Published online Oct 18, 2021. doi: 10.5312/wjo.v12.i10.781
Peer-review started: February 26, 2021
First decision: May 3, 2021
Revised: May 3, 2021
Accepted: August 4, 2021
Article in press: August 4, 2021
Published online: October 18, 2021
Processing time: 229 Days and 10.1 Hours
Unilateral patellofemoral pain syndrome (PFPS) is the most frequently diagnosed knee condition in populations aged < 50 years old. Although the treatment of myofascial trigger points (MTrPs) is a common and effective tool for reducing pain, previous studies showed no additional benefits compared with placebo in populations with PFPS. Percutaneous electrolysis is a minimally invasive approach frequently used in musculotendinous pathologies which consists of the application of a galvanic current through dry needling (DN).
To evaluate changes in sensitivity, knee pain perception and perceived pain during the application of these three invasive techniques.
A triple-blinded, pilot randomized controlled trial was conducted on fifteen patients with unilateral PFPS who were randomized to the high-intensity percutaneous electrolysis (HIPE) experimental group, low-intensity percutaneous electrolysis (LIPE) experimental group or DN active control group. All interventions were conducted in the most active MTrP, in the rectus femoris muscle. The HIPE group received a 660 mA galvanic current for 10 s, the LIPE group 220 mA × 30 s and the DN group received no galvanic current. The MTrP and patellar tendon pain pressure thresholds (PPTs) and subjective anterior knee pain perception (SAKPP) were assessed before, after and 7 d after the single intervention. In addition, perceived pain during the intervention was also assessed.
Both groups were comparable at baseline as no significant differences were found for age, height, weight, body mass index, PPTs or SAKPP. No adverse events were reported during or after the interventions. A significant decrease in SAKPP (both HIPE and LIPE, P < 0.01) and increased patellar tendon PPT (all, P < 0.001) were found, with no differences between the groups (VAS: F = 0.30; η2 = 0.05; P > 0.05; tendon PPT immediate effects: F = 0.15; η2 = 0.02; P > 0.05 and tendon PPT 7-d effects: F = 0.67; η2 = 0.10; P > 0.05). A significant PPT increase in rectus femoris MTrP was found at follow-up in both the HIPE and LIPE groups (both, P < 0.001) with no differences between the groups (immediate effects: F= 1.55; η2 = 0.20; P > 0.05 and 7-d effects: F = 0.71; η2 = 0.10; P > 0.05). Both HIPE and LIPE interventions were considered less painful compared with DN (F = 8.52; η2 = 0.587; P < 0.01).
HIPE and LIPE induce PPT changes in MTrPs and patellar tendon and improvements in SAKPP, and seem to produce less pain during the intervention compared with DN.
Core Tip: Percutaneous electrolysis is a minimally invasive approach frequently used in lower limb musculotendinous pathologies which consists of the application of a galvanic current through a dry needling (DN) or acupuncture needle which acts as a negative electrode, increasing the pH and cellular necrosis by a local electrochemical reaction. However, the current evidence regarding its application in myofascial trigger points (MTrPs) is limited. Therefore, the aim of this study was to assess the efficacy of percutaneous electrolysis compared with DN in patients with unilateral patellofemoral pain syndrome to improve rectus femoris MTrP and patellar tendon pain pressure thresholds, subjective anterior knee pain perception and induced pain during interventions.
- Citation: Valera-Calero JA, Sánchez-Mayoral-Martín A, Varol U. Short-term effectiveness of high- and low-intensity percutaneous electrolysis in patients with patellofemoral pain syndrome: A pilot study. World J Orthop 2021; 12(10): 781-790
- URL: https://www.wjgnet.com/2218-5836/full/v12/i10/781.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i10.781
In patients with knee complaints younger than 50 years, patellofemoral pain syndrome (PFPS) is the most frequently diagnosed condition[1] and is characterized by the high rates of recurrence and chronicity (up to the 90%)[2]. The current evidence suggests a multifactorial etiology[3]. Although the incidence is still unknown and sociodemographic features [e.g., height, weight, body mass index (BMI), and age] are not clearly identified as risk factors, women are more likely to develop PFPS (Odds ratio: 2.23)[4]. In addition, psychological conditions, physical conditioning, larger medial tibial intercondylar distance, vertical ground reaction force, plantar pressure features, onset timing of vastus medialis and lateralis, muscle flexibility (e.g., hamstring, quadriceps and gastrocnemius) and general joint laxity are clinical risk factors for developing PFPS[1].
With regard to PFPS management, although a systematic review and meta-analysis considered that trigger point dry needling (DN) is a common and (in general) effective technique in clinical practice[5] for reducing pain, the evidence shows no additional improvements compared with placebo in patients with PFPS[6]. DN consists of inserting a solid and thin needle into a myofascial trigger point (MTrP) to reduce the muscle stiffness, relieve pain and improve muscle function[7]. MTrPs are located in taut bands of skeletal muscles and course with pain and motor and neurovegetative dysfunctions[8]. At least one part of the MTrP nociceptive input is derived from blood capillary compression by these taut bands, inducing ischemia and hypoxia in the MTrP area[9]. Reduced levels of oxygen result in decreased pH (to 4.5), activation of acid-sensing ion channels, inhibition of acetylcholinesterase, and liberation of ATP, bradykinins, tumor necrosis factor-alpha, interleukins, serotonin, noradrenaline, substance P and calcitonin gene-related peptide[10-13].
Percutaneous electrolysis is a minimally invasive approach frequently used in lower limb musculotendinous pathologies[14] as preliminary evidence has suggested that it is more effectiveness when compared with DN[15], which consists of the application of a galvanic current through a DN or acupuncture needle which acts as a negative electrode, increasing the pH and cellular necrosis by a local electrochemical reaction[16]. Although the application of this procedure in a MTrP is limited, a previous clinical trial demonstrated greater improvements in pain and function compared with DN in patients with temporomandibular disorders[17].
As a previous study proposed that treatment of MTrP may be an effective way to diminish the pain associated with PFPS[6,18], the aim of this study was to assess the efficacy of percutaneous electrolysis compared with DN in patients with unilateral PFPS for improving rectus femoris MTrP and patellar tendon pain pressure thresholds (PPTs), subjective anterior knee pain perception (SAKPP) and perceived pain during interventions.
A parallel-group, controlled, triple-blinded, randomized pilot clinical trial comparing the effects of a single session of high-intensity percutaneous electrolysis (HIPE), low-intensity percutaneous electrolysis (LIPE) and DN applied to the rectus femoris most active MTrP in patients with unilateral PFPS was conducted. This clinical trial followed the Consolidated Standards of Reporting Trials for pragmatic clinical trials[19]. This study was conducted according to the Declaration of Helsinki and approved by the Institutional Ethics Committee of Clinical Research of Alfonso X el Sabio University (UAX 26-02-2020). All participants signed a written informed consent prior to their participation in this study.
A consecutive sample of patients with unilateral PFPS was screened for eligibility criteria from September 2020 to December 2020 from a private university located in Spain (Camilo José Cela University). To be eligible, participants had to report anterior knee pain of at least 6 mo duration, unilateral pain location, aged 18 to 50 years, with at least one active MTrP present in the rectus femoris muscle. Exclusion criteria included being under pharmacological (e.g., analgesics) or physiotherapy treatment 7 d prior to their participation or during the study, needle fear, prior lower extremity or spine surgery, absence of pain, any musculoskeletal or neuropathic conditions (e.g., peripheral compressive neuropathy, radiculopathy, sarcopenia, fiber ruptures…), traumatic injuries (e.g., fractures or fissures), or any medical condition or contraindication for needling treatment (e.g., anticoagulants).
Participants were randomly assigned to the HIPE experimental group, the LIPE experimental group or the DN active control group. Concealed allocation was conducted using a random-number generator (Research Randomizer Vr.4.0). Individual and sequentially numbered cards with the random assignment were folded in sealed opaque envelopes. One external researcher selected the envelope and proceeded with appropriate allocation. Then, the participants’ allocation was revealed after baseline data collection. Participants, examiner and rater were blinded to the allocation group.
All interventions were performed by an experienced assessor (more than 10 years of experience) in invasive physiotherapy procedures and MTrP management.
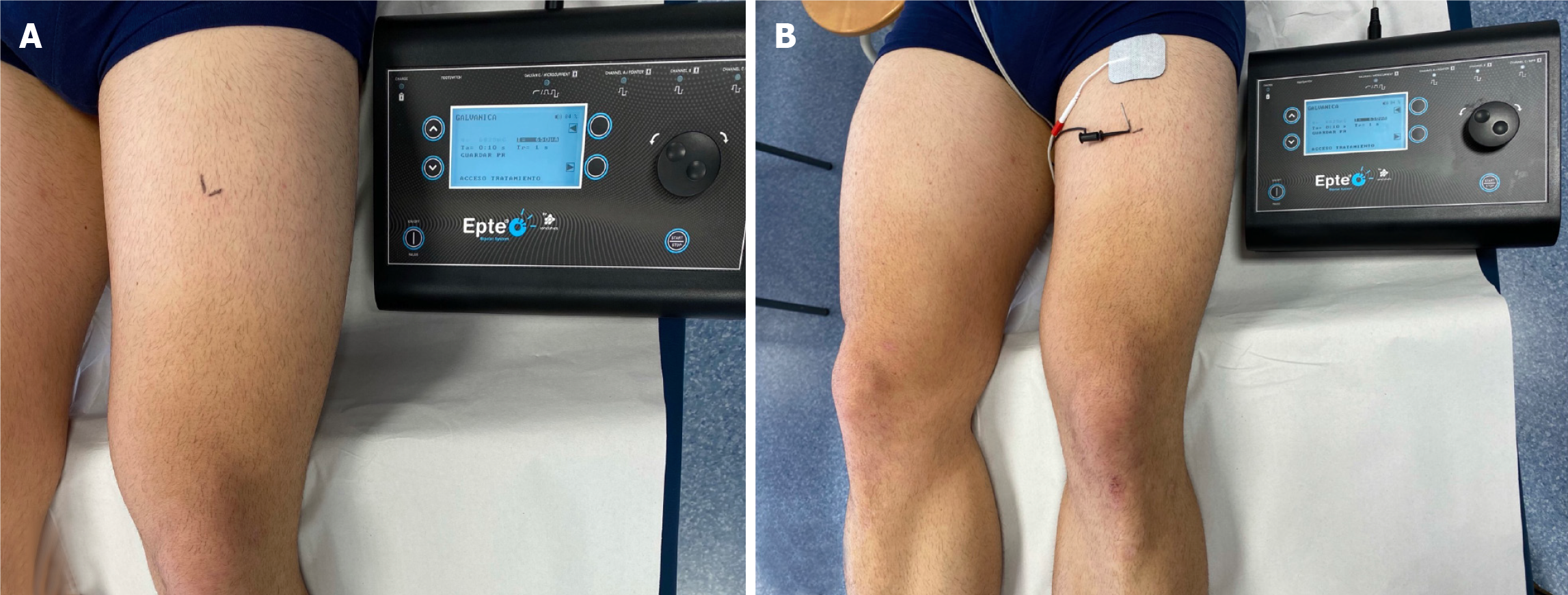
As MTrP diagnosis is most commonly conducted by manual palpation, active MTrPs were located following the instructions provided by Fernández-de-las-Peñas and Dommerholt[20]. Palpation evaluation can be used for the clinical diagnosis of MTrPs in this specific location as it shows acceptable reliability if experienced examiners are involved[21]. All participants were placed in the supine position with their knee passively flexed at 30º. The single intervention was conducted on the most painful active MTrP of the rectus femoris ipsilateral to the affected area. This MTrP was marked with a grid of 2 perpendicular lines and considered to be the one that elicited the highest recognized pain sensation under the same palpation pressure[22] (Figure 1A).
The same procedure was conducted for all groups as follows: After cleaning the skin with chlorhexidine (Lainco® 2%), a DN 0.30 × 40 needle (Agupunt, Barcelona, Spain) was inserted using an in-plane approach with a 70-80º angle to the skin surface until it produced the first local twitch response following a multiple rapid insertion technique, pain response and recognized MTrP referred pain pattern. The needle was statically placed in this location for 30 s in all groups. After placement, the needle was connected to a modified electrosurgical scalpel from an EPTE device (Ionclinics, Valencia, Spain) which acted as a cathode while a surface anode was placed 10 cm proximal to the location of the MTrP (Figure 1B).
For both HIPE and LIPE groups, a Q = 0.0066 coulombs (C) current was set. From the total 30 s intervention time in all groups: (1) The HIPE group received a galvanic current of 660 mA × 10 s and 20 s with no current; (2) The LIPE group received 220 mA × 30 s; and (3) The DN group, although the needle was connected to the device, received no current during the 30 s. Finally, hemostasis using a cotton swab was performed for 1 min in order to avoid post-needling soreness[20].
To ensure participants, examiner and rater blindness, one external assessor set the device settings according with the group allocation (660 mA × 10 s; 220 mA× 30 s; or none) and the same sounds were emitted for all groups at the start of the intervention and after 30 s.
Outcomes were evaluated before, immediately after and 7 d after the single intervention by an assessor blinded to the subject allocation group.
The primary outcome measure was the PPT of the most active MTrP. In addition, patellar tendon PPT, SAKPP and perceived pain during the intervention were the secondary outcomes.
As patients with PFPS showed lower PPTs compared with controls, PPTs were considered a pain sensitivity indicator[24]. First, PPTs were assessed using the analogic algometer Fischer FPN100. Two locations were unilaterally examined by the same rater: (1) MTrP; and (2) Patellar tendon (at the midpoint between the lower edge of the patella and tibial tuberosity)[25]. We performed three evaluations at each point with a 30 s rest, increasing the pressure at a rate of 1 kg/s and the average (kg/cm2) was recorded for analysis. Prior to the evaluation, the patients received standardized instructions to signal the first change from pressure to pain[26].
Second, SAKPP was assessed as an indicator of subjective pain perception using a Visual Analogue Scale (VAS). Patients were asked to identify their level of pain in a 100 mm VAS, where 0 was “no pain” and 100 was the worst imaginable pain[27]. The mean of 3 scores was calculated: The maximum pain perceived during the last 7 d, the minimum pain perceived during the last 7 d, and the current pain[28].
Finally, to assess the tolerability of all the techniques, the pain perceived during intervention was assessed using a VAS. Participants were asked to identify their mean level of pain during the 30 s interventions in a 100 mm VAS.
Participants were asked to report any adverse events experienced during or after the interventions (up to the 1-mo duration of this study). Adverse events were defined as sequelae of short-medium term symptoms perceived as unacceptable to the patient and required further treatment using a self-reported document provided to the participants and informed to an external clinician during the study[29].
All statistical analyses were performed using IBM SPSS Statistics Version 22 (IBM Corporation, Armonk, NY, United States), with a significance level of P < 0.05. After verifying the normal distribution of the data, descriptive statistics were used to summarize the sociodemographic and clinical variables. Normal-distributed data were described by means, SD, and 95%CI.
Comparability of groups at baseline was assessed using a one-way analysis of variance (ANOVA) test (Bonferroni post-hoc correction). To assess the effects of the three types of treatment on the primary and secondary outcomes, between-group differences in response to the interventions (HIPE, LIPE or DN) were analyzed using AN(C)OVA repeated measurement (groups vs time). For SAKPP, within-groups differences were assessed with the Student t-test. The effect size was estimated using η2 when significant. An effect size of 0.01 was considered small, 0.06 medium and 0.14 large. P values were assumed to be significant only at < 0.017 (Bonferroni correction: 0.05/3) level[30].
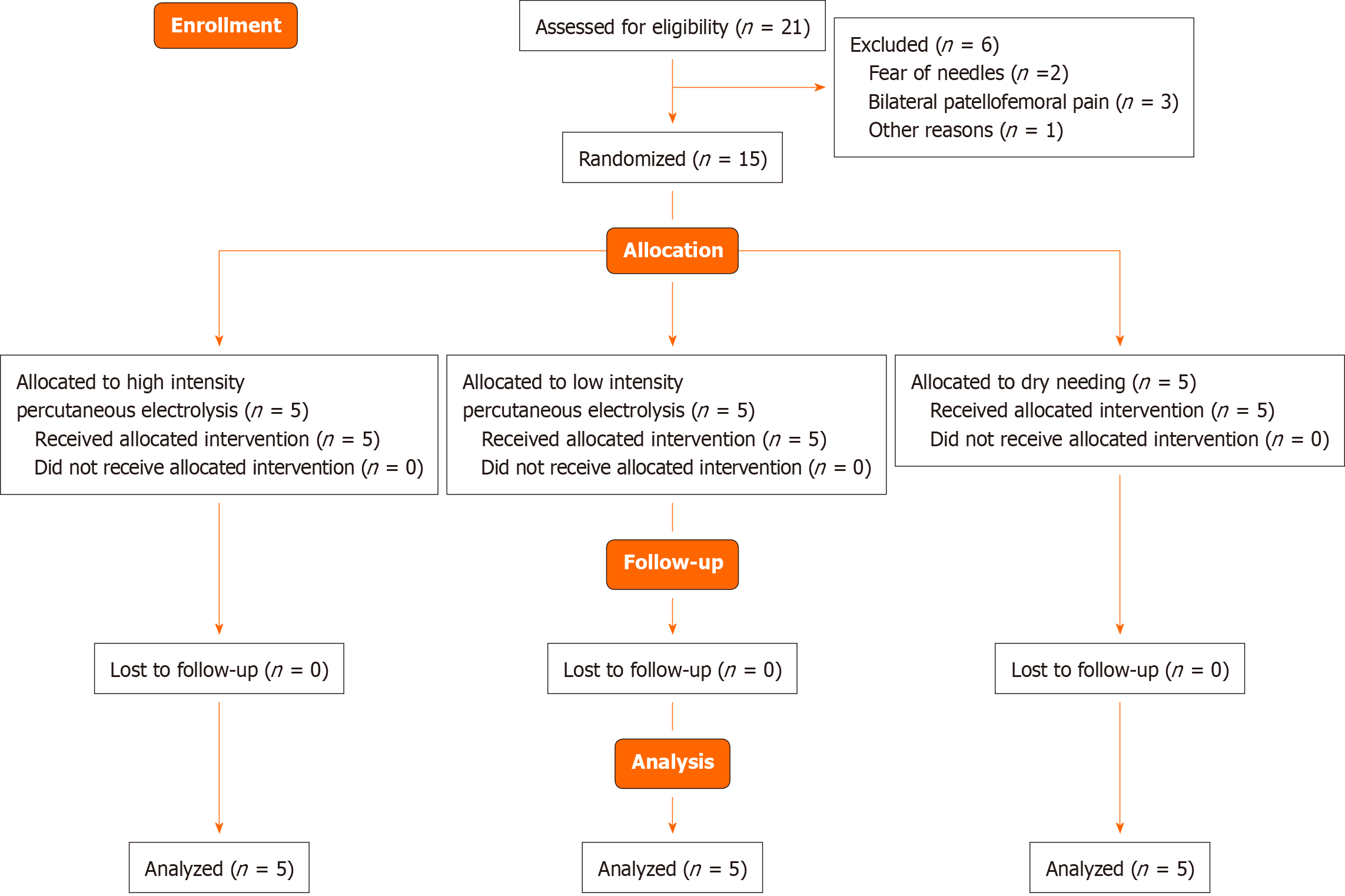
Twenty-one patients with PFPS were initially recruited in September 2020. Six participants were excluded for the following reasons: Fear of needles (n = 2), bilateral PFPS (n = 3), and refused to participate for personal reasons (n = 1). Fifteen patients with unilateral PFPS were finally included and randomized into one of three groups: HIPE (n = 5), LIPE (n = 5) or DN (n = 5). None of the participants in these groups were lost at 7 d follow-up (Figure 2). None of the participants reported adverse effects during the study. Both groups were comparable at baseline as no significant differences in the variables assessed were observed (Table 1).
| Subjects, n (%) | Age (yr) | Height (m) | Weight (kg) | BMI (kg/m2) | |
| Sample | 15 (100) | 25.6 ± 1.9 | 1.73 ± 0.05 | 73.5 ± 6.7 | 24.4 ± 1.6 |
| Intervention group | |||||
| HIPE | 5 (33.3) | 25.4 ± 2.3 | 1.71 ± 0.05 | 72.0 ± 7.7 | 24.5 ± 2.1 |
| LIPE | 5 (33.3) | 26.8 ± 1.4 | 1.75 ± 0.04 | 75.9 ± 6.1 | 24.6 ± 1.4 |
| DN | 5 (33.3) | 24.8 ± 1.8 | 1.73 ± 0.05 | 72.8 ± 6.9 | 24.1 ± 1.6 |
The mixed-model ANCOVA revealed no significant group * time interactions for the outcomes assessed in this study (all, P > 0.05). Post hoc analyses revealed significant improvements in both MTrP (HIPE and LIPE, P < 0.001) and patellar tendon (all groups, P < 0.001) PPTs and SAKPP (HIPE and LIPE, P < 0.05) at follow-up with no significant within-group immediate changes (P > 0.05) (Tables 2 and 3).
| Variable | Time of measurement | HIPE | LIPE | DN | Mean difference | ANOVA interaction effect | Bonferroni post-hoc analysis | |
| MTrP (kg/cm2) | Pre | 4.20 ± 0.57 | 4.10 ± 0.54 | 3.80 ± 0.67 | 0.10 (-0.95-1.15)1; 0.40 (-0.65-1.45)2; 0.30 (-0.75-1.35)1 | F = 0.65; P = 0.562; η2 = 0.09 | Group | NA |
| Post | 3.50 ± 0.61 | 3.60 ± 0.41 | 4.00 ± 0.35 | 0.10 (-0.73-0.93)1; 0.50 (-0.33-1.33)2; 0.40 (-0.43-1.23)3 | F = 1.55; P = 0.251; η2 = 0.20 | Time | ||
| 7 d follow-up | 5.00 ± 1.00 | 5.00 ± 0.50 | 4.50 ± 0.70 | 0.00 (-1.34-1.34)1; 0.50 (-0.84-1.84)2; 0.50 (-0.84-1.84)3 | F = 0.71; P = 0.509; η2 = 0.10 | Follow up > post, P < 0.0014,5 | ||
| Patellar tendon (kg/cm2) | Pre | 5.20 ± 0.83 | 5.30 ± 0.27 | 5.20 ± 0.83 | 0.10 (-1.13-1.33)1; 0.00 (-1.23-1.23)2; 0.10 (-1.13-1.33)3 | F = 0.03; P = 0.967; η2 = 0.00 | Group | NA |
| Post | 4.90 ± 1.19 | 4.70 ± 0.44 | 5.00 ± 0.79 | 0.20 (-1.32-1.72)1; 0.10 (-1.42-1.62)2; 0.30 (-1.22-1.82)3 | F = 0.15; P = 0.858; η2 = 0.02 | Time | Follow-up > pre, P < 0.0014,5,6 | |
| 7 d follow-up | 9.10 ± 0.82 | 9.50 ± 0.35 | 9.00 ± 0.86 | 0.40 (-1.66-0.86)1; 0.10 (-1.16-1.36)2; 0.50 (-0.76-1.76)3 | F = 0.67; P = 0.526; η2 = 0.10 | Follow-up > post, P < 0.0014,5,6 | ||
| Variable | Time of measurement | HIPE | LIPE | DN | Mean difference | ANOVA interaction effect | Group: Bonferroni post-hoc analysis; time: Student t-test | |
| VAS (0-10) | Pre | 4.2 ± 0.5 | 4.5 ± 1.0 | 4.6 ± 1.3 | 0.3 (-1.6-2.1)1; 0.3 (-1.5-2.2)2; 0.1 (-1.8-1.9)3 | F = 0.14; P = 0.868; η2 = 0.02 | Group | NA |
| 7 d follow-up | 2.9 ± 0.9 | 2.8 ± 0.7 | 3.2 ± 0.9 | 0.1 (-1.5-1.6)1; 0.3 (-1.2-1.9)2; 0.4 (-1.1-1.9)3 | F = 0.30; P = 0.741; η2 = 0.05 | Time | Follow up < pre, P < 0.054,5 | |
Finally, participants who received the HIPE and LIPE interventions experienced less pain during the intervention compared with the DN group (HIPE vs DN and LIPE vs DN, P < 0.01) (Table 4).
The aim of this study was to assess the efficacy of two different protocols of percutaneous electrolysis at different intensities and time periods (applying the same electric charge in both groups) compared with DN to improve subjective pain and PPTs at the 7 d follow-up after a single intervention. In addition, perceived pain during the intervention was also assessed. Local twitch responses were found in all the participants during the interventions.
Several findings in this pilot clinical trial were observed. First, the results showed similar improvements in patellar tendon PPTs in all the groups at the 7 d follow up. Second, significant changes in the active rectus femoris MTrP after both electrolysis procedures were observed at the 7 d follow-up, but no changes were found after DN. Third, both electrolysis procedures showed lower SAKPP compared with DN. Fourth, surprisingly, both percutaneous electrolysis procedures were perceived as “less painful” when compared with DN. Finally, several statistical estimates for sample size calculation are reported to develop further research with proper statistical power.
Current evidence recommends a multidisciplinary therapeutic approach, including MTrP management to reduce exacerbated mechano-sensitivity and SAKPP and improve knee function[6,18]. Although several invasive procedures have been compared (e.g., DN with MTrP infiltration (with no significant differences between the methods)[31], and superficial vs deep DN)[32], the available evidence comparing DN with percutaneous electrolysis applied to MTrPs is limited. To our knowledge, only one clinical trial has compared percutaneous electrolysis and DN in patients with temporomandibular disorders[17]. Although this study reported greater improvements in pain reduction and function recovery, these results cannot be extrapolated (as just one pathology was assessed). In addition, as only one electrolysis procedure was assessed, studies evaluating the same electric charges with different application intensity and time or different electric charges are needed.
Available evidence on the efficacy of DN in pain and disability management of patients with PFPS is also limited with controversial findings[33,34]. The use of DN on quadriceps active MTrPs showed no additional pain or function improvements compared with placebo in a single session[6]. However, although VAS and PFPS disability questionnaires were assessed, it should be noted that PPTs were not included and samples sizes are not representative.
One possible explanation for our results regarding better PPT improvements in the active MTrP following HIPE or LIPE compared with DN could be the combined effect of both mechanic (twitch response) and electric stimuli (electrolysis)[14-16]. Further research is needed to analyze the association between clinical improvements and pH-induced changes.
This study had several limitations. First, this was a pilot study. Therefore, our results should be carefully interpreted as the sample size was small and type II errors should be considered. This pilot study was designed to calculate the effect size and provide the sample size needed to obtain appropriate power. Considering the PPT as our primary outcome and setting the effect size f to 0.314 (since eta-squared = 0.09); a = 0.05; 3 groups; and 3 measurements and correlation among repeated measures = 0.3 in the G*Power software V.3.1 for Mac OS, a sample size of 39 subjects is needed to obtain > 0.90 of power. Second, we applied a single session with a limited follow-up. Further research with a larger sample size, number of interventions and longer follow-up is needed to confirm the clinical significance of these study findings.
This triple-blinded, randomized clinical pilot study suggests that a single session of high- or low-intensity percutaneous electrolysis, if the same electric charge is applied, induced similar SAKPP and PPTs improvements in patients with unilateral PFPS. Furthermore, both HIPE and LIPE interventions seemed to be better tolerated compared with DN. However, no differences between-groups were found for SAKPP or PPTs. Further research including larger sample sizes, number of sessions and longer follow-up are needed to confirm these findings.
Dry needling (DN) has shown no additional improvements compared with placebo in patients with patellofemoral pain syndrome (PFPS).
Previous evidence suggested that percutaneous electrolysis could be more effective than DN for managing musculoskeletal pain. However, evidence is limited regarding its efficacy in different conditions and locations.
The efficacy of percutaneous electrolysis compared with DN in patients with unilateral PFPS for improving pain pressure thresholds, subjective anterior knee pain perception and perceived pain during interventions were assessed.
A parallel-group, controlled, triple-blinded, randomized pilot clinical trial was conducted to compare high-intensity percutaneous electrolysis, low-intensity percutaneous electrolysis and DN applied to the most active myofascial trigger points located in the rectus femoris.
Both percutaneous electrolysis modalities induced similar short-term effects on pain perception and sensitivity in patients with unilateral patellofemoral pain syndrome. However, percutaneous electrolysis was better tolerated compared with DN.
Percutaneous electrolysis could be a potential less-painful alternative to DN for reducing pain in patients with unilateral PFPS.
Further research including larger sample sizes, number of sessions and longer follow-up is needed.
Manuscript source: Invited manuscript
Specialty type: Rehabilitation
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jayaseelan DJ S-Editor: Wang JL L-Editor: Webster JR P-Editor: Wang LYT
| 1. | Lankhorst NE, Bierma-Zeinstra SM, van Middelkoop M. Risk factors for patellofemoral pain syndrome: a systematic review. J Orthop Sports Phys Ther. 2012;42:81-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Stathopulu E, Baildam E. Anterior knee pain: a long-term follow-up. Rheumatology (Oxford). 2003;42:380-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Davis IS, Powers CM. Patellofemoral pain syndrome: proximal, distal, and local factors, an international retreat, April 30-May 2, 2009, Fells Point, Baltimore, MD. J Orthop Sports Phys Ther. 2010;40:A1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Boling MC, Padua DA, Marshall SW, Guskiewicz K, Pyne S, Beutler A. A prospective investigation of biomechanical risk factors for patellofemoral pain syndrome: the Joint Undertaking to Monitor and Prevent ACL Injury (JUMP-ACL) cohort. Am J Sports Med. 2009;37:2108-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 5. | Kietrys DM, Palombaro KM, Azzaretto E, Hubler R, Schaller B, Schlussel JM, Tucker M. Effectiveness of dry needling for upper-quarter myofascial pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2013;43:620-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 6. | Sutlive TG, Golden A, King K, Morris WB, Morrison JE, Moore JH, Koppenhaver S. Short-term effects of trigger point dry needling on pain and disability in subjects with patellofemoral pain syndrome. Int J Sports Phys Ther. 2018;13:462-473. [PubMed] |
| 7. | Dommerholt J. Dry needling - peripheral and central considerations. J Man Manip Ther. 2011;19:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Simons DG, Travell JG, Simons LS. Protecting the ozone layer. Arch Phys Med Rehabil. 1990;71:64. [PubMed] |
| 9. | Sikdar S, Ortiz R, Gebreab T, Gerber LH, Shah JP. Understanding the vascular environment of myofascial trigger points using ultrasonic imaging and computational modeling. Annu Int Conf IEEE Eng Med Biol Soc. 2010;2010:5302-5305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, Gerber LH. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 403] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 11. | Gerwin RD, Dommerholt J, Shah JP. An expansion of Simons' integrated hypothesis of trigger point formation. Curr Pain Headache Rep. 2004;8:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Abat F, Gelber PE, Polidori F, Monllau JC, Sanchez-Ibañez JM. Clinical results after ultrasound-guided intratissue percutaneous electrolysis (EPI®) and eccentric exercise in the treatment of patellar tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2015;23:1046-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Money S. Pathophysiology of Trigger Points in Myofascial Pain Syndrome. J Pain Palliat Care Pharmacother. 2017;31:158-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Arias-Buría JL, Truyols-Domínguez S, Valero-Alcaide R, Salom-Moreno J, Atín-Arratibel MA, Fernández-de-Las-Peñas C. Ultrasound-Guided Percutaneous Electrolysis and Eccentric Exercises for Subacromial Pain Syndrome: A Randomized Clinical Trial. Evid Based Complement Alternat Med. 2015;2015:315219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Rodríguez-Huguet M, Góngora-Rodríguez J, Lomas-Vega R, Martín-Valero R, Díaz-Fernández Á, Obrero-Gaitán E, Ibáñez-Vera AJ, Rodríguez-Almagro D. Percutaneous Electrolysis in the Treatment of Lateral Epicondylalgia: A Single-Blind Randomized Controlled Trial. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Valera-Calero JA. Percutaneous Electrolysis: A Potential Tool for Myofascial Pain Syndrome Treatment? Biomed J Sci Tech Res. 2020;27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Lopez-Martos R, Gonzalez-Perez LM, Ruiz-Canela-Mendez P, Urresti-Lopez FJ, Gutierrez-Perez JL, Infante-Cossio P. Randomized, double-blind study comparing percutaneous electrolysis and dry needling for the management of temporomandibular myofascial pain. Med Oral Patol Oral Cir Bucal. 2018;23:e454-e462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Samani M, Ghaffarinejad F, Abolahrari-Shirazi S, Khodadadi T, Roshan F. Prevalence and sensitivity of trigger points in lumbo-pelvic-hip muscles in patients with patellofemoral pain syndrome. J Bodyw Mov Ther. 2020;24:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, Oxman AD, Moher D; CONSORT group; Pragmatic Trials in Healthcare (Practihc) group. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337:a2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1258] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 20. | Fernández-de-Las-Peñas C, Dommerholt J. International Consensus on Diagnostic Criteria and Clinical Considerations of Myofascial Trigger Points: A Delphi Study. Pain Med. 2018;19:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 21. | Rozenfeld E, Finestone AS, Moran U, Damri E, Kalichman L. Test-retest reliability of myofascial trigger point detection in hip and thigh areas. J Bodyw Mov Ther. 2017;21:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Velázquez-Saornil J, Ruíz-Ruíz B, Rodríguez-Sanz D, Romero-Morales C, López-López D, Calvo-Lobo C. Efficacy of quadriceps vastus medialis dry needling in a rehabilitation protocol after surgical reconstruction of complete anterior cruciate ligament rupture. Medicine (Baltimore). 2017;96:e6726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Mayoral O, Salvat I, Martín MT, Martín S, Santiago J, Cotarelo J, Rodríguez C. Efficacy of myofascial trigger point dry needling in the prevention of pain after total knee arthroplasty: a randomized, double-blinded, placebo-controlled trial. Evid Based Complement Alternat Med. 2013;2013:694941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | van der Heijden RA, Rijndertse MM, Bierma-Zeinstra SMA, van Middelkoop M. Lower Pressure Pain Thresholds in Patellofemoral Pain Patients, Especially in Female Patients: A Cross-Sectional Case-Control Study. Pain Med. 2018;19:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Martin-Alguacil JL, Arroyo-Morales M, Martin-Gómez JL, Lozano-Lozano M, Galiano-Castillo N, Cantarero-Villanueva I. Comparison of knee sonography and pressure pain threshold after anterior cruciate ligament reconstruction with quadriceps tendon versus hamstring tendon autografts in soccer players. Acta Orthop Traumatol Turc. 2019;53:260-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Úbeda-D'Ocasar E, Valera-Calero JA, Hervás-Pérez JP, Caballero-Corella M, Ojedo-Martín C, Gallego-Sendarrubias GM. Pain Intensity and Sensory Perception of Tender Points in Female Patients with Fibromyalgia: A Pilot Study. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Karcioglu O, Topacoglu H, Dikme O. A systematic review of the pain scales in adults: Which to use? Am J Emerg Med. 2018;36:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 600] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 28. | Valera-Calero A, Lluch Girbés E, Gallego-Izquierdo T, Malfliet A, Pecos-Martín D. Endocrine response after cervical manipulation and mobilization in people with chronic mechanical neck pain: a randomized controlled trial. Eur J Phys Rehabil Med. 2019;55:792-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Capili B, Anastasi JK, Geiger JN. Adverse event reporting in acupuncture clinical trials focusing on pain. Clin J Pain. 2010;26:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Malfliet A, Lluch Girbés E, Pecos-Martin D, Gallego-Izquierdo T, Valera-Calero A. The Influence of Treatment Expectations on Clinical Outcomes and Cortisol Levels in Patients With Chronic Neck Pain: An Experimental Study. Pain Pract. 2019;19:370-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Ay S, Evcik D, Tur BS. Comparison of injection methods in myofascial pain syndrome: a randomized controlled trial. Clin Rheumatol. 2010;29:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Griswold D, Wilhelm M, Donaldson M, Learman K, Cleland J. The effectiveness of superficial versus deep dry needling or acupuncture for reducing pain and disability in individuals with spine-related painful conditions: a systematic review with meta-analysis. J Man Manip Ther. 2019;27:128-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Behrangrad S, Abbaszadeh-Amirdehi M, Kordi Yoosefinejad A, Esmaeilnejadganji SM. Comparison of dry needling and ischaemic compression techniques on pain and function in patients with patellofemoral pain syndrome: a randomised clinical trial. Acupunct Med. 2020;38:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Zarei H, Bervis S, Piroozi S, Motealleh A. Added Value of Gluteus Medius and Quadratus Lumborum Dry Needling in Improving Knee Pain and Function in Female Athletes With Patellofemoral Pain Syndrome: A Randomized Clinical Trial. Arch Phys Med Rehabil. 2020;101:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |