Published online Dec 18, 2021. doi: 10.5312/wjo.v12.i12.961
Peer-review started: January 30, 2021
First decision: October 17, 2021
Revised: November 3, 2021
Accepted: December 9, 2021
Article in press: December 9, 2021
Published online: December 18, 2021
Processing time: 318 Days and 12.1 Hours
Far lateral lumbar disc herniations (FLLDH) represent a separate category of disc pathology which includes both intraforaminal and extraforaminal lumbar disc herniations, that are characterized by a peculiar clinical presentation, diagnostic and treatment modalities as compared to the more frequent median and paramedian disc hernias. Surgical treatment often represents the only effective weapon for the cure of this disease and over the years different approaches have been developed that can reach the region of the foramen or external to it, with different degrees of invasiveness. The diagnosis is more demanding and still underestimated as it requires a more detailed knowledge in the spine anatomy and dedicated radiological studies. Computerized tomography and in particular magnetic resonance imaging are the appropriate tools for the diagnosis of FLLDH. Despite the widespread use of these diagnostic tests, many cases of FLLDH are overlooked due to insufficiently detailed radiological examinations or due to the execution of exams not focused to the foraminal or the extraforaminal region. Neurophysiological studies represent a valid aid in the diagnostic classification of this pathology and in some cases they can facilitate the differential diagnosis with other types of radiculopathies. In the present study, a comprehensive review of the clinical presentation, epidemiology, radiological study and the neuro
Core Tip: Far lateral lumbar disc herniations constitute a distinct category of lumbar disc herniations. Clinical presentation, diagnosis and treatment are more demanding and require specific knowledge. A comprehensive review of the clinical presentation, epidemiology, radiological study, and neurophysiological aspects is presented in the present study.
- Citation: Berra LV, Di Rita A, Longhitano F, Mailland E, Reganati P, Frati A, Santoro A. Far lateral lumbar disc herniation part 1: Imaging, neurophysiology and clinical features. World J Orthop 2021; 12(12): 961-969
- URL: https://www.wjgnet.com/2218-5836/full/v12/i12/961.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i12.961
Approximately 10% of symptomatic lumbar disc herniations are located within the neural foramen or lateral to it. These intraforaminal and extraforaminal lumbar disc herniations, usually referred to as far-lateral lumbar disc herniations (FLLDH), can compress the spinal nerve and dorsal root ganglion leading to severe, sometimes excruciating pain that often does not respond to conservative management and requires surgery.
FLLDH represent therefore a distinct category of lumbar disc herniation, which are characterized by unique clinical manifestations and require a greater diagnostic and therapeutic effort than the more usual median and paramedian localizations of disc hernias.
In this review, we analyze the clinical features, the radiological imaging aspects and the neurophysiological characteristics.
More than 90% of lumbar disc herniations happen at the disc's posterior edge, which is located within the spinal canal. There are two types of intracanalicular herniations: median and paramedian (or postero-lateral). By impinging the nerve root in the lateral recess, shortly as it emerges from the thecal sac, they can produce radiculopathy. As a result, the root that exits the canal through the foramen of the caudal interspace at the afflicted disc is the one that is involved (e.g., in the case of a far lateral L4-L5 her
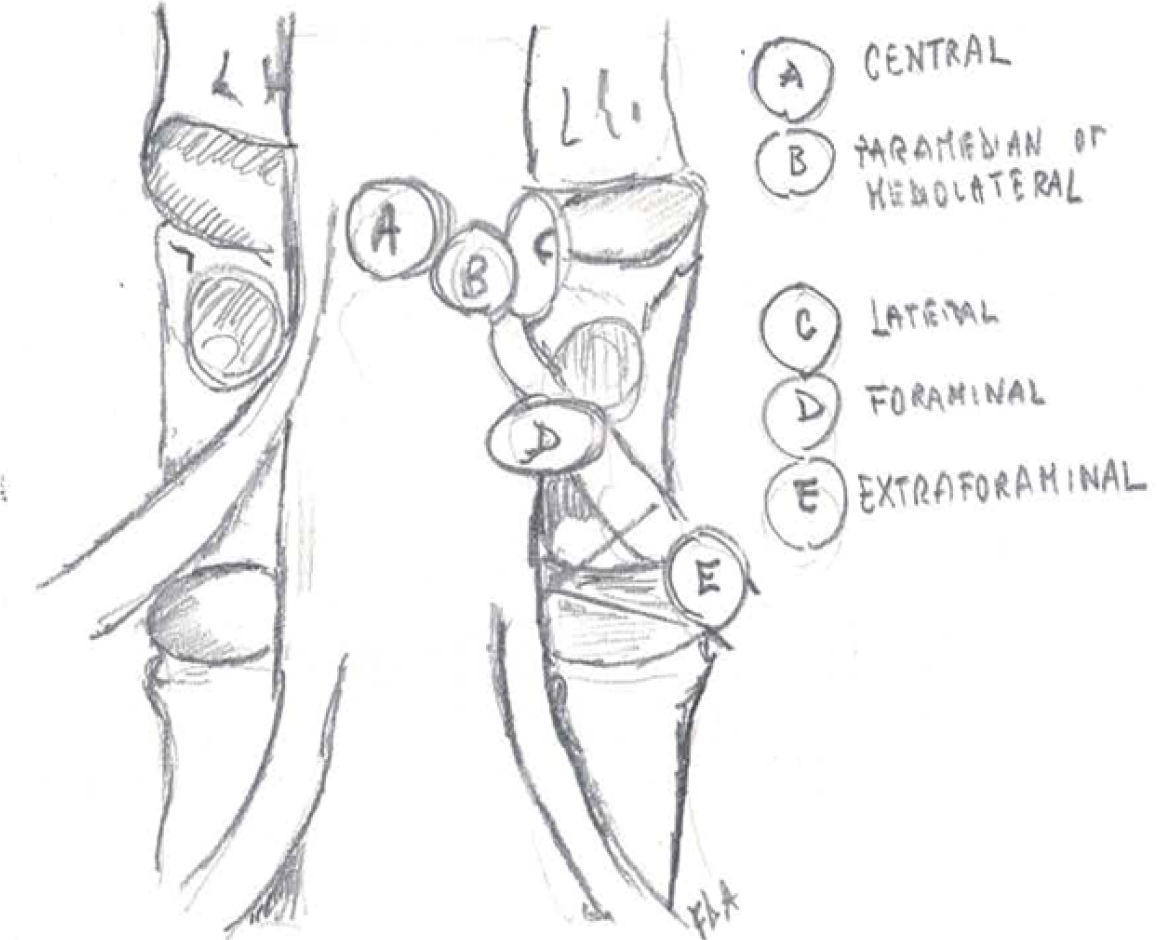
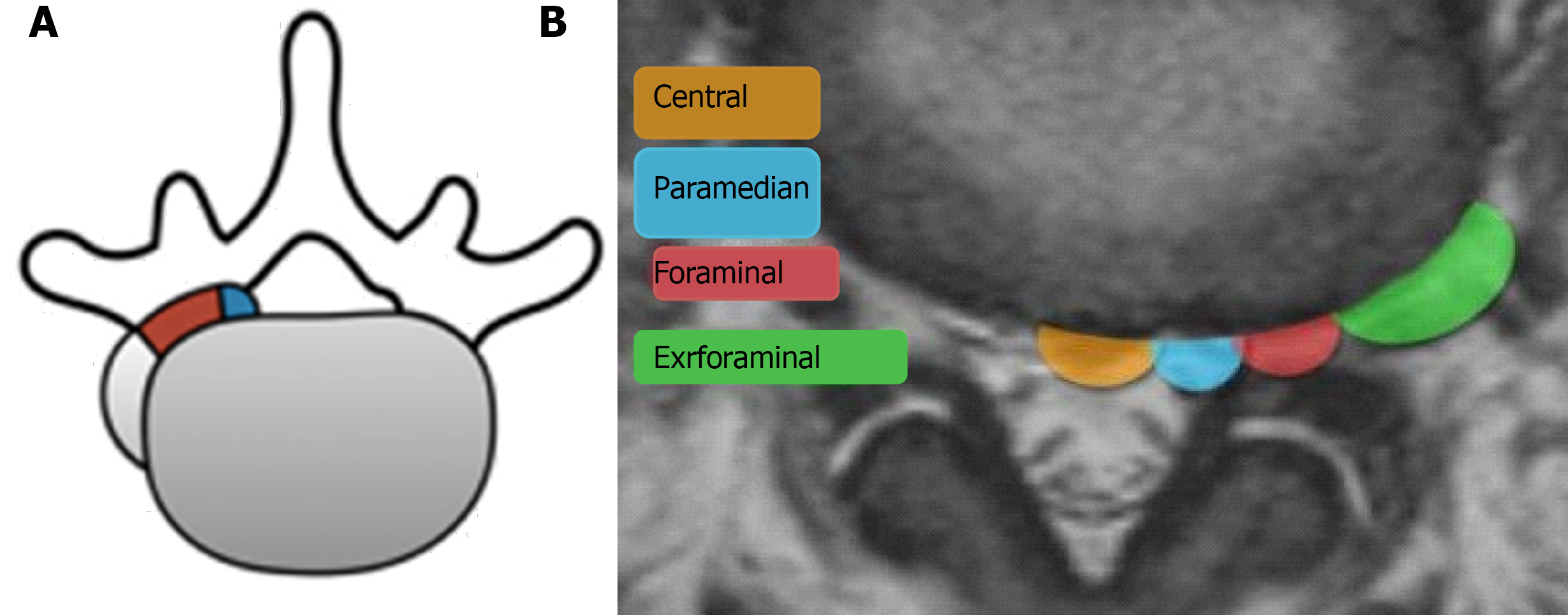
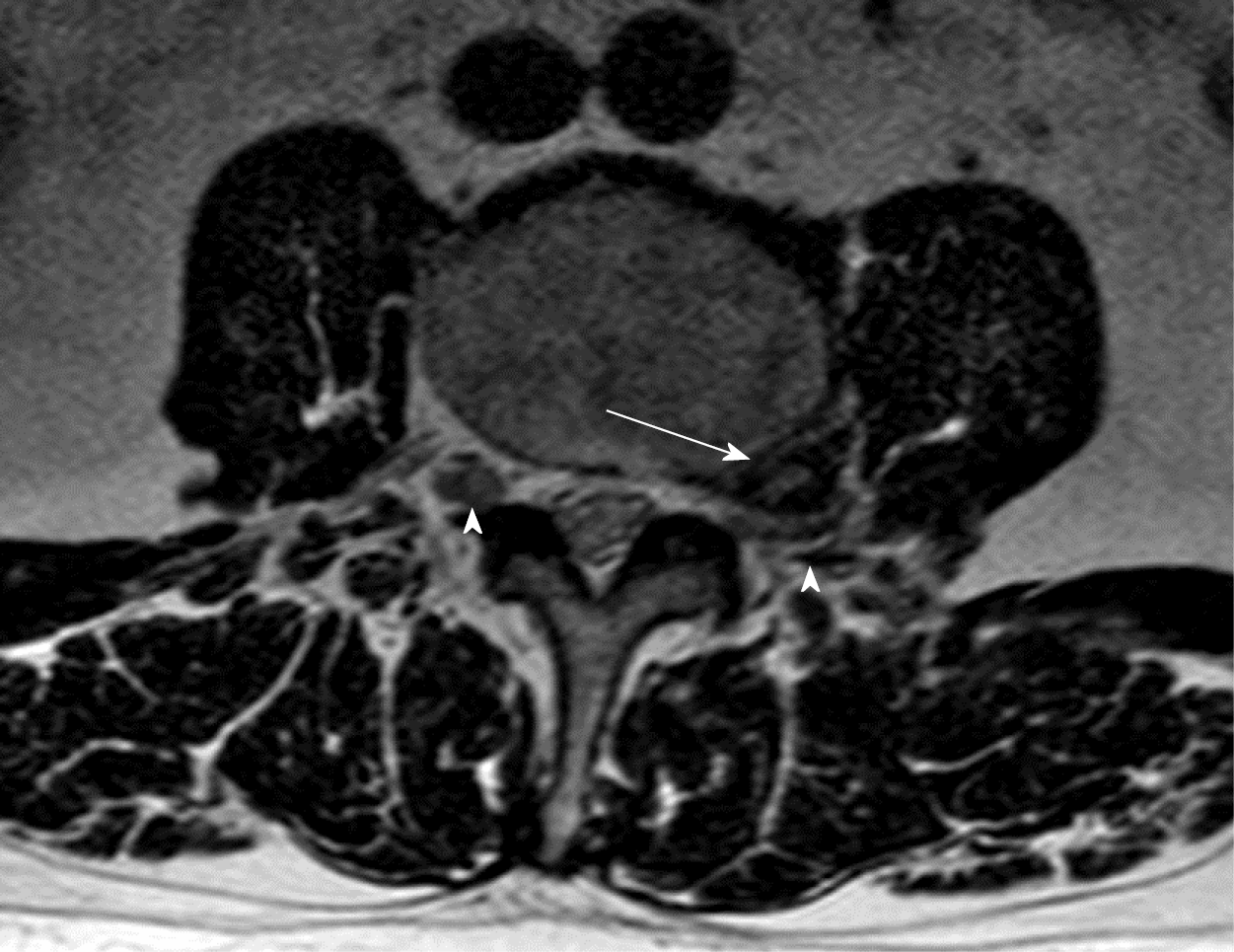
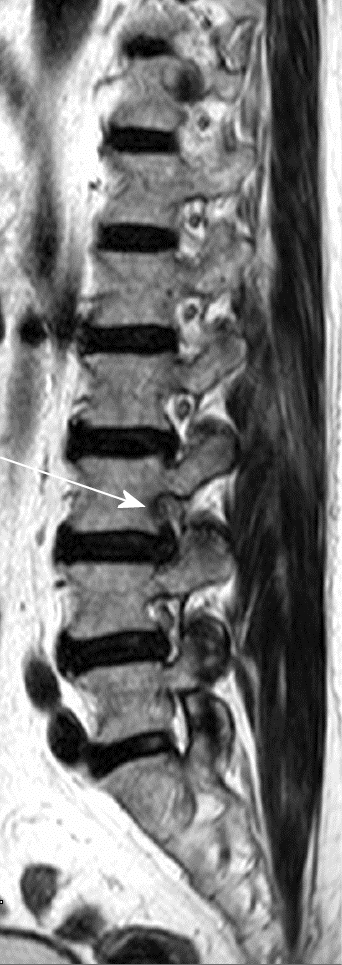
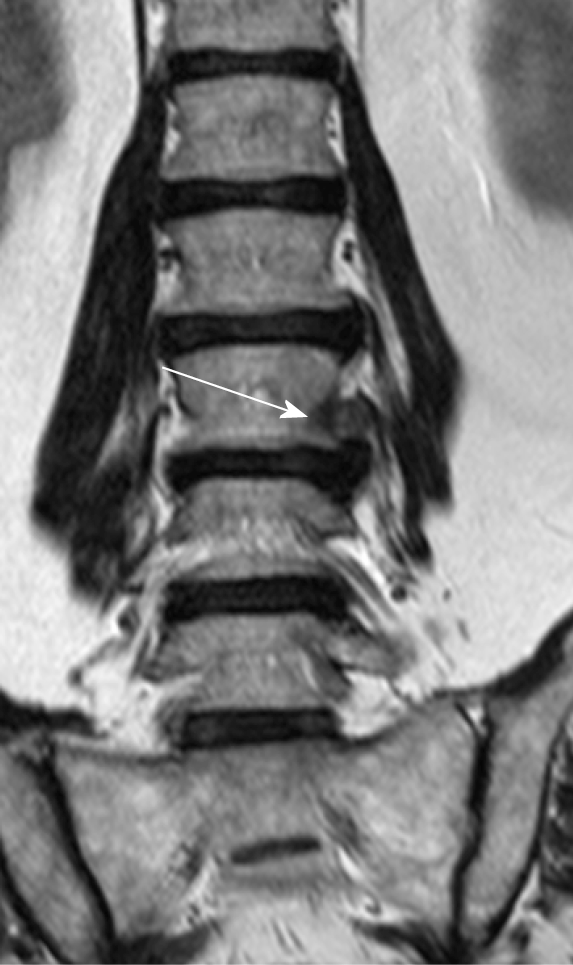
FLLDH are herniations that occur outside the spinal canal, within the neural foramen (the space bounded cranially and caudally by the pedicles), or in the extraforaminal area (the space beyond the lateral margin of the pedicles).
The herniation involves the root that exits into the foramen of the same intervertebral space (e.g., in the case of an L4-L5 paramedian herniation, the L4 root) (Figures 1-3).
Macnab described extraforaminal disc herniations and the associated symptoms caused by compression of the exiting nerve root in his 1971 paper about negative surgical disc space explorations in patients with radiculopathy[2].
Lateral disc herniation has different clinical features from medial disc herniation. Patients with lateral disc herniation may manifest with more severe clinical symptoms, like severe radicular pain, and motor and sensory neurologic deficits are more frequent than those with medial disc herniation. The cause is that the herniated disc fragment compresses the nerve root inside a narrow radicular foramen, resulting in direct compression of the dorsal radicular ganglion, which is a pain-sensitive structure.
In 1975, Abdullah and colleagues[3] published a detailed description of the clinical syndrome caused by FLLDH. The "extreme lateral" syndrome described by Abdullah is well defined and includes severe pain due to dorsal root ganglion involvement, as well as a higher risk of neurological deficits than common posterolateral herniations.
FLLDH is responsible for 6.5% to 12% of all lumbar disc herniations[4,5]. Intraforaminal and extraforaminal lesions appear to be almost equally common (3 percent and 4%, respectively)[6]. L3-L4 and L4-L5 are the most involved levels, followed by L5-S1. With a reported frequency of 28 percent of all FLLDH, proximal levels (L2-L3 and L1-L2) are less prevalent but comparatively more common than typical postero-lateral herniations. The average patient age is between 50 and 78 years old, with a male to female ratio ranging from 1:1 to 2:1[7,8]. Extreme radicular pain is the most prevalent clinical manifestation, which is commonly accompanied by sensory and motor dysfunction as well as a reduced patellar reflex[9]. Back pain is a common symptom in intracanalicular herniations, but it is usually less severe. The femoral stretch test (reverse - Laségue) may show a significant positive result. By bending to the side of the lesion, radicular pain and paresthesia can be replicated, and this is thought to be a sign of intraforaminal root compression[3]. FLLDH at the L3-L4 Level, causing compression of the L3 root, result in pain in the anterior aspect of the tight. FLLDH at the L4-L5 Level, causing compression of the L4 root, are associated with pain in the anterior aspect of the tight, medial malleolus, and medial foot. FLLDH at the L5-S1 Level, where the compressed root is L5, are associated with pain in the postero-lateral aspect of the tight and leg. The clinical characteristics of postero-lateral and far-lateral herniations are summarized in Tables 1 and 2. Despite the heavy clinical manifestations of FLLDH, the natural history is favorable with a reported cure rate with conservative treatment of approximately 71%[10].
| Clinical findings | PLH | FLH |
| Nerve root invovled | At the level below the disc herniation | At the same level of disc herniation |
| Femoral stretch test | Not always significantly reliable | Markedly positive |
| Lateral bending | Do not reproduce radicular symptoms | Usually reproduces pain and paresthesia |
| Severity of pain | Variable | Strong, related to dorsal root ganglion compression |
| Root | PLH level | FLH level | Pain/radiation/sensory involvement | Motor involvement | Deep tendon reflex | Radicular stretching test |
| L3 | L2-L3 | L3-L4 | Anterior aspect of the tigh | Iliopsoas and/or quadriceps | Patellar | Femoral |
| L4 | L3-L4 | L4-L5 | Anterior aspect of the tigh, medial malleolus and medial foot | Quadriceps and anterior compartment fo the leg | Patellar | Femoral |
| L5 | L4-L5 | L5-S1 | Postero-lateral tigh and leg | Extensor hallucis longus and dorsiflexors | None | Lasègue |
| S1 | L5-S1 | Posterior thigh and leg, foot (plantar) | Triceps surae | Achilles | Lasègue |
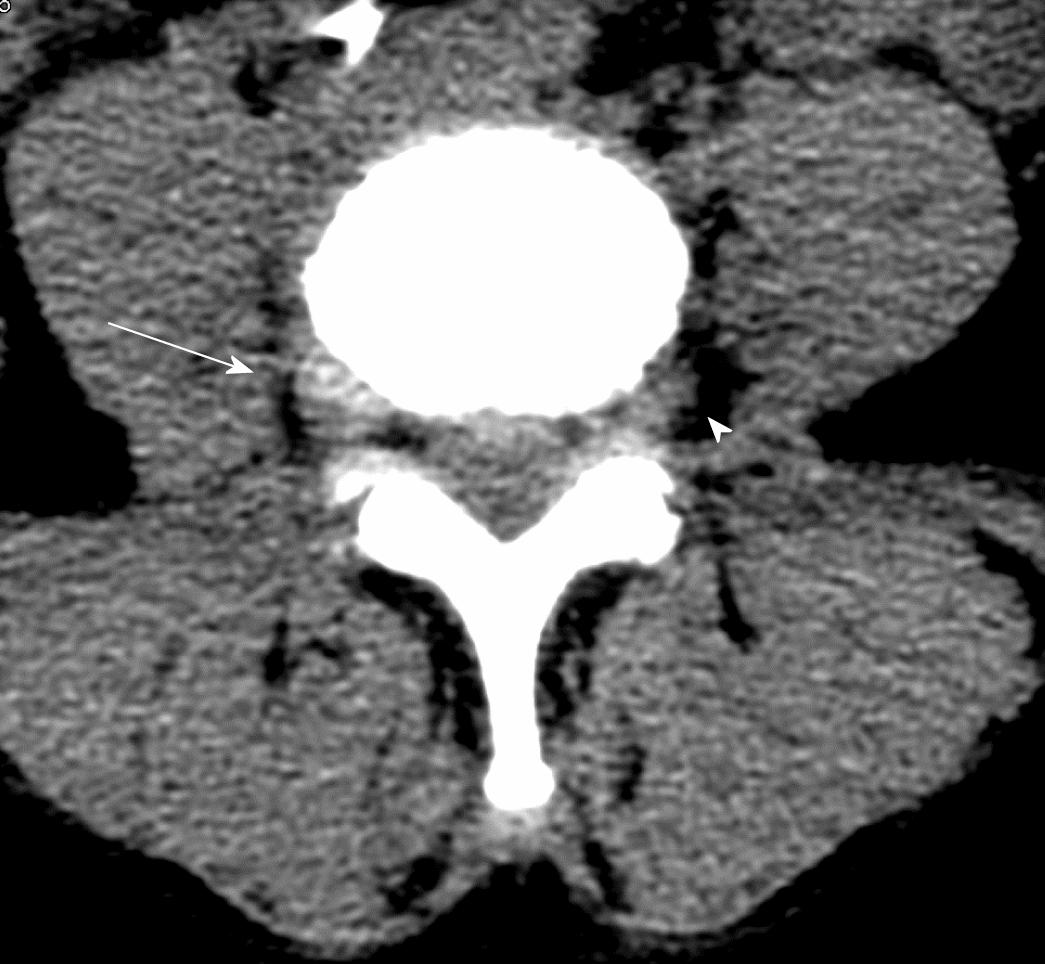
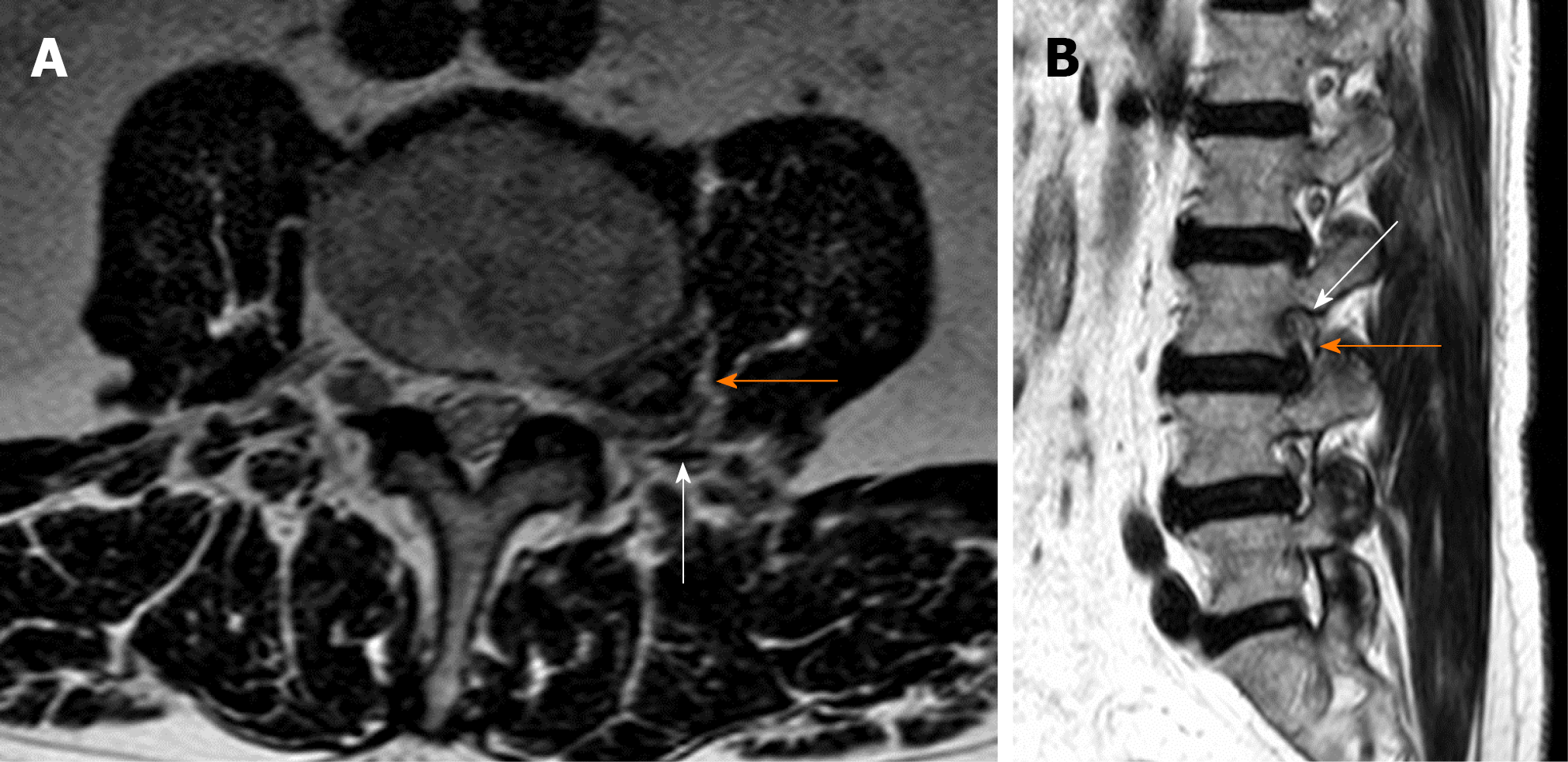
The correct surgical strategy depends on a preoperative diagnosis and thorough location of an extracanalicular herniated disc. FLLDH were difficult to detect until the advent of computed tomography (CT): In fact, root compression lies beyond the lateral extension of the subarachnoid space, therefore it cannot be seen on myelographic images[2]. Both magnetic resonance imaging (MRI) and computed tomography (CT) can now show disc herniations in intra- and extraforaminal locations in high detail. Despite advancements in neuroimaging, however, diagnosing FLLDH may be difficult. In fact, routine spine imaging is frequently limited by slice thickness and field lateral extension. Furthermore, concurrent degenerative alterations like stenosis or intracanalicular disc bulging might make radicular compression inside or laterally to the foramen difficult to visualize[11]. Osborn and colleagues discovered a 30% probability of misdiagnosis on the first CT or MR report in one study. Intracanalicular herniations, on the other hand, are rarely ignored[12]. Osteophytes, nerve root sheath pathologies (such as conjoined roots, arachnoid, perineural, and synovial cysts), and schwannomas, neurofibromas, and ectatic epidural venous plexuses are among the differential diagnoses for FLLDH[12]. When compared to the adjacent intersomatic non herniated disc, the extruded disc material is frequently hyperdense on CT images. (Figure 4). Bone windows make it easier to identify osteophytes. The herniation to the intersomatic disc is often hypointense in T1 and hyperintense in T2 on MR; osteophytes show a signal void in both sequences (Figure 5). The best imaging approach for detecting FLLDH is magnetic resonance imaging (MRI). CT detects radicular compression less reliably than MR and has lesser resolution for spinal and paraspinal soft tissues (Figures 6-8). CT imaging, on the other hand, can be effective in detecting osteophytes and calcifications[11,12]. There may be one or more of the following MR findings: (1) disc margin focal eccentricity; (2) perineural fat tissue obliteration; (3) nerve root thickness alterations; and (4) nerve root dislocation The herniated disc compresses the nerve roots directly, causing thinning, whereas edema can produce thickening. Furthermore, a closer examination usually indicates that epidural fat tissue obliteration is predominantly medial to the root in exclusively intraforaminal herniations, whereas it is observed both medially and laterally to the root in intra-extraforaminal herniations. As previously stated, standard MR examinations are frequently not focused on extraforaminal locations, and imaging this region can be particularly difficult at L5-S1 since the sacral alae and iliac bones' bony features tend to overlap. Furthermore, degenerative changes to the L5-S1 disc are common, reducing its height and making research difficult. Misdiagnosis is frequently caused by an improper MR methodology. Axial slices must be parallel to the intersomatic disc when centered on the sagittal plane. This is necessary in order to detect even minor disc margin focal eccentricities and distinguish real root dislocations from non-pathological asymmetries between the two sides' roots. In order to locate the route of roots and proximal spinal nerves, paracoronal sections (angled 15 to 30 degrees) as well as sagittal sections reaching far laterally and spanning the entire length of the foramina are necessary in the search for a far-lateral herniation[11,12]. Contrast agent administration is not usually required. Differentiating a sequestered disc fragment from other diseases such as schwannomas may need contrast-enhanced imaging. In such circumstances, fat-saturation pulse T1-weighted spin-echo sequences with axial and sagittal T1-weighted spin-echo can be employed. The sequestered fragment normally improves in the periphery, most likely as a result of an inflammatory reaction in the surrounding area[13].
Neurophysiology is a complimentary yet crucial tool in the diagnosis of FLLDH, as it aids in the differential diagnosis of radiculopathy and other disorders, as well as the verification of the implicated level. It may also reveal the extent of the damage to the brain. This evaluation is aided by a variety of ways. Electromyography, as well as findings from nerve conduction tests, H reflex, and F wave studies, are used to determine the appropriate workout. (1) signs of neurogenic injury in muscles pertaining to the same spinal root with normal (or relatively spared) findings in muscles pertaining to nearby roots; (2) involvement of the proximal part of the peripheral nervous system; and (3) exclusion of other possible sites of injury that can mimic a radicular lesion, such as the lumbo-sacral plexus or single nerves.
The pattern distribution of anomalies is commonly used to identify the afflicted root. As a result, needle electromyography is done on a large number of muscles, looking for anomalies in muscles belonging to a single root and normal findings in muscles belonging to other roots. Normal results in muscles innervated by distinct roots but belonging to the same nerve or plexus may also assist in distinguishing nerve or plexus injury from radiculopathy. Unfortunately, each muscle is frequently assigned to one of several nearby roots, and each root feeds multiple muscles, making differential diagnosis difficult. Because the motor regions of roots L2, L3, and L4 are significantly overlapping, this is especially noticeable when examining upper lumbar radiculopathies[14]. In such cases, assessing the paraspinal muscles can be helpful in determining the affected level. This should concentrate on the multifidus muscle, which, unlike other paraspinal muscles, is thought to be innervated by a single root[15]. In any event, there are certain limits to paraspinal muscle examination: fibrillation can be absent in paraspinal muscles in some cases of root injury, and these muscles can be difficult to assess, especially in obese individuals or those who are unable to relax the target muscles. Furthermore, after back surgery, residual neurogenic alterations due to local trauma can be found in paraspinal muscles, making postoperative testing useless[13] (Daube, 2009). Electromyography can also reveal information about the disease's progression and severity. The first expected observation after acute axonal injury is a decrease in motor unit potential (MUP) recruitment proportionate to the amount of the lesion. After 2-3 weeks, fibrillation potentials arise, and their quantity is a good measure of the amount of destroyed motor axons. Denervated muscle fibers will gradually be recruited in surviving motor units, resulting in distinct modifications in the weeks and months ahead (at first an increase in MUP duration and number of phases, and then of MUP amplitude)[16]. Increased duration and amplitude of compound potentials are a static finding that lasts indefinitely (assuming the larger motor units aren't successively harmed), therefore they shouldn't be considered indicative of continuous root injury since MUP changes are secondary to motor unit remodeling[17]. If the axonal loss is so minor that MUP changes aren't noticeable, fibrillation potentials may be the only aberrant finding in some radicular lesions[17]. Fibrillation generally fades and eventually vanishes when the motor unit remodels, but it can be recorded indefinitely in severe or continuing lesions. In isolated injuries that do not permanently damage axons, recruitment alterations can return to normal (like neurapraxic or myelin lesions). The discovery of fibrillation potentials, recruitment deficits, and MUP changes all occur at the same time, which aids in determining the initiation of injury and the severity of axonal loss. As a result, the presence of fibrillation in the lack of MUP changes usually indicates an acute injury, whereas MUP changes in the absence of fibrillation indicate a static or slowly progressive injury.
Even in the face of a clinical sensory impairment, involvement of the dorsal root between the spine and the dorsal root ganglion might spare sensory nerve action potential (SNAP) amplitudes, demonstrating radicular involvement and possibly excluding plexus or nerve lesions. Far lateral disc herniations, on the other hand, typically compress the dorsal root in the intervertebral canal and/or extraforaminal region, causing a lesion of the dorsal root ganglion or even a more distant component of the root. As a result, the amplitude of the SNAP signal may be reduced. As a result, sensory conduction tests can be deceiving, and they are insufficient to distinguish radicular from more distant sites of injury. They will, in any case, provide information that will help identify or rule out additional PNS illnesses. In muscles belonging to the afflicted root, motor conduction investigations can reveal a drop in compound muscle action potential (CMAP) amplitude, especially if the axonal loss is extensive and the muscle is weak. The CMAP and distal nerve conduction velocity can be unchanged in lesser root injuries or if the lesion does not produce axonal loss (i.e. in a neurapraxic lesion). It is important to remember that acute lesions involving both sensory and motor axonal loss cause changes in CMAP only after a period of time has passed (CMAP and SNAP amplitudes halve by 5-7 days after injury), i.e. when the nerve fiber and the neuromuscular endplate become unexcitable due to Wallerian degeneration[18].
The H reflex and the F wave may be relevant in the diagnosis of FLLDH on rare occasions. The H reflex is the myotactic tendon reflex's neurophysiological counterpart. It's a potential recorded from muscle fibers that's induced by electrical stimulation of a motor nerve at a lesser intensity than the CMAP[16]. It's easy to assess in the soleus muscle, and it's generally aberrant with S1 radicular lesions, but it's less reliable in other limb muscles[19]. Changes in a modified H reflex from the tibialis anterior muscle were only anecdotally linked to L4 and L5 radiculopathies (after stimulation of peroneal nerve). This explains why the H reflex isn't very useful in determining whether or not someone has FLLDH. The F wave, on the other hand, may be detected in almost all muscles. It's a minor potential measured from muscle fibers that occurs after the CMAP and is caused by anterior horn cells activating in an antidromically conducted stimulus backfiring. The F wave is a method of assessing conduction along proximal nerve segments that can be recorded from any nerve. Theoretically, clear aberrant F wave values paired with normal distal conduction parameters can detect injuries in proximal PNS sites. Unfortunately, this technique's sensitivity is poor, and normal results do not rule out a radicular lesion. Furthermore, in normal persons, the response from some nerves, such as the peroneus profundus, may be absent. As a result, the F wave's utility in the identification of radicular lesions is regarded as restricted[20]. Finally, when radiculopathy is suspected, a neuro
Far-lateral disc herniations differ from their more common postero-lateral counterparts in the following ways: (1) they involve the nerve root exiting at the same level; (2) they may have a positive femoral stretch test; (3) pain and paresthesia can be reproduced by lateral bending to the side of the disc herniation; and (4) pain is often more severe than in central disc herniations, possibly due to direct compression of the dorsal root ganglion. If an appropriate procedure is followed, MR is the best imaging modality for identifying FLLDH. If an MR scan is not possible, a multi-slice CT scan is a good option. The distinction between intraforaminal and extraforaminal herniations must be correctly diagnosed before the right surgical strategy can be chosen. Despite their limitations, neurophysiological tests are a useful tool in the diagnosis and follow-up of FLLDH patients.
The authors wish to express their gratitude to Pompeo D'Ambrosio for the illustrations and to Federica D'Ambrosio for the editing.
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: Italian society of neurosurgery; Congress of Neurological Surgeons.
Specialty type: Orthopedics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma X, Peng BG S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Di Rita A, Levi V, Gribaudi GL, Casaceli G, Di Leo G, Berra LV, Egidi M. The interlaminar contralateral approach to far-lateral lumbar disc herniations: a singlecenter comparison with traditional techniques and literature review. J Neurosurg Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. 1971;53:891-903. [PubMed] |
| 3. | Abdullah AF, Ditto EW 3rd, Byrd EB, Williams R. Extreme-lateral lumbar disc herniations. Clinical syndrome and special problems of diagnosis. J Neurosurg. 1974;41:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 110] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Epstein NE. Foraminal and far lateral lumbar disc herniations: surgical alternatives and outcome measures. Spinal Cord. 2002;40:491-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 5. | Li P, Yang F, Chen Y, Song Y. Percutaneous transforaminal endoscopic discectomy for different types of lumbar disc herniation: A retrospective study. J Int Med Res.. 2021;49:3000605211055045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Siebner HR, Faulhauer K. Frequency and specific surgical management of far lateral lumbar disc herniations. Acta Neurochir (Wien). 1990;105:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Ebeling U, Reichenberg W, Reulen HJ. Results of microsurgical lumbar discectomy. Review on 485 patients. Acta Neurochir (Wien). 1986;81:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 106] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Epstein NE. Evaluation of varied surgical approaches used in the management of 170 far-lateral lumbar disc herniations: indications and results. J Neurosurg. 1995;83:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Berra LV, Foti D, Ampollini A, Faraca G, Zullo N, Musso C. Contralateral approach for far lateral lumbar disc herniations: a modified technique and outcome analysis of nine patients. Spine (Phila Pa 1976). 2010;35:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Rust MS, Olivero WC. Far-lateral disc herniations: the results of conservative management. J Spinal Disord. 1999;12:138-140. [PubMed] |
| 11. | van Rijn JC, Klemetso N, Reitsma JB, Bossuyt PM, Hulsmans FJ, Peul WC, den Heeten GJ, Stam J, Majoie CB. Observer variation in the evaluation of lumbar herniated discs and root compression: spiral CT compared with MRI. Br J Radiol. 2006;79:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Osborn AG, Hood RS, Sherry RG, Smoker WR, Harnsberger HR. CT/MR spectrum of far lateral and anterior lumbosacral disk herniations. AJNR Am J Neuroradiol. 1988;9:775-778. [PubMed] |
| 13. | Chen CY, Chuang YL, Yao MS, Chiu WT, Chen CL, Chan WP. Posterior epidural migration of a sequestrated lumbar disk fragment: MR imaging findings. AJNR Am J Neuroradiol. 2006;27:1592-1594. [PubMed] |
| 14. | Albeck MJ, Taher G, Lauritzen M, Trojaborg W. Diagnostic value of electrophysiological tests in patients with sciatica. Acta Neurol Scand. 2000;101:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Campbell WW, Vasconcelos O, Laine FJ. Focal atrophy of the multifidus muscle in lumbosacral radiculopathy. Muscle nerve. 1998;21:1350-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Daube JR, Rubin DI. Clinical Neurophysiology. (Oxford University Press, USA: 2009). |
| 17. | Wilbourn AJ, Aminoff MJ. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1998;21:1612-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Chaudhry V, Cornblath DR. Wallerian degeneration in human nerves: serial electrophysiological studies. Muscle Nerve. 1992;15:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 126] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Kimura J. Electrodiagnosis in diseases of nerve and muscle: principles and practice. (Oxford University Press: 2001). |
| 20. | Fisher MA. The contemporary role of F-wave studies. F-wave studies: clinical utility. Muscle Nerve. 1998;21:1098-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Ogura T, Shikata H, Hase H, Mori M, Hayashida T, Osawa T, Mikami Y, Kubo T. Electrophysiologic evaluation of lumbosacral single nerve roots using compound muscle action potentials. J Spinal Disord Tech. 2003;16:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |