly reduced PJI rates in high infection risk patients receiving dual ALBC strongly argue for the latter option. This review summarizes the literature and evaluates the evidence from preclinical and clinical studies for the use of dual ALBC for PJI prevention in risk for infection patients and orthopaedic risk procedures. For that purpose, the PubMed and EMBASE literature databases were screened for publications pertaining to the clinical utilization of dual antibiotics in cement for infection prophylaxis. Use of dual ALBC in treatment of septic cases was excluded from the evaluation. Only four in vitro and five original clinical studies were identified which met the inclusion criteria. The latter were also stratified by level of clinical evidence (I-IV). The combination of gentamicin and clindamycin in commercial bone cement was the only referenced dual ALBC in these clinical studies. To the best of our knowledge there are no clinical outcome studies published which have compared the PJI rate in hand-made (theatre-admixed) dual ALBC vs single ALBC.
COMMERCIALLY PREMIXED VS HAND-MIXED DUAL ALBC
There are several Food and Drug Administration and European Medicines Agency approved ALBC which are available as “ready-to-use” commercial products. According to their antibiotic contents they can be grouped in single low dose ALBC [e.g., impregnated with either 0.5 g or 1 g of gentamicin or loaded with 1 g of tobramycin in 40 g polymethyl methacrylate (PMMA) powder] or in dual high dose ALBC (e.g., impregnated with 1 g of gentamicin and 1 g of clindamycin or loaded with 0.5 g of gentamicin and 2 g of vancomycin). In addition, there is widespread non-standardized, off-label and surgeon-directed use involving hand-mixing various antibiotics into bone cement. Reasons for this practice are economic considerations, lack of availability of specific ALBC, limited local regulatory approval or need for specific customized solutions in septic revision arthroplasty[18]. However, manual admixture of antibiotics into bone cement has raised some concerns with regards to unknown elution kinetics, toxicity, efficacy and mechanical stability of such in-theatre made ALBC[18]. The latter aspect is particularly important if the cement is intended for fixation. In fact, the manual addition of higher amounts of some antibiotics in powder- or in liquid-form has been shown to affect the fatigue strength of PMMA prompting fears of premature aseptic loosening of the joint[19]. It should also be noted that some antibiotics are not stable at the bone cement curing temperature (e.g., many beta-lactam antibiotics) or chemically interfere with the polymerization process (e.g., rifampicin)[20]. Given these uncertainties, the majority of surgeons still prefer the use of commercial single or dual ALBC for prosthesis fixation.
STRONGER ANTIMICROBIAL ACTIVITY WITH THE DUAL ALBC COPAL GENTAMICIN + CLINDAMYCIN IN VITRO
Gentamicin is the most frequently used antibiotic for impregnating bone cement because of its broad and concentration-dependent bactericidal effect, its relatively good elution in comparison to other antibiotics and its ability to withstand the high temperatures reached during polymerization of the bone cement[21]. Its antimicrobial spectrum covers non-gentamicin resistant gram-positive staphylococci, enterococci and several gram-negative bacilli[22]. Clindamycin is also an attractive antibiotic for local delivery which shares several features of gentamicin, but shows in addition a potent antimicrobial activity against intraosteoblastic Staphylococcus aureus[23]. Its spectrum overlaps with gentamicin on staphylococci and furthermore covers non-clindamycin resistant streptococci and anaerobic bacteria[24]. In combination, both antibiotics may target up to 90% of all pathogens typically found in PJI[25,26]. Given these antibiotic properties it is therefore not surprising that a dual ALBC bone cement using these antibiotics has been developed. This bone cement COPAL G+C (gentamicin + clindamycin) (Heraeus-Medical GmbH, Wehrheim, Germany) is simultaneously loaded with 1.68 g of gentamicin sulfate (= 1 g of active gentamicin) and 1.18 g of clindamycin hydrochloride (= 1 g of active clindamycin) within the polymer basis of the successful PALACOS bone cement. Soon after the commercialization of COPAL G+C, Kuehn et al[7] and Neut et al[27] compared the antibiotic elution from this product with several single antibiotic loaded low dose cement brands on the market in two independent studies. It was found that COPAL G+C exhibited a much stronger synergistic release of both antibiotics exceeding that of gentamicin alone in single ALBC by a factor of at least 10[7,27].
Ensing et al[28] then combined these elution experiments with antimicrobial growth inhibition tests comparing the dual high dose COPAL G+C and the single low dose PALACOS R+G cement (containing 0.5 g of gentamicin). For that purpose, antibiotic-containing eluates from bone cement samples were collected at different time points and spotted onto agar plates which had been priorly inoculated either with a gentamicin-sensitive Staphylococcus aureus or with a gentamicin-resistant coagulase-negative Staphylococcus epidermidis strain. Both bacterial test strains were originally derived from PJI patients. COPAL G+C was observed to inhibit bacterial growth much more strongly when compared to PALACOS R+G. In more detail, the single low dose ALBC was effective in inhibiting growth of the gentamicin-sensitive Staphylococcus aureus for a period of 72 h of elution. However, the G+C containing cement yielded a stronger and more prolonged bacterial inhibition for at least 28 d, which was the entire duration of the experiment. In case of the gentamicin-resistant Staphylococcus epidermidis strain PALACOS R+G was not able to inhibit the bacteria while COPAL G+C prevented growth of these bacteria at all times after elution.
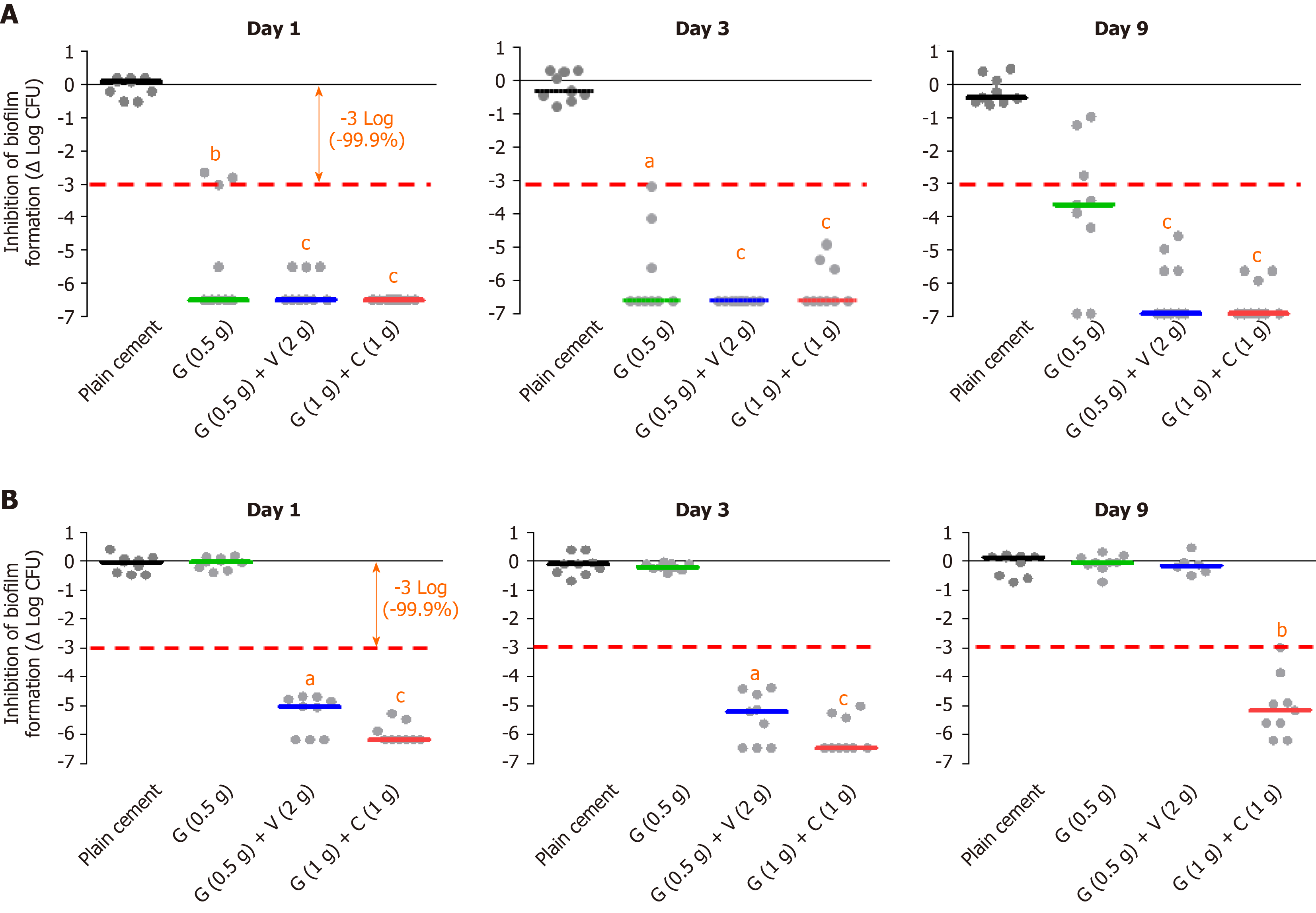
Cara et al[29] expanded on these studies and compared the inhibitory effect on staphylococcal biofilm formation of plain cement (no antibiotic) with the three ready-to-use commercial ALBC brands PALACOS R+G, COPAL G+C and COPAL G+V (the latter contains a combination of 0.5 g gentamicin and 2 g vancomycin, Heraeus Medical GmbH, Wehrheim, Germany). In total, ten different strains of Staphylococcus aureus and Staphylococcus epidermidis, some with specific resistance to gentamicin, were analyzed. It was observed that all the tested ALBC can inhibit biofilm formation of methicillin-susceptible staphylococci (without antibiotic resistances) up to day 9 (end of observation period). However, the inhibition of the dual ALBC brands at day 9 appeared more potent and sustained than that of the single ALBC product (Figure 1A). Strong antimicrobial effect of all 3 ALBC - at least up to day 3 - was also evident for methicillin-resistant staphylococci if they were still susceptible to gentamicin. However, a strong difference could be noticed for such strains which were highly resistant to gentamicin. In these cases, only the dual loaded products were able to exert a potent anti-biofilm activity with a tendency of even stronger and longer lasting inhibition for the G+C combination (Figure 1B). The most reliable and most sustained inhibition effect of the G+C combination against gentamicin-resistant coagulase-negative staphylococci is of important clinical relevance since regular antibiotic surveillance data from several countries point to an increasing gentamicin resistance level of these bacteria[30].
Figure 1 In vitro biofilm inhibition experiments with different bone cement types (plain, single and dual antibiotic-loaded bone cement).
A: Prophylactic anti-biofilm effect of three different antibiotic-loaded bone cements against a gentamicin and methicillin-susceptible Staphyloccus aureus strain at day 1, day 3 and day 9 on basis of three independent experiments; B: Prophylactic anti-biofilm effect of three different antibiotic-loaded bone cements against a gentamicin- and methicillin-resistant Staphylococcus epidermidis strain on basis of three independent experiments. aP < 0.05, bP < 0.01, or cP < 0.001 respectively in comparison with PALACOS R (cement without antibiotic). G: Gentamicin; C: Clindamycin; V: Vancomycin.
LOWER PJI RATE WITH DUAL ALBC (COPAL G+C) - HEMIARTHROPLASTY IN FNF PATIENTS
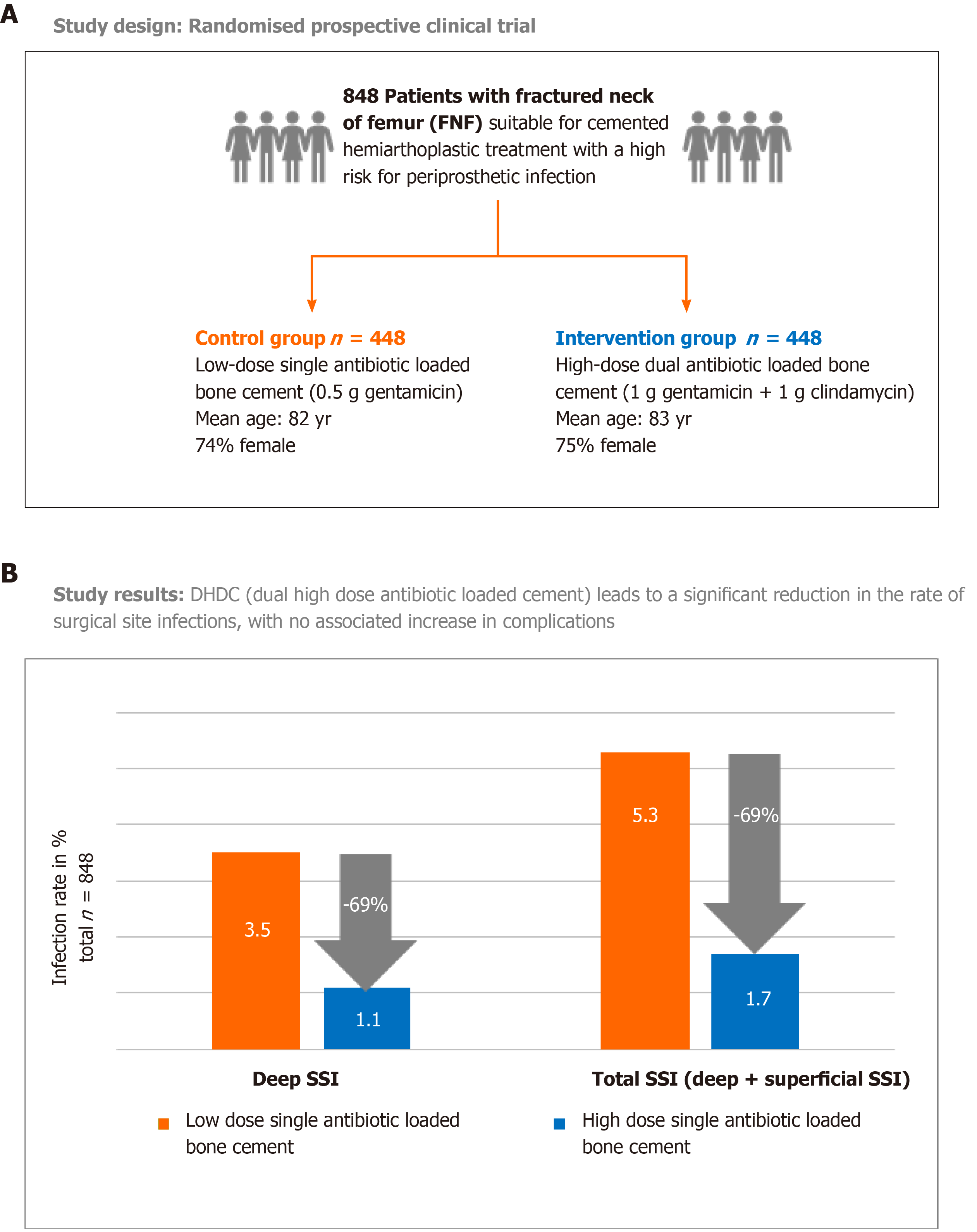
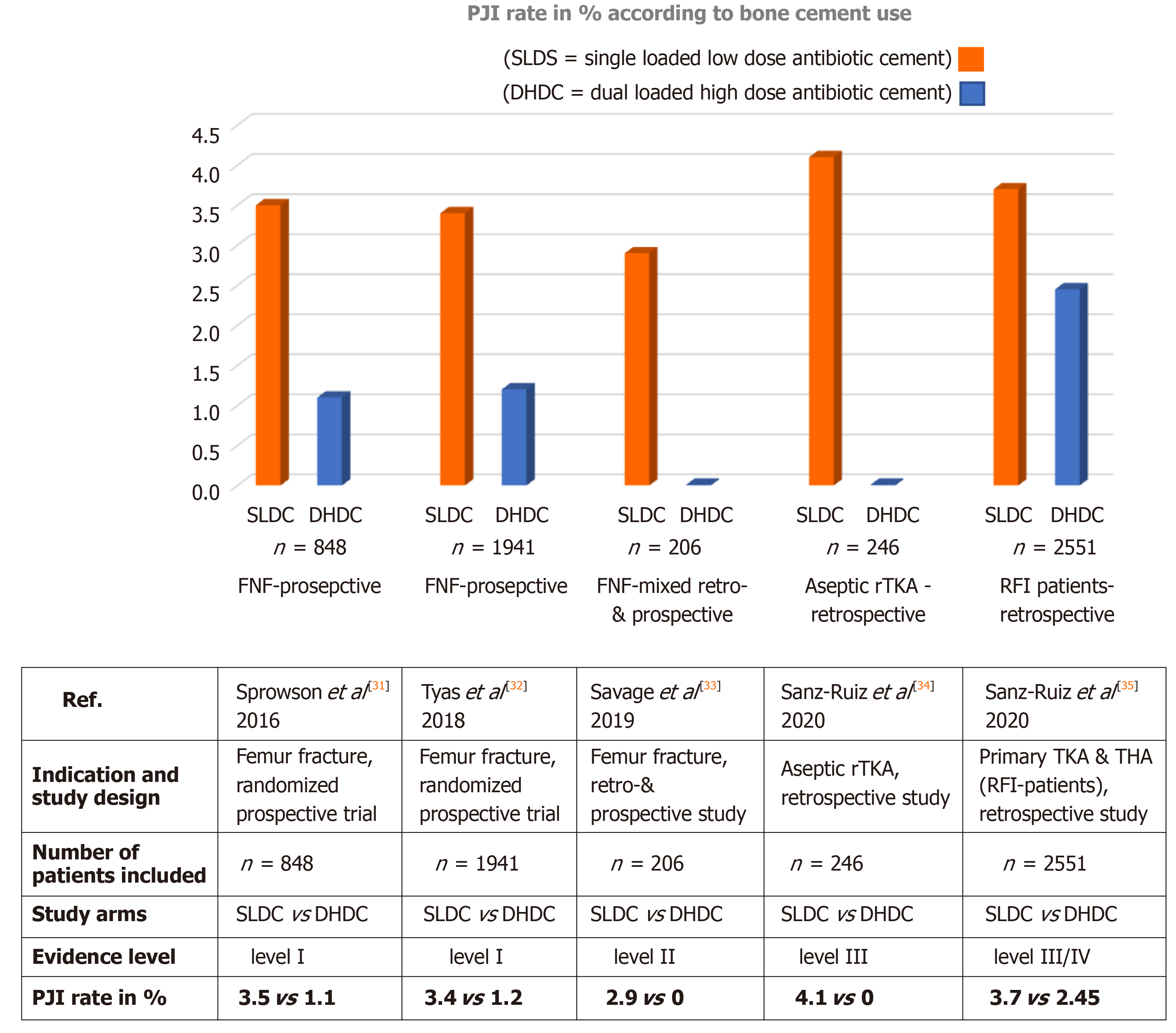
These promising in vitro observations with dual ALBC prompted surgeons at the Northumbria NHS Trust hospitals in the United Kingdom to test the hypothesis of a clinically more meaningful infection prophylaxis with COPAL G+C in the setting of a randomized clinical trial. For this they chose the particularly frail patient cohort of FNF patients known to suffer from higher infection risks. The study comprised of 848 patients with intracapsular fractures who were treated with cemented hemiar-throplasty according to the United Kingdom trauma guidelines. It was found that the primary study endpoint, incidence of deep surgical site infections (SSI), was significantly lower in the intervention group receiving the dual ALBC COPAL G+C (1.1% deep SSI rate) compared to the standard group receiving the single low dose ALBC PALACOS R+G (3.5% deep SSI rate, P = 0.041, evidence level I, Figure 2)[31]. If also considering the number of superficial SSI occurring in both groups, the difference was even more significant (1.7% in the intervention group vs 5.3% in control group). Tyas et al[32] later extended the patient number from this randomized study and analyzed 1941 FNF-patients in the same way. The lower PJI rate in the dual ALBC group was maintained (1.2% vs 3.4%). Savage et al[33] independently reported a PJI rate of 0% in the dual ALBC FNF-patient cohort vs 2.9% in the single ALBC group. This study compared bone cements from two different manufacturers in a mixed prospective and retrospective study design (n = 206), (evidence level II).
Figure 2 Randomized clinical trial in femoral neck fracture patients comparing prosthetic joint infection rate in low dose single antibiotic loaded bone cement group with high dose dual antibiotic loaded bone cement group.
A: Study design, 848 patients were randomised to receive either hemiprostheses cemented with a low dose single antibiotic-loaded bone cement (PALACOS R + gentamicin = control group) or with a high dose dual antibiotic-loaded bone cement (COPAL gentamicin + clindamycin = intervention group); B: Study results: Primary endpoint was the deep surgical site infection rate (SSI) in the observation period of ≥ 1 yr in each group. Secondary endpoint was the rate of superficial SSI. For the calculation of the total SSI, both deep and superficial SSI cases in each group were combined. SSI: Surgical site infection.
Concerns that the use of a dual antibiotic loaded cement with higher drug content may trigger more antibiotic-mediated side effects in these fragile patient cohorts could not be confirmed. In fact, the comparison of complications including renal failure or percentage of Clostridium difficile infections did not reveal differences between the standard and intervention group[31]. There was even a statistically significant decrease in the need for critical care treatment in the COPAL G+C group (0.5% vs 4.7%) reflecting the clinical impact of the much lower PJI rate in the intervention group receiving dual ALBC[31].
LOWER PJI RATE WITH DUAL ALBC (COPAL G+C) - ASEPTIC KNEE REVISION ARTHROPLASTY
Inspired by the promising results from the FNF studies, Sanz-Ruiz et al[34] tested the study hypothesis of a more potent infection prophylaxis with the dual ALBC COPAL G+C in the field of aseptic revision knee arthroplasty. All septic and oncologic revision causes were excluded in this study. On basis of 246 patients analyzed in this retrospective study no case of PJI was observed in the COPAL G+C group compared to six cases occurring in the PALACOS R+G group (PJI rate = 4.1%, P = 0.035, evidence level III). The use of the dual ALBC in all patients undergoing aseptic revision arthroplasty was further found to be cost-effective despite the additional cost of dual ALBC. A hospital saving of approximately 1200 € per patient was calculated due to 3.9 avoided PJI cases per 100 aseptic knee revision patients[34].
LOWER PJI RATE WITH DUAL ALBC (COPAL G+C) - RISK FOR INFECTION PATIENTS IN PRIMARY ARTHROPLASTY
Sanz-Ruiz and Berberich[35] further analyzed the infection rate in presumed risk for infection patients by comparing the influence of single ALBC vs dual ALBC on the PJI incidence after primary cemented joint replacement. Patients were defined as risk for infection individuals if they presented a combination of at least two or three major risk factors for total hip arthroplasty and total knee arthroplasty, respectively, using a simple scoring system. The risk algorithm included specific patient-related comorbidities (e.g., severe anemia, severe obesity, diabetes mellitus, chronic immunosuppression) and further general risk factors (e.g., hip-fractures or prior arthroplasty surgeries)[35]. The study analyzed 2551 patients and found a trend towards fewer PJI cases in the dual ALBC (COPAL G+C) group containing exclusively patients at higher infection risk compared to the mixed risk profile (low and high risk) in the single ALBC (PALACOS R+G) group (PJI rate 2.45% vs 3.7%) (level of evidence III/IV). This was a particularly interesting observation as one would expect an even higher PJI incidence in the higher infection risk cohort of patients. Further studies are needed to confirm whether this trend to fewer PJI cases in presumed risk for infection patients can be generalized on a broader basis for dual ALBC.
LOWER RATE OF RE-REVISIONS WITH DUAL ALBC IN SPACER AND/OR FIXATION CEMENT FOR REVISION PROSTHESIS - SEPTIC REVISION ARTHROPLASTY
For many surgeons it is common clinical practice to use ALBC for the manufacture of block or articulating spacers in staged PJI treatment protocols and/or for the fixation of the revision prosthesis. Such ALBC spacers are meant to prevent bacterial recolonization of the foreign body and assist in the successful eradication of the infected joint in combination with systemic antibiotics. In order to increase the depot effect of the local antibiotics and to counteract the risk of antibiotic resistances in septic cases, the use of combinations of local antibiotics has been suggested[36]. The selection of antibiotics should be based on the antibiogram of the PJI organisms found after culture of synovial fluid and tissue biopsies. Vancomycin is the most common antibiotic added to aminoglycoside-containing single ALBC either in form of commercial dual ALBC brands or manually admixed in the theatre. The rationale of its use is to further target gentamicin-resistant methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis (MRSE). pathogens which are frequent in some regions, such as the United States[37]. Although several questions remain regarding optimal dosage for the possible therapeutic effects on biofilm-bacteria and/or contribution to renal injury if admixing large amounts of antibiotics into cement[18,36], its contribution to successful infection eradication appears conclusive[38]. Wouthuyzen-Bakker et al[39] have recently demonstrated on a large number of PJI cases to which extent the addition of vancomycin into the cement spacer influences the amount of growth-positive cultures taken at reimplantation of the revision prosthesis. The rate of unsterile biopsies dropped from 21.7% to 9.5% if combinations of vancomycin and gentamicin in the cement were used instead of aminoglycoside monotherapy spacers. On a single bacteria level, the strongest antimicrobial effect by such dual ALBC spacers was evident for coagulase-negative staphylococci (reduction of growth-positive samples from 13.3% to 2.5%).
Abdelaziz et al[40] have also provided evidence that the strategy of using the broad spectrum dual ALBC COPAL G+C (with or without additional admixing of vancomycin or ofloxacin) led to complete cure of PJI in one-stage treatment protocols. At five years follow-up, no patient required a repeated revision arthroplasty with exchange of the cemented prosthesis because of either infection or loosening. This was particularly remarkable as 33% of the included PJI cases in this study were caused by polymicrobial infections.
CONCLUSION
The current literature including in vitro and in vivo studies supports the additional benefits of dual ALBC, with synergy of drug elution and improved antibacterial activity on a wide range of pathogens related to orthopedic infections. While its therapeutic efficacy on mature biofilm-bacteria is still not entirely clear, more and more data have now demonstrated that it may confer better protection from infection in particularly vulnerable patients or in higher risk procedures (see Figure 3 for summary of clinical evidence). However, this conclusion is based on a mix of prospective and retrospective cohort studies, the latter with a lower evidence level and inherent limitations with regard to possible study bias and higher risk of confounding factors. A generalization of the observed effect of a stronger antibiotic prophylaxis by dual ALBC may also be problematic given that the ready-to-use brands of bone cements differ in their antibiotic elution properties as well as in the nature and amount of pre-mixed antibiotics.
Figure 3 Overview of published clinical study results comparing prosthetic joint infection rate in patients in single low dose cement and dual high dose cement group across different indications.
The table below lists the main study authors, indication and study design, number of patients included, evidence level of clinical study and prosthetic joint infection rate in % in both study groups. PJI: Prosthetic joint infection; SLDC: Single low dose cement = PALACOS R+G (containing 0.5 g of gentamicin); DHDC: Dual high dose cement = COPAL G+C (gentamicin + clindamycin); FNF: Femoral neck fracture; rTKA: Revision total knee arthroplasty; RFI: Risk for infection; THA: Total hip arthroplasty.
The idea of an infection risk-adapted antibiotic prophylaxis strategy may be one interesting option among other preoperative optimization protocols to decrease the burden of PJI. In addition to the use of dual ALBC this can also be achieved by temporary or permanent antibacterial implant coatings including surface modifications with silver ions or manual spreading of a fast-resorbable, antibiotic-loaded hydrogel[41]. Both strategies have been shown to reduce early post-surgical infections in uncemented implants in orthopedic surgery. Further studies are needed to truly elucidate the effect of dual ALBC and other local antibiotic delivery systems for infection prevention and to weigh possible benefits against potential adverse effects and costs.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Veltman ES, Weng X S-Editor: Gao CC L-Editor: A P-Editor: Xing YX