Published online Nov 18, 2021. doi: 10.5312/wjo.v12.i11.867
Peer-review started: June 17, 2021
First decision: July 28, 2021
Revised: July 28, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: November 18, 2021
Processing time: 151 Days and 7.9 Hours
Defect treatment with tendon autograft in osteochondral lesions has been published in the literature with an experimental study in dogs. To demonstrate that it is possible to treat knee osteochondral lesions with the technique of autologous tendon transplantation.
To evaluate the clinical and radiological results of patients with knee osteochondral lesions who were treated with autologous tendon transplantation.
Twenty patients (22 knees) with osteochondritis dissecans (OCD) lesions involving the knee were treated with autologous tendon transplantation between 2005-2018. All lesions were International Cartilage Repair Society grade IV. All patients were evaluated clinically at final follow-up with knee injury and osteoarthritis outcome score (KOOS); and radiologically with magnetic resonance observation and cartilage repair tissue (MOCART) and Kellgren-Lawrence (KL) classification.
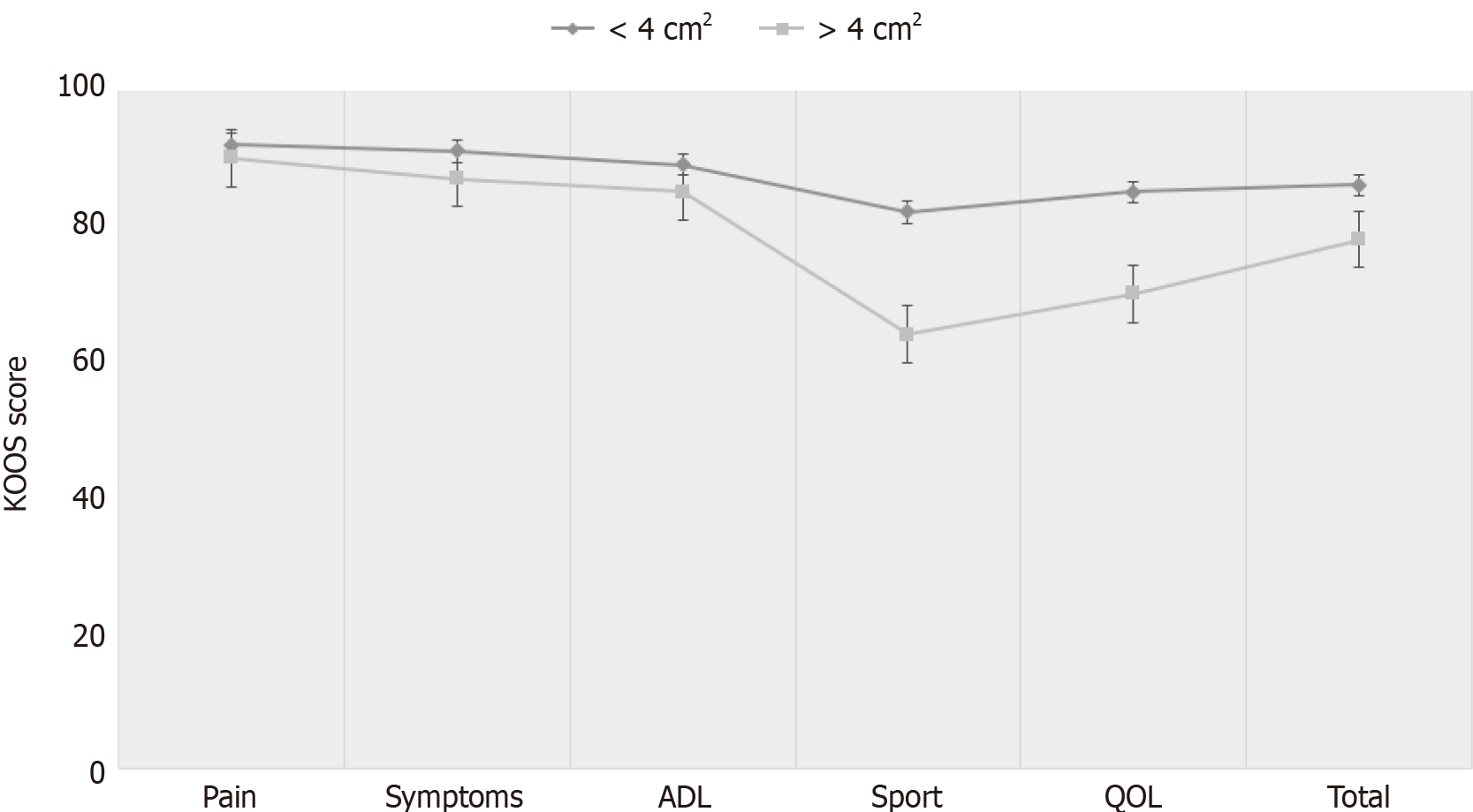
A total of 20 patients (22 knees) with a mean age of 25.5± 6.8 years were included. The average defect size was 4.2 ± 2.1 cm2, and the average defect depth was 0.9 ± 0.4 cm. Total KOOS score was preoperatively 29.4 ± 5.5 and was later found to be 81.5 ± 5.9 after an average of 68.7 ± 37.7 mo follow-up. The mean MOCART score was 56.2 ± 10.7. Preoperatively, all of the patients had KL grades of 0–1; during the follow-up period, 80% of the patients showed no radiological progress of osteoarthritis. Patients with less than 4 cm2 lesion had statistically significantly better overall KOOS than patients whose more than 4 cm2 lesion, particularly in sport and quality of life subscales.
The autologous tendon transplantation is a single-step, safe, simple, cost-effective method for the treatment of knee OCD with satisfactory clinical and radiological outcomes, particularly in patients with less than 4 cm2 lesion.
Core Tip: Defect treatment with tendon autograft in osteochondral lesions has been published in the literature with an experimental study in dogs. However, to date, only one case in the capitellum of the elbow has been scientifically published in humans. This retrospective study shows that knee osteochondral lesions are possible with tendon autograft transplantation technique.
- Citation: Turhan AU, Açıl S, Gül O, Öner K, Okutan AE, Ayas MS. Treatment of knee osteochondritis dissecans with autologous tendon transplantation: Clinical and radiological results. World J Orthop 2021; 12(11): 867-876
- URL: https://www.wjgnet.com/2218-5836/full/v12/i11/867.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i11.867
Knee osteochondritis dissecans (OCD) is a common pathology affecting the articular cartilage and resulting in the delamination of subchondral bone[1,2]. The exact causes are unknown, however, biological (ischemia, osteonecrosis) and mechanical factors (trauma, overuse) has been proposed[3,4]. The OCD can cause a very wide range of clinical presentation from completely asymptomatic to functional impairment. If left untreated, these lesions may lead to the development of osteoarthritis[5].
The management of OCD in young patients who may not be good candidates for arthroplasty remains a challenge for the knee surgeons. Many treatments have been proposed including fragment fixation, microfracture, osteochondral autograft /allograft transplantation, matrix-induced chondrogenesis, and autologous chondro
Osteochondral autograft transplantation and mosaicplasty are common surgical procedures for treating symptomatic International Cartilage Repair Society (ICRS) grade 3 or 4 defects smaller than 3 cm2[8]. These techniques has several advanteges including resurface the defect with normal cartilage and replace concurrently the subchondral bone. But, the donor site morbidity is a major disadvantage. Furthermore, obtaining a congruent surface with donor grafts requires technically challenging skill[9]. To address these problems, we have proposed a new graft source, the peroneus longus tendon, hypotesizing that the tendon autograft with elastic structure can enable easly joint congruence and with solid structure can provide early weight-bearing. Encouraged by the success of tendon autograft in a dog model[10], the technique was applied in a series of 20 patients with a OCD.
The purpose of this study is to retrospectively evaluate the clinical and radiological results of autologous tendon transplantation using the peroneus longus tendon in patients with ICRS grade 3 or 4 defects.
This study was approved by the institutional review board of our hospital. Patients were retrospectively followed up for a minimum of 2 years and data were evaluated retrospectively. Twenty-two consecutive knees (20 patients, 2 bilateral) who under
Preoperatively, all patients underwent a thorough physical examination, including knee passive and active range of motion, ligamentous stability and knee specific tests. Standard radiography were acquired in every patient to evaluate the osteochondral lesions and knee osteoarthritis. The magnetic resonance images (MRI) were obtained routinely to evaluate the size and depth of the OCD lesion. Patients were assessed with Knee Injury and Osteoarthritis Outcome Score (KOOS) before the operation and at the final follow-up. The radiological outcomes were assessed with magnetic resonance observation of cartilage repair tissue (MOCART) score and with Kellgren-Lawrence (KL) classification.
The osteochondral defect repair was radiologically evaluated by MRI using the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART). Images were evaluated according to defect filling, integration into the border region, surface of the repair tissue, structure of the repair tissue, signal intensity of the repair tissue, subchondral lamina status, integrity of the subchondral bone, and joint adhesion and infusion[11]. Integrity assessment of the subchondral bone could not be made because the defect area was restored with autologous tendon in the treatment procedure. Therefore, the score ranged from 0 (worst result) to 90 (best possible result).
MRI was performed on a 1.5 Tesla system (Avanto; Siemens Medical Solution, Erlangen, Germany) using ankle coil. The following sequences with axial, coronal and sagittal plains were used: T1-weighted turbo spin echo (T1W TSE) [TR/TE = 777/12 ms, matrix = 320 x 224, field of view (FOV) = 16 cm, excitations = 1, slice thickness = 3 mm, spacing = 0.6 mm], fat saturated proton density weighted turbo spin echo (PDW TSE FS) (TR/TE = 3330/47 ms, matrix = 320 x 224, FOV = 16 cm, excitations = 1, slice thickness = 4 mm, spacing = 1.2 mm), and fat saturated T2-weighted turbo spin echo (T2 TSE FS) (TR/TE = 5200/75 ms, matrix = 208 x 256, FOV = 16 cm, excitations = 1, slice thickness = 4 mm, spacing = 1.2 mm).
KOOS is a specific questionnaire form about the knee containing 42 questions in 5 individual subheadings. These 5 subgroups are: pain, symptoms, activities of daily living, sports and quality of life. KOOS is a recommended scoring system in cartilage repair patients and is a reliable test being used in patients after their surgical treatments of focal cartilage lesions in recent years[12].
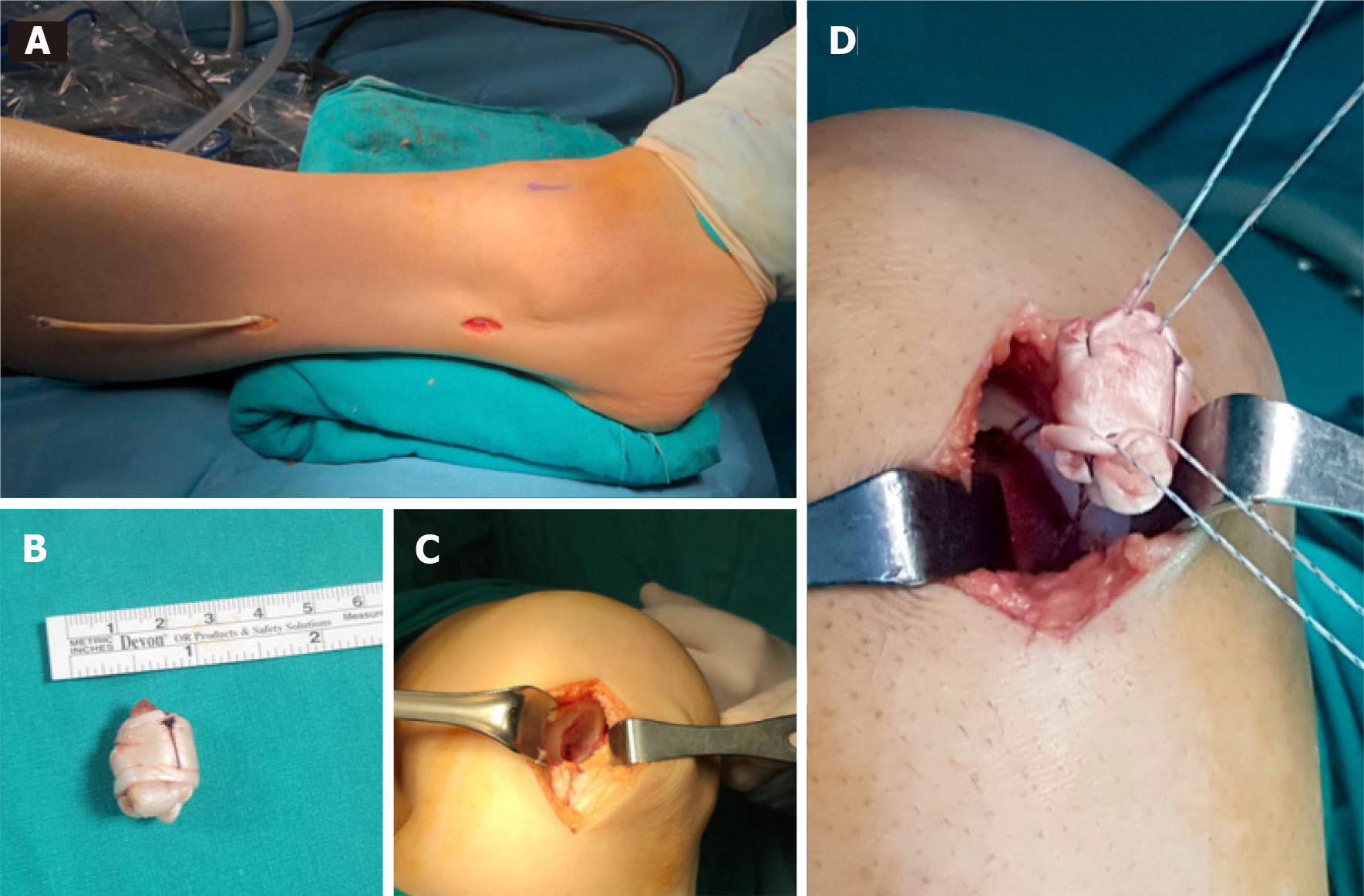
All procedures were performed in a supine position under general or spinal anesthesia and a thigh tourniquet was applied. A diagnostic arthroscopy was performed, intraarticular conditions were evaluated, associated meniscal injuries were treated and any loose bodies were removed. A mini-arthrotomy was used, depending on the size and site of the lesion. The osteochondral lesion was removed and debrided until viable bleeding bone was reached. The subchondral bone was drilled at 2-4 mm intervals with 1 mm thick K-wire. Following this, a longitudinal posterolateral mini-incision was opened over the peroneus longus tendon, 10 cm proximal to the lateral malleolus on the ipsilateral side. A split portion of tendon was harvested from the peroneus longus. The graft was folded back on itself and held with absorbable suture, creating a “ball” and then fixed into the defect with suture anchor (Figure 1). The spherical congruency of the joint was checked. Finally, a cylindrical cast or orthosis was applied at 15 degrees of knee flexion.
Post-operative follow-up; Immediate isometric quadriceps exercise was started postoperatively. All patients were mobilized with weight-bearing on postoperative day 1. At the end of the 4th week, cylindrical cast was removed and active and passive knee joint movements were prescribed. All patients followed the same rehabilitation protocol for 6 mo, respectively, based on current knowledge of the graft healing biology: protect the transplant from excessive loads and shearing forces, gain full extension and gradual recovery of knee flexion, progressive recovery in daily functional activities, increase the strength of the quadriceps and hamstrings, recovery of full range of motion, further increase in strength of quadriceps and flexors muscles, further increase in functional activities level, prepare athlete for a return to team and competition with good recovery of the aerobic endurance, maintain a good quality of life, avoiding excess of body fat and preventing risk of reinjury.
All patients were followed up in the outpatient clinic at the 2nd week, 4th week, 8th week, 12th week, 6th month, at the end of 1 year and at the last clinical follow-up. Clinical follow-ups; It was performed together with a surgeon and a physiotherapist in the surgical team, and the data were documented at each clinical control.
All patients were evaluated radiologically with MRI at the end of the first year and at the last follow-up. Radiological evaluations were evaluated by 2 independent radiologists in consensus.
The statistical analysis was performed using SPSS Version 22.0 statistical analysis software. Percentage, rate, average and SD were used as descriptive statistics. The compliance of the quantitative data with normal distribution was evaluated using the Kolmogorov–Smirnov test. The parametric data were compared using the Student t-test and the nonparametric data were compared using the Mann-Whitney U test. P value less than 0.05 was considered to be statistically significant.
Twenty-two knees of 20 patients underwent autologous tendon transplantation who had knee OCD were included in the study. The mean patient age was 25.5 ± 6.8 years (range, 19-42 years) and the follow-up period was 68.7± 37.7 mo (range, 30-182 mo). Detailed patients demographics are shown in Table 1.
| Age at time of surgery, yr, mean ± SD (range) | 25.5 ± 6.8 (19-42) |
| Sex, M/F, n (%) | 15 (75%)/5 (25%) |
| BMI, kg/cm2, mean ± SD (range) | 27.1 ± 3.5 (21.6-32.4) |
| Side, R/L, n (%) | 13 (59.9%)/9 (40.1%) |
| Location, MFC/LFC, n (%) | 18 (81.8%)/4 (18.2%) |
| Size, cm2, mean ± SD (range) | 4.2 ± 2.1 (2.1-9.0) |
| Depth, cm, mean ± SD (range) | 0.9 ± 0.4 (0.4-1.7) |
| Follow-up duration, mo, mean ± SD (range) | 68.7 ± 37.7 (30-182) |
Preoperatively, the average KOOS score was 32.2 ± 5.8 for pain, 28.2 ± 6.4 for symotom, 44.5 ± 8.1 for activity of daily living, 22.4 ± 4.6 for sport, and 24.5 ± 5.8 for quality of life. Postoperatively, the average KOOS score was 91.3 ± 4.2 for pain, 89.1 ± 7.2 for symptom, 85.1 ± 6.8 for activity of daily living, 74.5 ± 7.2 for sport, and 72.4 ± 8.1 for quality of life. Preoperative total KOOS score was 29.4 ± 5.5 (range, 21.4-40.5). KOOS total score was found to be increased to 81.5 ± 5.9 (range, 74.2-92.7). All parameters of the KOOS score improved significantly (P < 0.001) (Table 2).
| Preoperative, mean ± SD (range) | At final follow-up, mean ± SD (range) | P value | ||
| Total KOOS score | 29.4± 5.5 (21.4-40.5) | 81.5 ± 5.9 (74.2-92.7) | < 0.01 | |
| Pain | 32.2 ± 5.8 | 91.3 ± 4.2 | < 0.01 | |
| Symptoms | 28.2 ± 6.4 | 89.1 ± 7.2 | < 0.01 | |
| ADL | 44.5 ± 8.1 | 85.1 ± 6.8 | < 0.01 | |
| Sport | 22.4 ± 4.6 | 74.5 ± 7.2 | < 0.01 | |
| QOL | 24.5 ± 5.8 | 72.4 ± 8.1 | < 0.01 | |
| MOCART score | NM | 56.2 ± 10.7 (40-75) | NM | |
| Kellgren-Lawrence grade | 0.4 ± 0.1 (0-1) | 0.6 ± 0.1 (0-1) | NS | |
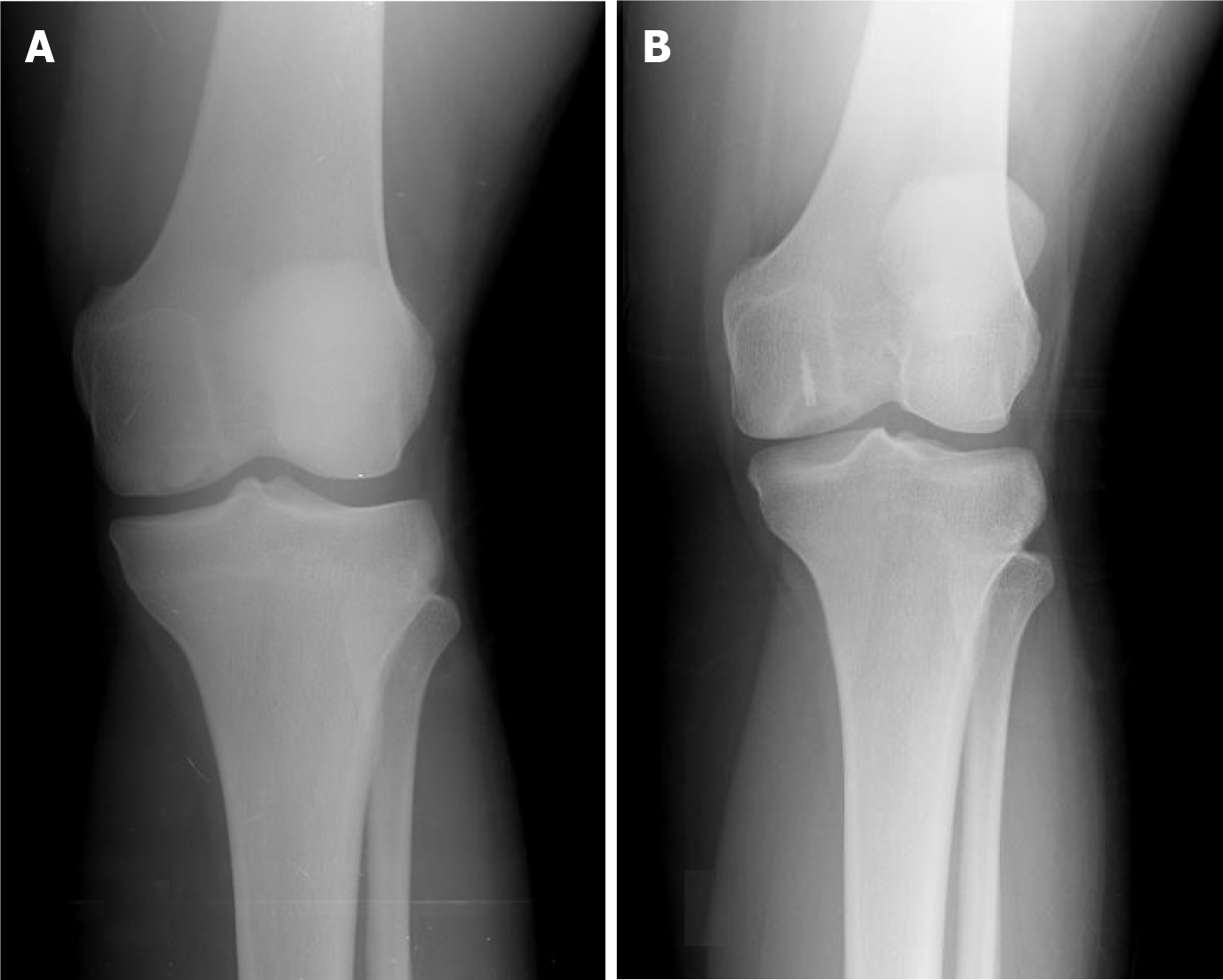
The mean MOCART score was found 56.2 ± 10.7 (range, 40-75) at last follow-up. There was no significant correlation between the total MOCART and KOOS scores. Preoperatively, all of the patients had Kellgren–Lawrence grades of 0–1; during the follow-up period, 80% (n = 16) of the patients no showed radiographical progression of osteoarthritis (Figure 2). Patients with less than 4 cm2 lesion had statistically significantly better overall KOOS (P < 0.01) than patients whose more than 4 cm2 lesion. In terms of the KOOS subscales, however, this was true only with sport (P = 0.01) and quality of life (P = 0.02). There were no statistically significant differences in the subscales pain, symptoms and activity of daily living (Figure 3).
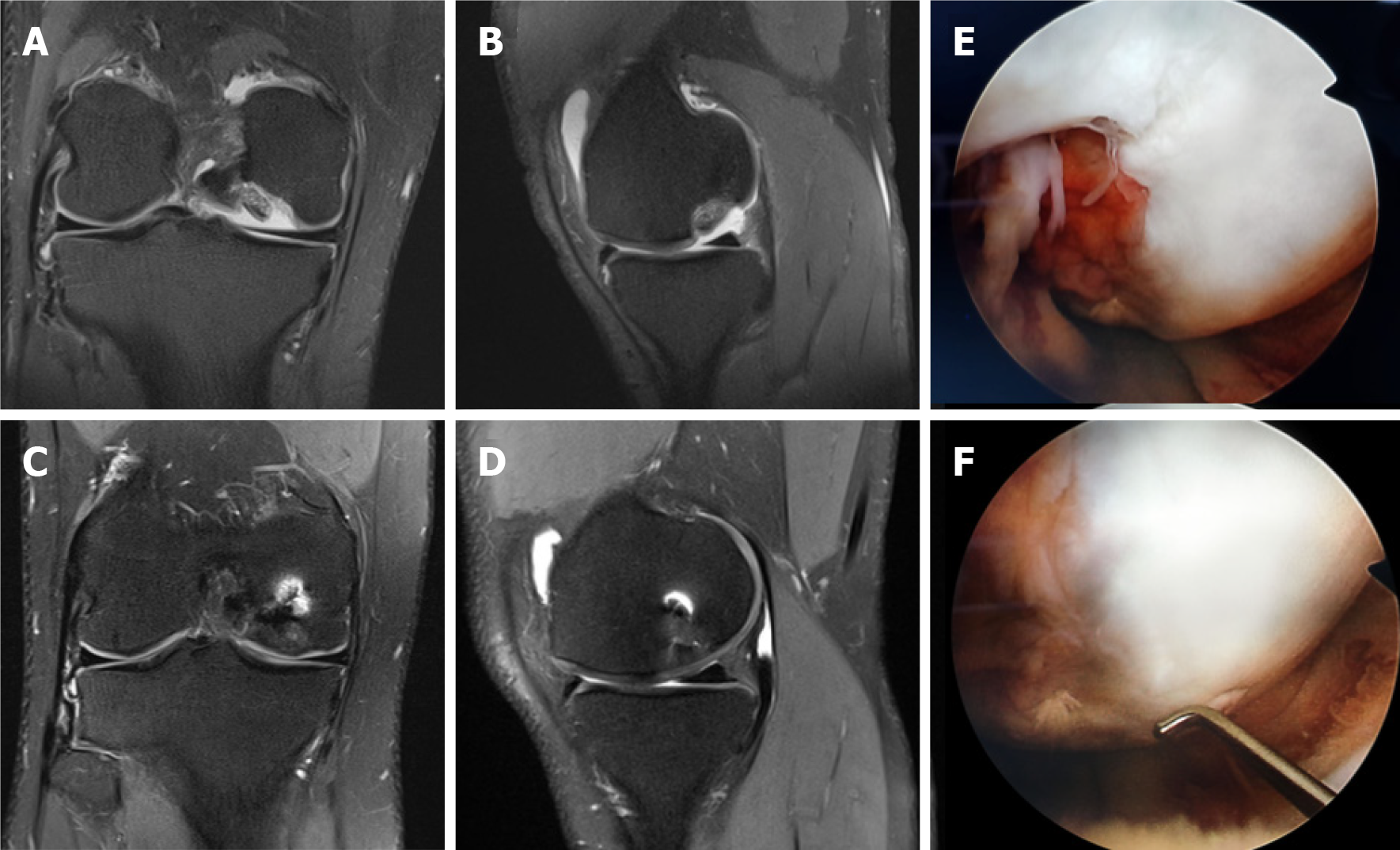
Second-look arthroscopy was performed during contralateral knee operation to the patients who was operated from both knees at one year intervals. Second look arthroscopy view at 1-year follow-up of ICRS grade 4 of medial femoral condyle showing filling of the defect with a well-integrated, smooth surfaced and stable regenerated cartilage. The graft adaptation and incorporation were assessed to be excellent (Figure 4).
Peroneus longus tendons were used as autologous grafts in all patients. No complications developed in any patient regarding donor site. In the graft donor site, no patient reported a neurological symptom. Hypertorphic scar tissue did not occur in any of the patients in the donor site. At the end of the 6th month, all patients returned to their daily work and daily activities without any restrictions.
The most important finding of the present study is that all of the patients treated with autologous tendon transplantation had “excellent and good” clinical and radiological outcomes with minimum 2 years follow-up. All parameters of the KOOS score improved significantly (P < 0.001). Patients with less than 4 cm2 lesion had statistically significantly better overall KOOS (P < 0.01) than patients whose more than 4 cm2 lesion.
The main aim in the treatment of osteochondral defect is to restore joint integrity by creating a tissue that is the same or similar to the biomechanical properties of the articular cartilage. This is the basic logic of this method. The reason we think of using tendons in the treatment of OCD is that the tendon has a viscoelastic and anisotropic structure that has main tasks of carrying energy from muscle to bone and storing energy with its highly organized hierarchy[13-15]. Tendons have been proven to increase their amounts of proteoglycan and glycosaminoglycan present in the matrix under compression conditions, and can resist compressive loads thanks to these two substances[13,15,16]. Another feature of the tendons is that they can change their structures and compositions in case of mechanical load changes. Cells in the tendon are responsible for adaptive changes and can alter gene expression, protein synthesis and cell phenotype against mechanical load. In addition, the extracellular matrix of the tendon acts as a scaffold, allowing cell adhesion, development, and differentiation[16,17]. Joint compliance is easily provided by the elastic structure of the tendon, hence solid structure forms a load carrying surface; thus, the pain is eliminated, joint functions are preserved and degeneration is stopped. The results of this technique we have described show that we have achieved this.
The technique we have described is actually a tissue-based cartilage repair technique. Therefore, it can be compared with osteochondral autograft or allograft repair techniques, which are tissue-based repair techniques. The most important advantage of the osteochondral autograft technique is that the graft is autograft and it enables the subchondral tissue to be restored[18]. But it has potential disadvantages such as donor site morbidity and poor joint compliance. In addition, the larger the defect area is, the higher the risk of complications are[19]. The osteochondral autograft technique may exhibit mismatch in connection geometry at the recipient site. In addition, the need for multiple grafts in large lesions complicates the provision of joint geometry and increases donor site morbidity. This can cause premature degeneration of the graft and joint, synovitis, and pain[20-23].
Another tissue-based cartilage repair technique is osteochondral allografts. These allografts have been used for many years in orthopedic surgery to reconstruct osteochondral defects[24]. It has also become an option in the treatment of cartilage abnormalities affecting subchondral bones, such as osteochondritis dissecans[25]. Osteochondral allograft is a highly beneficial procedure especially in the treatment of large lesions that are between the sizes 2-20 cm2 and in the treatment of a failed OCD[26-29]. Even if successful results have been reported in previous studies, the most important disadvantage of osteochondral allografts are their shorter storage life. Williams et al[30] in their study; reported that fresh human osteochondral allograft tissue that was kept for more than fourteen days, preserves its glycosaminoglycan content and biomechanical properties, yet it significantly loses its chondrocyte viability, viable cell density and metabolic activity. Other disadvantages of this method are the difficulty of accessibility to the graft, immunogenicity and graft rejection, the risk of disease transmission such as HIV and Hepatitis, disjointedness in joint geometry and collapse problems[25,31]. With the autograft we used, the disadvantages of osteochondral allografts are avoided.
The most important limitation of this study is the small number of patients. Even if we compare the study with previous studies, the absence of a control group is another limitation. Cartilage evaluation was evaluated with standart (1.5 Tesla system (Avanto; Siemens Medical Solution, Erlangen, Germany) MRI. Another shortcoming is that we did not perform imaging with gadolinium-enriched MRI.
This technique allows to avoid many of the disadvantages of other techniques. Important advantages; It is done in one step, it is an autograft, it does not require additional cost and it is easy to apply. Since it is an autograft, there is no risk of tissue rejection and contagious infection. Similar clinical and radiological results were obtained when compared to other treatment modalities. The autologous tendon transplantation is a single-step, safe, simple, cost-effective method for the treatment of knee OCD with satisfactory clinical and radiological outcomes, particularly in patients with less than 4 cm2 lesion.
This research was initiated by being inspired by the article titled "Treatment of osteochondral defects with tendon autografts in a dog knee model" made in 1999 and the articles titled "Tendon regeneration: an anatomical and histological study in sheep" published in 2004.
Our teacher Ahmet Uğur Turhan's interest in joint surgery and his publications in 1999-2004 inspired him.
In order to protect the knee joint, the authors share the new technique with the world, and share the results with the world and to inspire new publications.
A report of multiple patients with the same treatment, but no control group or comparison group.
All parameters of the knee injury and osteoarthritis outcome score (KOOS) score improved significantly in all patients. Patients with lesions less than 4 cm2 had a significantly better overall KOOS than patients with lesions greater than 4 cm2.
Autologous tendon transplantation has satisfactory clinical and radiological results in patients with osteochondral lesions of the knee.
The autologous tendon transplantation is a single-step, safe, simple, cost-effective method for the treatment of knee osteochondritis dissecans with satisfactory.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Orthopedics
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shetty UC, Zabrzyński J S-Editor: Ma YJ L-Editor: A P-Editor: Wang LYT
| 1. | Bruns J, Werner M, Habermann C. Osteochondritis Dissecans: Etiology, Pathology, and Imaging with a Special Focus on the Knee Joint. Cartilage. 2018;9:346-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Andriolo L, Candrian C, Papio T, Cavicchioli A, Perdisa F, Filardo G. Osteochondritis Dissecans of the Knee - Conservative Treatment Strategies: A Systematic Review. Cartilage. 2019;10:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Shea KG, Jacobs JC Jr, Carey JL, Anderson AF, Oxford JT. Osteochondritis dissecans knee histology studies have variable findings and theories of etiology. Clin Orthop Relat Res. 2013;471:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Andriolo L, Crawford DC, Reale D, Zaffagnini S, Candrian C, Cavicchioli A, Filardo G. Osteochondritis Dissecans of the Knee: Etiology and Pathogenetic Mechanisms. A Systematic Review. Cartilage. 2020;11:273-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Davies-Tuck ML, Wluka AE, Wang Y, Teichtahl AJ, Jones G, Ding C, Cicuttini FM. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Demirel M, Polat G, Erşen A, Aşık M, Kılıçoğlu Öİ. Internal fixation for osteochondritis dissecans lesions of the knee in patients with physeal closure. Acta Orthop Traumatol Turc. 2021;55:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Howell M, Liao Q, Gee CW. Surgical Management of Osteochondral Defects of the Knee: An Educational Review. Curr Rev Musculoskelet Med. 2021;14:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Solheim E, Hegna J, Øyen J, Harlem T, Strand T. Results at 10 to 14 years after osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee. Knee. 2013;20:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Kowalczuk M, Musahl V, Fu FH. Cochrane in CORR®: Surgical Interventions (Microfracture, Drilling, Mosaicplasty, and Allograft Transplantation) for Treating Isolated Cartilage Defects of the Knee in Adults. Clin Orthop Relat Res. 2018;476:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Turhan AU, Aynaci O, Turgutalp H, Aydin H. Treatment of osteochondral defects with tendon autografts in a dog knee model. Knee Surg Sports Traumatol Arthrosc. 1999;7:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Albano D, Martinelli N, Bianchi A, Giacalone A, Sconfienza LM. Evaluation of reproducibility of the MOCART score in patients with osteochondral lesions of the talus repaired using the autologous matrix-induced chondrogenesis technique. Radiol Med. 2017;122:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Collins NJ, Prinsen CA, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee Injury and Osteoarthritis Outcome Score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage. 2016;24:1317-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 480] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 13. | Böl M, Ehret AE, Leichsenring K, Ernst M. Tissue-scale anisotropy and compressibility of tendon in semi-confined compression tests. J Biomech. 2015;48:1092-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | LaCroix AS, Duenwald-Kuehl SE, Lakes RS, Vanderby R Jr. Relationship between tendon stiffness and failure: a metaanalysis. J Appl Physiol (1985). 2013;115:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Zabrzyński J, Gagat M, Paczesny Ł, Łapaj Ł, Grzanka D. Electron microscope study of the advanced tendinopathy process of the long head of the biceps brachii tendon treated arthroscopically. Folia Morphol (Warsz). 2018;77:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Wren TA, Beaupré GS, Carter DR. Mechanobiology of tendon adaptation to compressive loading through fibrocartilaginous metaplasia. J Rehabil Res Dev. 2000;37:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Wang JH. Mechanobiology of tendon. J Biomech. 2006;39:1563-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 553] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 18. | Schrock JB, Kraeutler MJ, Houck DA, McQueen MB, McCarty EC. A Cost-Effectiveness Analysis of Surgical Treatment Modalities for Chondral Lesions of the Knee: Microfracture, Osteochondral Autograft Transplantation, and Autologous Chondrocyte Implantation. Orthop J Sports Med. 2017;5:2325967117704634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Gowd AK, Cvetanovich GL, Liu JN, Christian DR, Cabarcas BC, Redondo ML, Verma NN, Yanke AB, Cole BJ. Management of Chondral Lesions of the Knee: Analysis of Trends and Short-Term Complications Using the National Surgical Quality Improvement Program Database. Arthroscopy. 2019;35:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Miniaci A, Tytherleigh-Strong G. Fixation of unstable osteochondritis dissecans lesions of the knee using arthroscopic autogenous osteochondral grafting (mosaicplasty). Arthroscopy. 2007;23:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Sgaglione NA, Miniaci A, Gillogly SD, Carter TR. Update on advanced surgical techniques in the treatment of traumatic focal articular cartilage lesions in the knee. Arthroscopy. 2002;18:9-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Yoshizumi Y, Sugita T, Kawamata T, Ohnuma M, Maeda S. Cylindrical osteochondral graft for osteochondritis dissecans of the knee: a report of three cases. Am J Sports Med. 2002;30:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Wang CJ. Treatment of focal articular cartilage lesions of the knee with autogenous osteochondral graftsA 2- to 4-year follow-up study. Arch Orthop Trauma Surg. 2002;122:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Filardo G, Andriolo L, Soler F, Berruto M, Ferrua P, Verdonk P, Rongieras F, Crawford DC. Treatment of unstable knee osteochondritis dissecans in the young adult: results and limitations of surgical strategies-The advantages of allografts to address an osteochondral challenge. Knee Surg Sports Traumatol Arthrosc. 2019;27:1726-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, Kon E. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18:434-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 258] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 26. | Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | Levy YD, Görtz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 28. | Lyon R, Nissen C, Liu XC, Curtin B. Can fresh osteochondral allografts restore function in juveniles with osteochondritis dissecans of the knee? Clin Orthop Relat Res. 2013;471:1166-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, Sah RL, Bugbee WD. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85:2111-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Enneking WF, Campanacci DA. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg Am. 2001;83:971-986. [PubMed] |