INTRODUCTION
The rising prevalence of diabetes represents a major public health and socioeconomic burden on society. Diabetic foot is one of the clinical manifestations of diabetes. Diabetic foot may develop in some diabetic patients as a consequence of advanced disease. It is defined as a structural or functional alteration of the foot that may manifest as ulcers, osteomyelitis, or gangrene, as a result of the interaction of different factors induced by sustained hyperglycemia and previous traumatic causes[1,2]. Diabetes-related foot complications have been identified as the single most common cause of morbidity among diabetic patients[3]. Recurrence rates of diabetic foot ulcers are high, and they remain an unresolved issue for many patients[4,5]. Pinpointing the exact cause is difficult; however, this is crucial for the management of diabetic foot as well as for the prevention of recurrences.
Neuropathy, deformity, callus, increased peak plantar pressure, peripheral arterial disease, penetrating trauma, and ill-fitting shoes account for 64% of all new diabetic foot ulcers; hence, their prevention is paramount in comprehensive diabetes management[6,7]. Once it occurs, diabetic foot may be managed with offloading, wound care utilizing various dressings, skin grafting or formal debridement.
One of the common causes of foot ulceration is ankle equinus, which increases the pressures on the plantar surface of the foot during ambulation[8]. Among diabetic patients, contracture of the triceps surae is thought to occur and this contributes to ulcer formation[9]. In patients with a high risk of recurrent ulcers and when traditional offloading methods have failed, surgical offloading may be an alternative option[10-12]. However, equinus resulting from limb contracture is known to be a complication of prolonged static positioning, specifically due to foot wounds, as the ulcer can be initially caused by external injuries as well[13]. In the late stage, it is difficult to determine which one came first: The contracture or the ulceration.
It is not clear in the current literature if the pathophysiology leading to equinus is the same between diabetic and nondiabetic patients[8]. In this paper, we review and analyze the contribution of equinus contracture to diabetic foot ulcers and vice versa. We also sought to formulate the possible timelines of both conditions during the natural course of diabetic foot ulcers.
CLINICAL CONSIDERATIONS
An equinus ankle is an ankle that will not go into more than 5 degrees of dorsiflexion with the knee extended. Most cases of equinus are associated with other biomechanically related disorders[14]. There are several possible causes for the limited range of ankle motion, including a bony block or soft tissue contracture. Limb contracture itself is a condition of shortening and hardening of the muscles, tendons, or other tissues, often leading to deformity and rigidity of the joints. In diabetic foot, contracture may ensue following prolonged immobilization due to the ulcers[13]. This is made worse by macro and microangiopathy, further deteriorating the soft tissue quality.
Initial management of ankle equinus involves stretching and physical therapy, balancing flexibility and power. However, in severe cases, surgery may be chosen to provide an immediate increase in the range of motion[15]. Several surgical techniques have been described. The type and level of surgery will be determined from a physical examination, specifically related to the affected structure. Achilles tendon lengthening is indicated if both the gastrocnemius and soleus are affected. Here, the tendon is either sectioned completely, Z-lengthened, or triply hemisectioned[15,16]. The increase in tendon length will reduce its tension and reduce the pressure on the plantar aspect of the foot by allowing it to dorsiflex. The pressure offloading relieves the abnormal pressure applied to the ulcer, promoting wound healing[17]. Lengthening the triceps surae complex should decrease the stress on the entire plantar forefoot. Lengthening other tendons may serve specific purposes: Peroneus longus tendon lengthening may relieve pressure on the plantar surface of the first metatarsal head, while lengthening of the posterior medial tendon should reduce plantar flexion of the fifth metatarsal head. The literature has attested to the virtue of tendon lengthening when indicated[18]. In addition, correction of static deformity through various methods, including resectional arthroplasty or osteotomy, is beneficial to decrease the pressure overload on the prominences of the plantar surface[19]. Bony correction, such as arthroplasty or excision, may be helpful were deemed necessary. The drawbacks include the changes in the foot anatomy as well as the possible relocation of points of focal pressure to a different area of the foot[20].
PATHOGENESIS OF FOOT ULCERATIONS
Regardless of the anatomical location, the etiology of a diabetic foot wound is considered multifactorial. While the underlying cause is poor glycemic control resulting in angiopathy and neuropathy, the wound can be precipitated by injury, deformities, improper foot care, or elevated plantar pressure due to increased biomechanical loading[21,22].
Diabetic foot development occurs in phases: The first phase is callus formation, followed by multiple foot traumatization due to loss of the protective sensations secondary to neuropathy. Dry skin on the diabetic foot caused by autonomic neuropathy only worsens the condition. The ensuing subcutaneous hemorrhaging delivers the final insult to the skin, resulting in skin ischemia and then ulceration[23].
The presence of localized elevations of plantar pressure has been conclusively identified as the primary determinant of plantar ulceration[24]. Localized increases in pressure are sufficient to initiate ulceration[25]. Tightness or contracture in the triceps surae and foot intrinsic muscles contributes to the initiation of ulceration; triceps surae contracture plantarflexes the foot, increasing stress on the forefoot, while hammertoe and clawtoe resulting in intrinsic tightness cause the migration of the plantar metatarsal head fat[26]. Prior studies demonstrated that plantar pressures are higher in cases with active diabetic foot ulcers despite having a longer stance phase duration, which would be expected to lower plantar pressure. Hence, taking these condition into consideration, the prevention of diabetic foot ulceration requires offloading of pressure during ambulation despite a longer stance phase[27].
Charcot neuropathic osteoarthropathy, for which diabetic neuropathy is the most common etiology, is a condition affecting the bones, joints, and soft tissues of the foot and ankle, characterized by inflammation in the earliest phase. This inflammatory condition may lead to varying degrees and patterns of bone destruction, subluxation, dislocation, or deformity. The hallmark deformity is midfoot collapse, described as a “rocker-bottom” foot, that can result in persistent foot ulceration due to the pressure[28]. The contributing forces for this deformity include the contracture of the peroneal, anterior tibialis, and Achilles tendon.
Biological impairment and vasculopathy also play a significant role in the formation of diabetic foot and its ability to heal. Endothelial dysfunction is an inflammation of the endothelial cells due to hyperglycemia. During the hyperglycemic state, the endothelial cells switch from the utilization of nitric oxide to metabolize glucose, the depletion of which results in the inability to vasodilate. The inability to vasodilate increases intravascular pressure, causing injury and inflammation to the endothelial cells, which in turn causes the migration of inflammatory cells subintimally, forming atherogenic foam cells. Lytic enzymes released by inflammatory cells further damage the vessels in a condition called vasculopathy, which is responsible for both the initiation of ulceration as well as its impaired healing. Impaired activity of the white blood cells involving both B and T cell types in diabetic patients may also complicate the healing and treatment of these wounds[19]. Furthermore, ankle joint equinus has been reported to be associated with diminished venous blood flow in the lower extremity that is detrimental to wound healing[29].
PATHOGENESIS OF EQUINUS CONTRACTURE
Tendon diseases are increasingly common fibrotic disorders and they account for a third of all musculoskeletal complaints[30-32]. Fibrosis itself is characterized by extracellular matrix (ECM) accumulation and often by a change in the quality of the ECM. The morphological and biochemical disturbances of the ECM are directly related to a loss of function in the target organs[33].
In general, several pathophysiologic mechanisms appear to be involved in the development of contracture. The most frequent cause is immobilization, but it can also be caused by congenital deformities, muscle problems, ulcers, local trauma, diabetes, and hormone deficiencies[34]. Prior studies confirmed that levels of physical activity are low in the population with diabetes, and this inactivity is more commonly seen in older patients[35]. This inactivity can lead to contracture development through some conditions, including static positioning, muscle imbalance, and the aforementioned fibrosis.
Static positioning indicates that the position in which a joint is statically positioned influences the number of sarcomeres present in any given muscle. A statically positioned limb developing fibrotic changes within the muscle will develop contracture formation in the position of immobilization. In a supine position, bulky posterior muscles are at a physiologic disadvantage in maintaining flexibility. The imbalance between the flexor and extensor muscle groups has not been shown to be a major factor leading to contracture formation, but contractures are frequently observed when major muscle imbalance is present[13,36]. Thus, inactive patients tend to develop plantarflexion contracture without regular stretching or splinting. Intrinsic muscle tissue alterations in dystrophic myopathies also contribute to contracture formation, due to replacement of the functioning muscle fibers with collagen and fatty tissue in concert with a chronically shortened resting muscle length[13]. A situation of concern is when soft tissue changes that contribute to contractures begin very early after the onset of immobility. Protein synthesis within muscle fibers is reduced within 6 h after immobilization. Shortening of muscle fibers occurs within 24 h, and after 48 h, collagen infiltration of the perimysium is increased[37].
In diabetes patients, chronic hyperglycemia is a major factor causing various complications, since strict glycemic control reduces the end-organ complication incidence and the rate of progression[33]. Hyperglycemia can work through both metabolic and hemodynamic pathways to affect ECM turn-over[33]. Hyperglycemia is responsible for the presence of high levels of advanced glycation end-products (AGEs), which are able to directly stimulate the production of ECM[38]. In addition, AGEs significantly interact with the renin angiotensin aldosterone system (RAAS). Angiotensin II, the main physiological effector molecule of the RAAS, mediates fibrosis by stimulating the synthesis of ECM components. Generally, RAAS is known to be an important contributor to the pathogenesis of diabetic micro- and macrovascular complications by inducing various tissue responses, including not only fibrosis but also vasoconstriction, inflammation, oxidative stress, cell hypertrophy and proliferation[33]. Pathogenesis of fibrosis is also affected by transforming growth factor β (TGF-β). TGF-β regulates the expression of many matrix proteins, including ECM. TGF-β has been previously reported to be the main pro-fibrotic factor in diabetic nephropathy[39]. In the diabetic environment, there is upregulated TGF-β1 expression and bioactivity in glomerular mesangial and proximal tubule cells[33]. Certain comorbidities have been known to affect TGF-β expression, for example, cigarette smoking, in which sustained oxidative stress induces chronic inflammation and causes a further release of active TGF-β1[40].
Our body’s repair mechanism during the healing cascade of the wound may also lead to fibrosis. Activated repair cells, myofibroblasts, are the main producers and organizers of ECM, which is needed to restore tissue integrity after injury. Too many fibroblasts working for too long can cause hypertrophic scarring and tissue contractures[41].
Nonhealing wounds resulting in an infection may cause a stress response in the body by increasing the amount of certain hormones, such as cortisol and adrenaline. In the liver, high cortisol levels increase gluconeogenesis and decrease glycogen synthesis[42]. These hormones work against the action of insulin, and as a result, the body’s production of glucose and blood sugar levels are increased. Inflammatory stimuli that activate macrophages enhance the release of active TGF-β complexes that are secreted by plasma cells and then release active TGF-β into the extracellular fluid[43]. It is well established that TGF-β1 functions as a wound healing promoting factor, and when in excess it may lead to overhealing outcomes, such as hypertrophic scarring and fibrosis[44].
It can be concluded that there are multifactorial etiologies of ankle equinus contracture. Intrinsically, hyperglycemia and wound healing mechanisms are known to play a role in contracture development. In addition, static positioning due to a systemic health problem or the presence of an established wound may result in contracture as well.
CONCLUSION
Diabetic foot ulcer has been known to be associated with various factors, including equinus deformity ensuing from a triceps surae contracture. Although some light has been shed on the mechanism of both, the exact mechanisms and their interactions are still unclear. This is a situation that may seem like a chicken-or-egg condition, and which one comes first is puzzling.
In our experience, the patients came at different stages without any strict pattern of the pathogenesis. Some patients presented ulcers without contracture, and vice versa. It is also suggested not to consider hyperglycemia status as a sole determinant of the ulcerations of contracture development, as it is also depended on many factors as mentioned above.
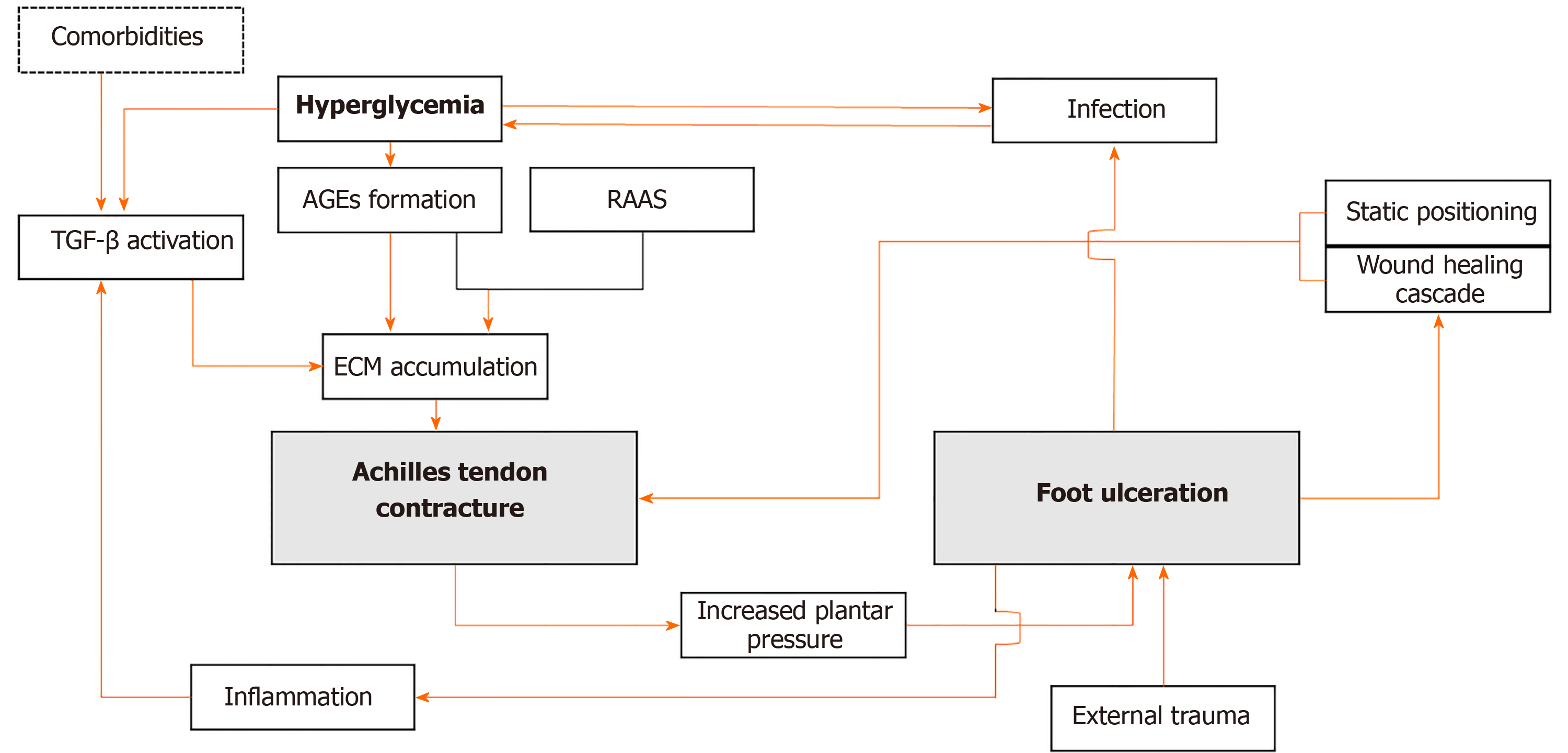
Determining the timeline of each pathology is crucial, as shown in Figure 1. A contracture may contribute to wound development in the ulcer-prone feet. Recognizing and addressing the source of a triceps surae contracture is important to prevent subsequent ulcers. While chronic hyperglycemia can directly cause contractures through AGEs accumulation, it is not the sole etiology for this condition. Thus, other contributing factors must be identified and taken into consideration. Prolonged immobilization due to diabetic foot is seen as the bridge to triceps surae contracture in nonambulant patients. The body’s wound healing cascade can contribute to further fibrosis that affects the joint. In patients presenting with long-standing wounds, it is difficult to determine which one came first. Thus, a thorough evaluation, including physical and adjunct examinations, must be conducted and interpreted wisely.
Figure 1 Schematic diagram indicating how a vicious cycle can occur between equinus contracture and foot ulceration in patients with diabetes.
TGF-β: Transforming growth factor β; AGEs: Advanced glycation end-products; RAAS: Renin angiotensin aldosterone system; ECM: Extracellular matrix.
Identifying the pathogenesis of a specific pathology can lead to the correct and timely treatment. Assessments and interventions are essential in breaking the vicious cycle that may disturb wound healing.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Indonesia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Valentini R, Yang Z S-Editor: Gao CC L-Editor: A P-Editor: Xing YX